Abstract
The investigation and study of cancer stem cells (CSCs) have received enormous attention over the past 5 to 10 years but remain topics of considerable controversy. Opinions about the validity of the CSC hypothesis, the biological properties of CSCs, and the relevance of CSCs to cancer therapy differ widely. In the following commentary, we discuss the nature of the debate, the parameters by which CSCs can or cannot be defined, and the identification of new potential therapeutic targets elucidated by considering cancer as a problem in stem cell biology.
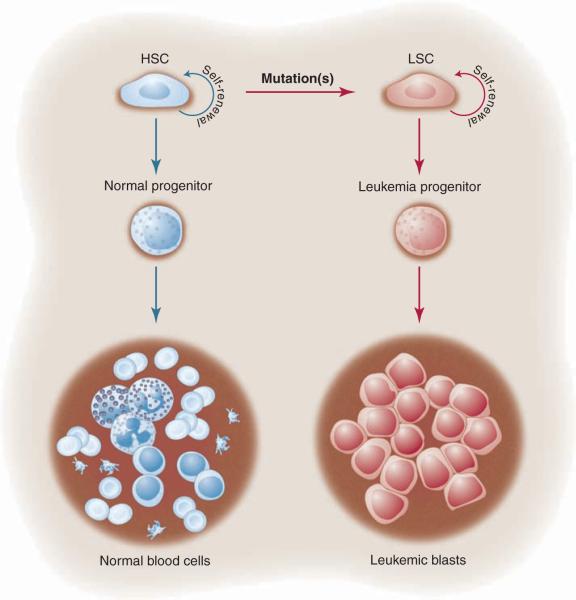
In 1994, John Dick and colleagues published their seminal paper that human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell, as shown in Fig.1 (1, 2). This report became the paradigm for later studies, which suggested that a similar model existed for solid tumors with cancer stem cells (CSCs) at the top of a hierarchical pyramid (3). These studies were based on a similar approach that uses fluorescence-activated cell sorting (FACS) of primary human cells with antibodies directed at defined cell-surface markers followed by limiting dilution transplantation, usually into an orthotopic site in immunocompromised mice (the xenograft model). Thus, the CSC paradigm refers to the ability of a subpopulation of cancer cells to initiate tumorigenesis by undergoing self-renewal and -differentiation, like normal stem cells, whereas the remaining majority of the cells are more “differentiated” and lack these properties.
Fig. 1.
Initial studies in leukemia provided the paradigm for the general CSC model. As shown on the left side of the figure, a hematopoietic stem cell (HSC) gives rise to normal progenitors and mature blood cells. The original model suggests that the HSC undergoes mutation(s) that give rise to its malignant counterpart, the leukemia stem cell (LSC). The LSC retains some degree of developmental potential, generating the leukemia progenitor and leukemic blast cells, which differ in their biological properties from the parent LSC. As in normal hematopoiesis, the stem cell maintains the ability to undergo self-renewal and thereby perpetuate the leukemia population.
Why Is There a Debate?
