Abstract
A Tesla type continuous flow left ventricular assist device (VAD) has been designed by Penn State and Advanced Bionics, Inc. (ABI). When a continuous flow device is employed, care must be taken to limit low pressures in the ventricle that can produce an obstruction to the inlet cannula or trigger arrhythmias. Design of an inexpensive, semi-conductor strain gage inlet pressure sensor to detect suction has been completed. The research and design analysis included finite element modeling of the sensing region. Sensitivity, step-response, temperature dependence and hysteresis tests have been performed on prototype units. All sensors were able to withstand the maximum expected strain of 82 μin/in at 500 mmHg internal pressure. Average sensitivity was 0.52 ±0.24 μV/mmHg with 0.5 V excitation (n=5 units). Step response time for a 0 to 90 mmHg step change averaged 22 milliseconds. Hysteresis was measured by applying and holding 75mmHg internal pressure for 4 hours, followed by a zero pressure measurement, and ranged from -15 mmHg to 4.1 mmHg (n=3 units). Offset drift varied between 180 and -140 mmHg over a four week period. (n=2 units). Span temperature sensitivity ranged from 18 to -21 μV/°C (n=5 units). Gain temperature sensitivity ranged from -7.4 to 4.9 μV/°C (n=5 units). With the inherent drift, it is currently not possible to use the transducer to measure actual pressures, but it can easily be used to measure pressure changes throughout the cardiac cycle. This signal can then be used in the control system to avoid ventricular suction events.
Keywords: LVAD, Pressure Sensor, Control
Introduction
Heart failure currently occurs in over 500,000 patients per year.1 While a heart transplant is a viable option for some of these patients, there are currently over 40,000 heart transplant eligible candidates and less than 2,200 hearts available.2 Today, a relatively common treatment includes implantation of a left ventricular assist device (LVAD) as either a bridge to transplant or as end destination therapy. According to The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), 483 LVADs were implanted at ninety-four different centers over a two year span.3 This number is expected to increase as more devices are approved, and more centers are incorporated into the database. Many of these implanted pumps are of a continuous flow scheme. By their design, axial, centrifugal, and mixed continuous flow pumps can create suction at the pump inlet, and care must be taken to avoid negative pressures during decompression of the left ventricle, since it can produce an obstruction to the inlet cannula. Suction on the inlet to the device can potentially trigger malignant arrhythmias, and even cause death.4 An inlet pressure control system with an integrated pressure sensor that can detect potential suction and signal the device controller to take corrective actions is required for these devices to operate at peak efficiencies. Other groups have used other means to estimate pressure including measuring displacement of the rotor5 and investigating pulsatility in the flow.4, 6 One study used a similar technique as the one employed here with good results.7
To facilitate the design of the control scheme, design of an inexpensive, semi-conductor strain gage inlet pressure sensor to detect suction in the inlet of a Tesla style continuous flow device has been completed. The Tesla style pump is unique as compared to other LVADs in that it is a shear flow pump that uses a series of rapidly rotating disc to impart momentum to the fluid.8-10 The transducer is incorporated into the lumen of the titanium inlet connector of the VAD.
Here, we describe in detail the design and evaluation of the sensor, as well as how it may be utilized to monitor pressure changes during the cardiac cycle and control the LVAD. Analytical analysis and finite element modeling of the sensing region has been completed. Sensitivity, step-response, temperature dependence and hysteresis tests have been performed on prototype units. Discussion regarding the end use of the signal will also be introduced.