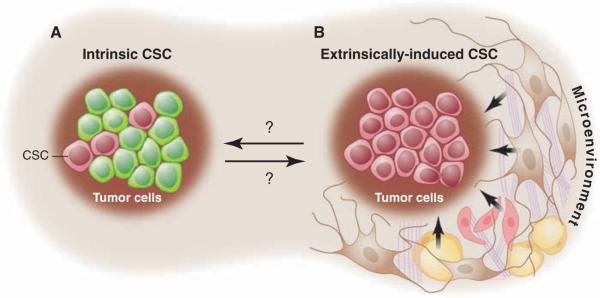
The concept that a specific subpopulation of tumor cells possesses distinct stem cell properties implies that CSCs arise as an intrinsic property of tumor biology and development (Fig. 2). However, the surrounding microenvironment (stromal fibroblasts, adipocytes, and endothelial cells, as well as the extracellular matrix) and the immune system are known to play important roles in cancer progression (4, 5). Consequently, one caveat to the intrinsic model in the context of a xenograft is the lack of an appropriate microenvironment because of differences between the mouse and human and the lack of an intact immune system when evaluating the tumor-initiating capacity of these human cancer cells. Thus, it is possible that the subpopulation of cells that appeared nontumorigenic might actually be tumorigenic in the presence of the appropriate microenviroment. In other words, tumor cells might be functionally homogeneous, with heterogeneous potential arising as a consequence of extrinsic cues or the lack thereof (Fig. 2). Strasser and colleagues attempted to test the original CSC hypothesis by using an alternative approach to the xenograft system. They used two transgenic mouse models in which the Eμ enhancer was used to express either the c-myc or N-ras oncogenes to induce B or T cell lymphomas, respectively (6). Upon the analysis of transplants, these authors concluded that “tumor growth need not be driven by rare cancer stem cells” based upon >10% of the transplanted cells, giving rise to tumors in syngeneic mice. Alternatively, recent studies by Guo et al. used a mouse model in which deletion of the Pten tumor suppressor gene in hematopoietic stem cells resulted in a myeloproliferative disorder followed by acute T-lymphoblastic leukemia (7). Using this model of a human leukemia, these investigators demonstrated by limiting dilution transplantation that a rare population of leukemia stem cells (LSCs) was responsible for leukemia development. Taken together, these findings indicate that the relative frequency and role of malignant stem cells can vary considerably as a function of the specific experimental system as well as perhaps the particular oncogenes or tumor suppressor genes used. However, for at least some forms of leukemia, stem cell properties reside within a relatively rare (<1%) subset of the tumor population, a finding that is consistent in both the xenograft and syngeneic models (8, 9).
Fig. 2.
CSC models. (A) The intrinsic model suggests that specific subpopulations within a tumor (pink cells) possess the functional properties of CSCs. (B) The extrinsic model proposes that all tumor cells are functionally equivalent and display heterogeneous behaviors as a function of extrinsic (microenviron-mental) cues.
What Is the Situation in Solid Tumors?
In 2003, Michael Clarke and colleagues adapted to breast cancer the methods (10) used in leukemias and demonstrated the existence of a subpopulation of tumorigenic cells isolated primarily from breast cancer pleural effusions by limiting dilution transplantation of CD44+/CD24−/lo/lineage− cells into the mammary fat pad of immunocompromised nonobese diabetic–severe combined immunodeficiency (NOD-SCID) mice. A year later, Peter Dirks and colleagues reported the identification of human brain tumor–initiating cells again with FACS analysis but using a different cell surface marker CD133 (also called prominin1) and orthotopic intracranial transplantation into NOD-SCID mice (11). These reports led to a flurry of similar studies in other solid tumors [reviewed in (3)].
FACS and xenotransplantation of viable single cells from solid tumors require modifications of the approaches used for hematologic cancers because of the need to dissociate solid tissues into single cells, which are larger and more fragile than the hematopoietic cells usually isolated by these methods. Such procedures require several manipulations that may affect cell viability or behavior. Thus, the frequency of CSCs calculated in limiting dilution experiments can be markedly influenced by these parameters as well as the duration of the experiment performed to assess tumorigenicity. In addition, the nature of the immunocompromised host can also play a critical role in the efficiency of tumor formation. This is illustrated by the recent studies on human melanoma cells in which modified xenotransplantation conditions, including the use of more highly immunocompromised NOD/SCID interleukin-2 receptor γ chain null mice, increased the detection of tumorigenic melanoma cells by several orders of magnitude (12). Approximately one in four of the unselected melanoma cells formed tumors in these studies, which argues that the CSC population is not necessarily always rare and leads investigators to question, “Have we been studying these cells all along?” These studies highlight that the relative frequency of CSCs may vary as a function of both the tumor type and the specific experimental system used.