Description
The sensor body is manufactured from 6Al-4V titanium. This material was chosen for its ease of machining, our prior experience utilizing this material, and biological response of the body to the material which has been well documented. Two diaphragm areas located 180° apart, where the sensor gages are mounted, are produced by wet grinding across the outside of the part, tangential to the inside lumen of the inlet cap to the pump. The diaphragm is a flat only on the outside of the sensor body; a non-obstructed circular path remains through the entire inlet to the device, thereby not disturbing the flow field in any manner. A mandrel is placed inside the inlet cap of the device to give mechanical support to the thin region during the manufacturing process. Once grinding has been completed, a thin region in the shape of an oval and tangential to the axis of rotation and inlet of the device is produced. It is intended that this region be no more than 0.001 inches thick in an area that lies along the central axis of the pump. Once fabrication of the inlet cap diaphragms is complete, the inside is polished to a mirror like finish. This eliminates any need for a coating to be applied to alleviate potential biological depositions. A picture of the prototype inlet cap may be seen in Figure 1 with the oval indicating the general shape of the diaphragm region. The long side of the oval is along the axis of flow through the sensor. Two strain gages are mounted on each side of the inlet of the device oriented 180° apart, one each along and transverse to the central axis of the device. The four gages on each sensor are cross wired in a Wheatstone bridge configuration to help compensate for bending in the inlet. It can be seen in the figure that as the inlet of the device is bent, a gage on one side of the inlet is in compression, while the other side will be in tension. When this occurs, one gage will exhibit an increase in resistance and the corresponding gage on the opposite side of the device will exhibit a decrease in resistance, thereby canceling one another in the output signal.
Figure 1.
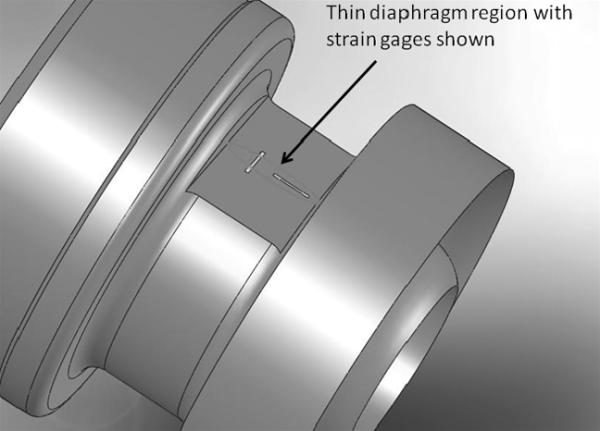
Thin region of the diaphragm as shown in the solid model representation of the inlet cap to the LVAD.
Materials and Methods
General Instrumentation
Instrumentation used with the pressure sensors remained fixed for all experiments, except as noted. Each bridge on the inlet pressure sensors was connected to a National Instruments Corporation (Austin, TX, USA, model SC-2345) data acquisition system (NI-DAQ). The NI-DAQ system had SCC-SG04 modules installed to acquire the output signal from the device. An external variable voltage source (Hewlett-Packard, Palo Alto, CA, USA Model #3630A DC) was set at 0.50 V to provide excitation to the bridge. Ambient temperature of the surrounding air was monitored using an Omega Chromega/Alomega Type-J thermocouple (Omega Engineering Incorporated, Stamford, CT, USA, model #5TC-TT-K-30-36) and connected to the NI-DAQ system using a SCC-TC01 Thermocouple Module. All modules contained a differential amplifier providing a gain of 100 with an error of ±0.8% full scale in the nominal temperature range (23° ± 2° C). The thermocouple module also includes a dual-pole 2 Hz filter onboard. Unless otherwise noted, all experiments were conducted in the nominal temperature range. For all tests, data were sampled at 1000 Hz for one second using a custom developed LabVIEW program. The lengths of time between data samplings varied for each experiment. Each of the sampling files was then averaged to compile a single data point.
Finite Element Analysis
Finite element analysis (FEA) was run on SolidWorks Cosmos Simulation Software (Dassault Systèmes SolidWorks Corporation, Concord, MA, USA). Several different scenarios were run on the solid model of the inlet cap to the LVAD. During all FEA analysis, the mesh for the simulation was tightly controlled in the region of the diaphragm. A close-up view of the mesh control may be seen in Figure 2. For all analyses, the large round cross sections in each figure were fixed and internal pressures of 10, 25 and 500 mmHg were applied. The rigidly mounted setup is similar to the arrangement that will be seen in the final clinical device. The 10 and 25 mmHg pressures were intended to simulate physiological conditions expected to be seen prior to a suction event, and FEA analysis indicated that negative pressures inside the device yielded identical results. The 500 mmHg pressure was intended for design purposes only. Fatigue life for the thin diaphragm region was considered using the FEA generated stress values at the maximum design criteria. The resultant von Mises stress of 1542 psi is well below the yield strength of the titanium material. As a result, at this stress level, the fatigue life of the diaphragm is assumed infinite.
Figure 2.
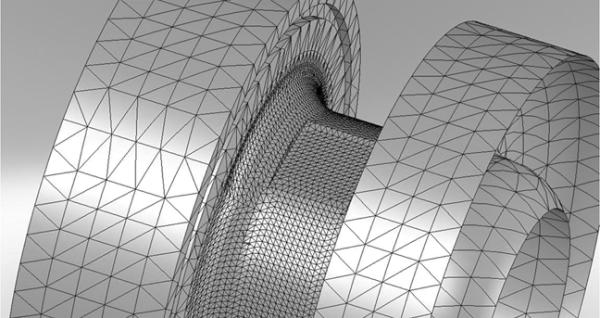
Close-up view of the controlled mesh for the FEA analyses.
Sensitivity Testing
All sensitivity data were acquired with five sensors connected in series by silicone tubing (Figure 3). An Argon Medical (Athen, TX, USA) CDXpress disposable pressure transducer was calibrated using a certified digital pressure manometer (Sper Scientific, Scottsdale, AZ, USA). An initial reading of each sensor output with zero pressure applied was acquired. After the initial reading, a small internal pressure (approximately 5 mmHg) was applied using a stopcock and syringe set-up as seen on the left side of in Figure 3. This process of adding 5 mmHg increments of pressure to the system was repeated until the overall internal pressure reading was 75 ± 5 mmHg. These tests were repeated at both room temperature (23 ± 2°C) and physiological body temperature (37 ± 1°C) for all five sensor prototypes.
Figure 3.
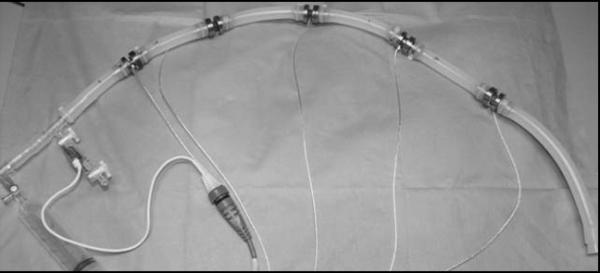
Inlet pressure sensors shown during setup for sensitivity testing. A similar setup with only three sensors was utilized for hysteresis and step testing.
Drift Testing
Two sensor units were placed under a plastic tub to maintain consistent atmospheric conditions, absent large fluctuations in temperature due to air currents. The sensors were allowed to remain undisturbed for a period of 4 weeks. Temperature was monitored using the Omega Chromega/Alomega Type-J thermocouple and data were taken for one second in half-hour intervals.
Hysteresis Testing
The experimental set-up for hysteresis testing was similar to the one that was used during the sensitivity testing described earlier and shown in Figure 3. Three of the prototype sensors were connected in series and run through two types of hysteresis tests. The first test involved holding 75 mmHg of internal pressure (relative to atmospheric pressure) for a prescribed period of time, and then allowing the system to return to zero pressure. The length of time that the system remained pressurized varied for each test. The holding times tested were one minute, fifteen minutes, one hour, and four hours. For all test conditions, an initial reading was taken with no pressure; the system was pressurized, and a reading was taken after 15-20 seconds. The system was allowed to remain pressurized for the prescribed time; another reading was captured, and the pressure was released. After another 15-20 second stabilization period, the final reading was taken with zero pressure in the system. For the two longer tests (one and four hour), an intermediate reading was also taken at the half-way point. For the second hysteresis test, the internal pressure applied to the gages was incrementally increased in 5 to 7 mmHg steps until a pressure of 75 mmHg was reached. Each reading was held for 15-20 seconds, allowed to stabilize, and data were then recorded. For each of the second type tests, at least 10 data points were taken on the way up to 75 mmHg and during the return to zero pressure.
Step Testing
Testing of pressure gages typically includes step testing, in which a pressure or load is suddenly applied to or removed from the gage. A standard type of step test for pressure sensors is the pop test.11 The pressure sensors were connected in series with an Argon pressure transducer and a standard 60cc Luer lock medical syringe (Figure 3) attached to the system via a 3-way stopcock. A negative pressure of -95 ± 5 mmHg was pulled onto the system using the syringe. The syringe rubber plunger was ‘popped’ free and removed from its housing allowing ambient pressure to rush into the system, creating the step input.