Once again, the use of syngeneic mouse models for analysis of CSCs has helped clarify the role of the microenvironment for solid tumors. As an example, two studies using mouse breast cancer models, the p53 null transplantation and the MMTV-Wnt1 models, have demonstrated by limiting dilution transplantation analysis that a small subpopulation of tumor cells are CSCs, whereas the bulk of the tumor cells are nontumorigenic (13, 14). The secondary tumors that arise from isolated CSCs phenocopy the original tumors and give rise to the heterogeneous cell types observed in the primary tumors. This type of cell heterogeneity is not observed in all genetically engineered mouse models of breast cancer, so it is probable that in some models the frequency of CSCs may be quite high, similar to the observations made by Strasser and his colleagues using the Eμ lymphoma models. Studies using the same p53 null mouse breast cancer model and improved FACS protocols found tumors formed with a more than 10-fold higher frequency than previously reported (14), thus illustrating that the absolute CSC frequencies are highly dependent on the experimental conditions used. Although the calculated frequencies of CSCs may therefore vary depending on the methods used to isolate and transplant these cells, the fold enrichment observed between the CSCs and bulk tumor population was quite similar. Most importantly, >90% of the bulk tumor cells were nontumorigenic even after more than a year after transplantation. Thus, like the findings from hematologic cancers, the data from solid-tumor analyses demonstrate a high degree of variability depending on the specific experimental system. However, despite concerns noted for the use of xenografts, data from syngeneic models demonstrate the existence of subpopulations of cells in at least some solid tumors, satisfying the functional criteria of CSCs.
Why Is the CSC Paradigm Becoming More Complex?
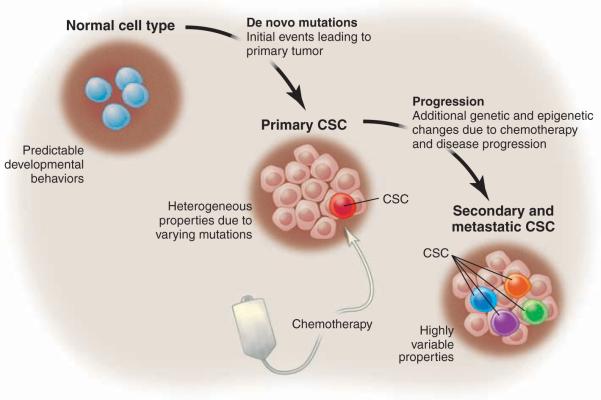
Perhaps the most challenging issue facing the field is the fact that CSCs in primary tumors do not always display the properties classically used to define normal stem cells, cells with the ability to self-renew and -differentiate into multiple cell types. The cell-surface immunophenotype of primary tumors, as well as the frequency of functionally defined CSCs, can vary dramatically among different patients. In some cases, CSCs are relatively rare, whereas in others CSCs can constitute a substantial proportion of the tumor mass (12). So, why are tumor CSCs so variable? In normal steady-state systems, one can expect reasonably well-conserved development behavior, but upon any kind of substantial genetic or epigenetic perturbation, the rules that define cell and tissue behavior are not easily predicted and must be defined empirically. Thus, in the context of inherently unstable conditions such as cancer, the fact that CSCs display varying behaviors is no surprise. Moreover, the properties of CSCs appear to be influenced by both the specific genetic aberrancies in a given tumor as well as the stage of disease progression and the types of drugs used to challenge tumor growth (Fig. 3). Consequently, for any particular type of cancer the patient-to-patient variability of CSCs may be quite substantial. Taken together, these issues make any consistent definition of CSC properties difficult and suggest that being overly rigid in how CSCs are defined is not realistic. Furthermore, the variability in CSC properties introduces problems when developing new therapies.
Fig. 3.
Stages of CSC evolution. For many tumor types, the de novo mutations leading to primary CSC are varied. Thus, one would expect that the biology of primary CSCs may also be heterogeneous. Properties such as CSC frequency, cell-surface phenotype, and drug sensitivity may vary as a function of the specific mutations as well as the nature of the normal cell type in which the primary events occur. Next, neoplastic progression may occur either as a consequence of intrinsic tumor pathogenesis and/or challenge with chemotherapy. Selective pressures associated with neoplastic progression may lead to a higher frequency of functionally defined CSC in secondary or metastatic stages as well inter-patient and intra-patient variability of CSC properties.