In Vitro Testing
Response of the sensor to detect potential suction events was also tested in vitro. On a Penn State mock circulatory loop, the Tesla type LVAD was plumbed in series with a Thoratec pneumatic VAD (which simulated the native ventricle). The custom pressure sensor was placed between the two devices, as was a fitting to allow the connection of an Argon commercial pressure transducer. Figure 4 shows the loop set-up for this test.
Figure 4.
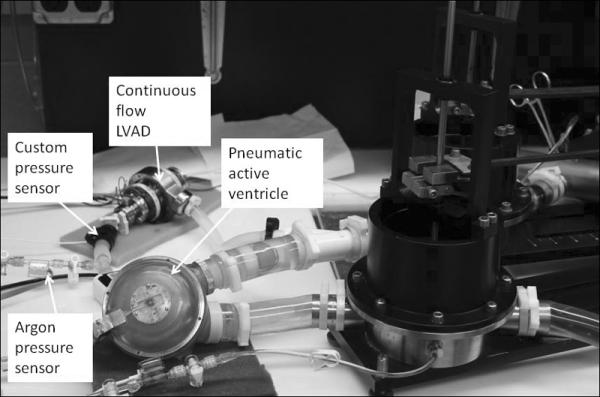
Loop setup for in vitro testing.
In Vivo Testing
A five-hour in vivo study using the Tesla type VAD with an inlet pressure sensor was conducted on a 105 kg calf. To compensate for baseline drift, the pressure trace was detrended by subtracting a 1 hour moving average of the input signal. The detrended inlet pressure signal was input to a custom control algorithm to automatically vary the pump speed. Suction was detected as a large drop in inlet pressure (-dP/dt). When a suction event was recognized, the control algorithm instructed the pump to reduce the set speed of the device.
Results and Discussion
FEA estimated strain for the 10 and 25 mmHg internal pressure on the inlet cap was 1.6 and 3.5 μm/m, respectively. Estimated strain at the design maxima criterion of 500 mmHg was 82 μm/m. FEA estimated pictorial representations of strain for the operating pressures may be seen in Figure 5a and b where the internal pressure was fixed at 10 mmHg (Figure 5a) and 25 mmHg (Figure 5b).
Figure 5.
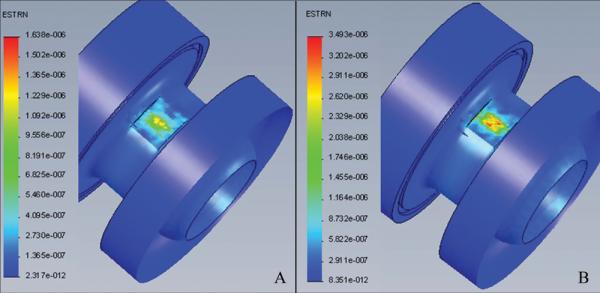
a) FEA model showing predicted strain at 10 mm Hg, and b) FEA model showing predicted strain at 25 mm Hg.
Drift tests revealed that offset (zero pressure) drift varied from -180 to 140 mmHg over a four week period (n = 2 units). Drift of this magnitude precludes the use of the sensor to measure true pressure at the VAD inlet (i.e., gage pressure relative to intrathoracic pressure). Low drift versions of these sensors of this type are available, and need to be investigated. Also, the use of a different approach to sensor gage bonding in which the gage is molecularly bonded to the diaphragm is currently being explored.
Average sensitivity for the prototype devices were 0.52 ± 0.24 μV/mmHg with 0.5 V excitation (n = 5 units). Larger values of excitation voltage were experimented with; however, this caused self heating of the gages and led to very erratic drift behavior. A custom dual-stage amplification box was also made for each sensor utilizing a commercially purchased low-drift precision amplifier (LT1013, Linear Technology, Milpitas, California, USA). The gain applied to each signal was approximately 10,000. Once this amplification was added to the set-up, the amplified NI module (SCC-SG04) was removed from the system and replaced by a voltage feed-through module (SCC-FT01). A comparison of the sensitivity tests at two temperatures (23 ± 2°C and 37 ± 1°C) yielded the gain drift for the sensors of -8.1 to 4.2 ± 45.3 μV/°C (n = 5 units).