A genetic program that might account in part for the diversity of abundance of CSCs in solid tumors is the ability of cells to undergo an epithelial-to-mesenchymal transition (EMT). Recent studies have suggested that induction of EMT in immortalized human mammary epithelia cells results in cells with stem-like properties, such as “ an increased ability to form mammo-spheres, a property associated with mammary epithelial stem cells” (15). A number of studies have suggested that cells at the leading invasive edge of solid cancers, such as colon, breast, and pancreatic tumors, exhibit more mesenchymal features and are characterized by the expression of CSC markers (16–18). Thus, there has been a convergence of the well-established concept that EMT is associated with tumor progression with the more recent hypothesis of migratory cancer stem cells (19, 20). So how might this process be regulated in CSCs? Recent studies have implicated transcription factors, such as Zeb1 and −2 and Twist, which are known inducers of EMT (21), and a negative feedback pathway involving transforming growth factor–β (TGFβ) (16, 22). So, changes in the surrounding microenvironment that influence expression of TGFβ family members as well as other cytokines expressed by mesenchymal stem cells or other cells in the microenvironment may influence both EMT and the reverse process of mesenchymal-to-epithelial transition, which is most likely critical for metastasis and colonization at distant sites (23). If true, then the CSC state may be transitory in certain circumstances, with tumor cells acquiring more of a stem-like phenotype upon stimulation with the appropriate environmental cues.
Studies of putative tumor stem cells have also served to highlight the potential clinical importance of the relationship between EMT and CSCs. For example, using the same sub-population of tumorigenic breast cancer cells identified in (10), we reported the intrinsic resistance of these cells to chemotherapy studied in paired breast cancer biopsies (24). We also identified a TGFβ-like tumorigenic gene signature in a molecular subtype of human breast tumors characterized by expression of many mesenchymal-associated genes. Tumors resistant to conventional treatments were enriched for cells bearing this signature, and increased mesenchymal markers were observed in the posttreatment specimens. These data support a growing body of evidence for a mesenchymal-like phenotype in breast and possibly other solid tumors that may be responsible for invasion, metastases, and even treatment resistance.
To understand the relationship between CSCs and normal stem cells, bioinformatic studies compared the transcriptional programs in embryonic stem cells (ESCs) with adult tissue stem cells and human cancers (21). The ESC-like transcriptional program shown to be activated in diverse human epithelial cancers was a predictor of metastasis and death. The oncogene c-Myc, but not other oncogenes, appeared to be sufficient to activate this ESC-like program and could increase the fraction of CSCs. These authors concluded that “Activation of an ESC-like transcriptional program in differentiated adult cells may induce pathologic self-renewal characteristic of cancer stem cells.” Further, Weinberg and colleagues have reported that a subset of ESC-associated transcriptional regulators are more frequently overexpressed in poorly differentiated tumors (25). These authors concluded “that these genes contribute to stem cell-like phenotypes shown by many tumors.” Such data imply that some poor-prognosis tumors may possess higher frequencies of CSCs.
New Directions Resulting from the Study of CSCs
The controversy about CSCs has had the unexpected benefit of stimulating research in areas that previously were not the focus of cancer therapeutics. Pathways known to be important for stem-cell self-renewal, such as the Wnt, Notch, and Hedgehog (Hh) (which were previously identified as relevant to cancer as well as developmental biology), are now of increased interest because of their potential role in CSCs. For example, the first clinical trial using gamma-secretase inhibitors to block the Notch pathway in combination with chemotherapy in the neoadjuvant setting for breast cancer has recently been initiated. Another example is the recent report that Hh signaling is essential for maintenance of CSCs in myeloid leukemia (26).
The indication that there were distinctive sub-populations of cells within tumors with stem-like properties, which could be isolated using a variety of cell-surface markers, has led investigators to identify the specific signaling pathways in these cells as compared with the bulk tumor cells and their normal counterparts. Studies have suggested there might be selective effects of inhibiting the Pten/Akt and nuclear factor κB(NF-κB) pathways in LSCs as compared with normal hematopoietic stem cells (27–29). These studies have been extended to solid tumors with the observation that brain (and possibly breast) CSCs may be preferentially sensitive to Akt inhibitors (30). Studies of the mechanisms by which parthenolide, an NF-κB pathway inhibitor, might induce apoptosis in LSCs have suggested a role for increased reactive oxygen species (ROS) (31). In fact, recent studies have suggested an association of ROS levels and radioresistance in CSCs (32). Variability in the response of CSCs to conventional chemotherapy and radiation therapy have led to investigations of the differences in cell-cycle checkpoint and DNA-repair pathways in CSCs versus the bulk of tumor cells (14, 33, 34). An exciting new approach is the use of chemical genetic screens of drug libraries against CSCs with the possibility of discovering drugs to target CSCs that may already be approved for alternative clinical use (35). Another interesting approach will be to use synthetic lethal screens to search for new agents that may sensitize resistant tumor-cell subpopulations to conventional chemoand radiation therapies (36). Finally, pharma and biotech companies now have active programs to develop therapeutics so as to target many of these pathways.