Pulsatile waveforms were also introduced to the sensors to measure span drift at multiple temperatures, room temperature (23 ± 2°C) and physiological body temperature (37 ± 1°C). The physiological temperature was introduced to the sensor through the use of a heated water bath and warmed fluid circulating through the devices. Comparing the two pulsatile waveforms, it was observed that maximum span drift ranged from 18 to -21 ± 19.7 μV/°C (n = 5 units).
Step response time for a 0 to 90 mmHg step change averaged 22 milliseconds (n = 5 units). The resulting unfiltered output signal from the two sensors may be seen in Figure 6. The remaining three sensors were not shown for clarity; however all remaining sensors exhibited similar response characteristics. As can be seen in Figure 6, the sensor responds almost instantaneously to the step input, similar to the Argon control transducer.
Figure 6.
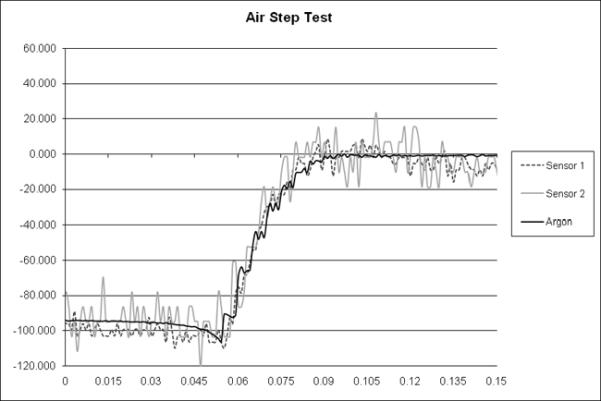
Graph of pressure versus time for two prototype sensors and the Argon Medical control transducer during the step test.
Hysteresis ranged from -15 mmHg to 4.1 mmHg (n = 3 units). These values were measured from the four hour test, during which the three sensors exhibited their most hysteresis. Hysteresis was not statistically significant during the shorter duration tests. While the exact cause of the hysteresis was not determined, it was speculated that the problem derives from the use of an epoxy to hold the gages to the diaphragm.
Transient response of the sensor appears to adequately detect impending suction events. In Figure 7, the top trace is the custom pressure sensor, while the bottom trace indicates the Argon control. A suction event was determined to be occurring through visual observation of the blood sac in the pneumatic Thoratec device (Figure 4). During a suction event, the blood sac would occlude the inlet to the ABI device and a simultaneous zero flow reading would result. As can be observed, three suction events occur in the first nine seconds of the experiment and both devices successfully register these events. After approximately 10 seconds, the suction events are fully resolved. Both sensors then return to “normal” trace. The negative pressure spikes are the result of rapid flow deceleration during the occlusion of the inlet cannula.
Figure 7.
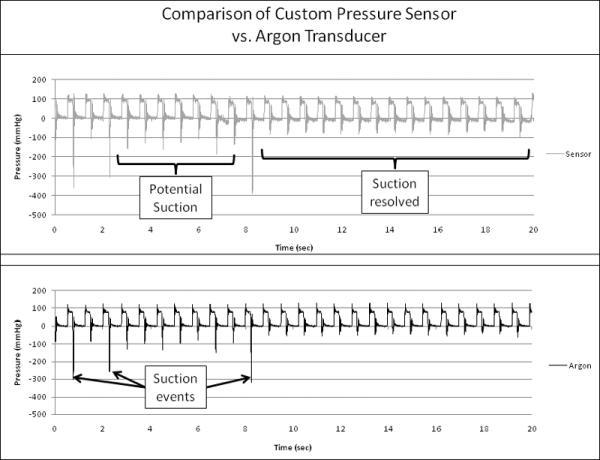
Graph showing the output trace from the custom pressure sensor (top) and the Argon transducer (bottom) during in-vitro testing. Both devices detect the potential impending suction events adequately.