Whether the CSC model is relevant to all cancers or not, it is clear that we need new approaches to target tumor cells that are resistant to current therapies and give rise to recurrence and treatment failure. Notwithstanding our ability to sequence the cancer genome(s) and to create personalized targeted therapies, it is apparent that combination therapies, which target the CSC subpopulation as well as the bulk of the tumor cells, will be required to effectively manage cancer treatment (37). One pressing need is the development of improved preclinical models to test these therapies because determining the appropriate doses and combinations as well as the order of addition of these agents will be critical for success in the clinic. The fact that these concepts are steadily making their way into the clinic is exciting and suggests that the recent interest in CSCs may yield beneficial outcomes in potentially unexpected ways.
Acknowledgments
The authors thank W. Woodward, M. Zhang, Y. Li, M. Guzman, and J. Dick for their helpful comments and apologize to investigators for the failure to cite many other important contributions in this field because of space limitations. These studies were supported by NIH grants R37-CA16303 and R01-CA122206.
References and Notes
- 1.Lapidot T, et al. Nature. 1994;367:645. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 2.Dick JE. Blood. 2008;112:4793. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 3.Visvader JE, Lindeman GJ. Nat. Rev. Cancer. 2008;8:755. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 4.Bissell MJ, Labarge MA. Cancer Cell. 2005;7:17. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A. Nature. 2009;457:36. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 6.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 7.Guo W, et al. Nature. 2008;453:529. doi: 10.1038/nature06933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnet D, Dick JE. Nat. Med. 1997;3:730. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 9.Neering SJ, et al. Blood. 2007;110:2578. doi: 10.1182/blood-2007-02-073031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Proc. Natl. Acad. Sci. U.S.A. 2003;100:3983. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh SK, et al. Nature. 2004;432:396. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 12.Quintana E, et al. Nature. 2008;456:593. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho RW, Clarke MF. Curr. Opin. Genet. Dev. 2008;18:48. doi: 10.1016/j.gde.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, et al. Cancer Res. 2008;68:4674. doi: 10.1158/0008-5472.CAN-07-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mani SA, et al. Cell. 2008;133:704. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burk U, et al. EMBO Rep. 2008;9:582. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginestier C, et al. Cell Stem Cell. 2007;1:555. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermann PC, et al. Cell Stem Cell. 2007;1:313. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Thiery JP. Nat. Rev. Cancer. 2002;2:442. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 20.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Nat. Rev. Cancer. 2005;5:744. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 21.Wong DJ, et al. Cell Stem Cell. 2008;2:333. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregory PA, Bracken CP, Bert AG, Goodall GJ. Cell Cycle. 2008;7:3112. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- 23.Karnoub AE, et al. Nature. 2007;449:557. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 24.Li X, et al. J. Natl. Cancer Inst. 2008;100:672. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Porath I, et al. Nat. Genet. 2008;40:499. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao C, et al. Nature. 2009;458:776. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Blood. 2003;102:972. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 28.Guzman ML, et al. Blood. 2001;98:2301. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 29.Yilmaz OH, et al. Nature. 2006;441:475. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 30.Eyler CE, et al. Stem Cells. 2008;26:3027. doi: 10.1634/stemcells.2007-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guzman ML, et al. Blood. 2005;105:4163. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diehn M, et al. Nature. 2009;458:780. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao S, et al. Nature. 2006;444:756. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 34.Woodward WA, et al. Proc. Natl. Acad. Sci. U.S.A. 2007;104:618. [Google Scholar]
- 35.Diamandis P, et al. Nat. Chem. Biol. 2007;3:268. doi: 10.1038/nchembio873. [DOI] [PubMed] [Google Scholar]
- 36.Whitehurst AW, et al. Nature. 2007;446:815. doi: 10.1038/nature05697. [DOI] [PubMed] [Google Scholar]
- 37.Woodward WA, Chen MS, Behbod F, Rosen JM. J. Cell Sci. 2005;118:3585. doi: 10.1242/jcs.02532. [DOI] [PubMed] [Google Scholar]