As can be seen in Figure 8, the inlet pressure sensor can also adequately detect suction on the inlet cannula during an in vivo experiment. Pressure sensor drift did not occur over time scales less than 1 hour, and therefore, deviations in the pressure trace on a beat-to-beat basis were due to physiologic changes in pressure and not due to baseline drift. The output signal from the inlet pressure sensor will be used in active control of the device. As a suction event is detected, the control algorithm reduces pump speed until the suction event has resolved.
Figure 8.
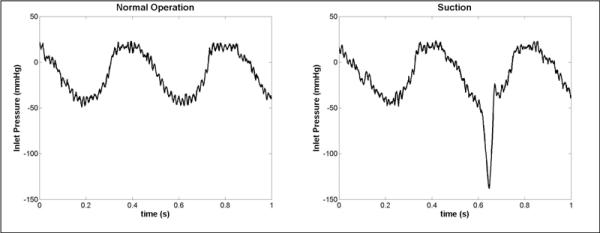
Pressure traces from the integrated inlet pressure sensor during an acute in-vivo experiment in a calf. Suction events (right trace) caused a sharp negative transient in the pressure during diastole. The pressure trace is shown detrended by the long term average inlet pressure in order to compensate for sensor drift.
Conclusion
An inexpensive, custom pressure sensor has been developed to detect potential suction events in the inlet to a new type of VAD. Drift currently remains an issue and alternative methods of gaging the system are being investigated. If drift in the system can be stabilized, a duplicate type of device may be placed on the outlet of the device to help in flow estimation. While the goal to measure physiological blood pressure may not have been achieved, the output is still indicative of pressure inside the lumen to the inlet of the device. This output signal will be fed into the control algorithm to determine if a suction event is occurring and reduce pump speed as necessary. Although at the present time the drift of the sensor limits pressure measurements, the signal is quite satisfactory as a control signal to reduce/eliminate ventricular suction events.
Acknowledgments
Disclosure: Work was supported by NIH Grant # R01HL81119, Development of an Innovatively Suspended TESLA Pump LVAD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. [June 1, 2009];Cardiovascular disease statistics. 2005 http://www.americanheart.org/presenter.jhtml?identifier=4478.
- 2. [May 25, 2009];Trends in hospitalization for heart failure by age group, 1979 - 2004, United States. 2006 http://www.cdc.gov/DHDSP/library/fs_heart_failure.htm.
- 3.Holman WL, Pae WE, Teutenberg JJ, et al. INTERMACS: interval analysis of registry data. J Am Coll Surg. 2009;208:755–761. doi: 10.1016/j.jamcollsurg.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Vollkron M, Voitl P, Ta J, Wieselthaler G, Schima H. Suction events during left ventricular support and ventricular arrhythmias. J Heart Lung Transplant. 2007;26:819–825. doi: 10.1016/j.healun.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Hetzer R, Weng Y, Potapov EV, et al. First experiences with a novel magnetically suspended axial flow left ventricular assist device. Eur J Cardiothorac Surg. 2004;25:964–970. doi: 10.1016/j.ejcts.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Vollkron M, Schima H, Huber L, Benkowski R, Morello G, Wieselthaler G. Development of a reliable automatic speed control system for rotary blood pumps. J Heart Lung Transplant. 2005;24:1878–1885. doi: 10.1016/j.healun.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Bullister E, Reich S, D'Entremont P, Silverman N, Sluetz J. A blood pressure sensor for long-term implantation. Artif Organs. 2001;25:376–379. doi: 10.1046/j.1525-1594.2001.025005376.x. [DOI] [PubMed] [Google Scholar]
- 8.Tesla N. Inventor. Turbine. US patent 1,061,2061913.
- 9.Izraelev V. Inventor. Blood pump having rotor with internal bore for fluid flow. US patent 5,938,4121997.
- 10.Izraelev V, Weiss WJ, Fritz B, et al. A passively suspended Tesla pump left ventricular assist device. ASAIO J. 2009;55:556–561. doi: 10.1097/MAT.0b013e3181bae73e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols WW, O'Rourke MF. McDonald's blood flow in arteries: theoretic, experimental and clinical principles. Hodder Arnold; London, UK: 1998. [Google Scholar]


