Abstract
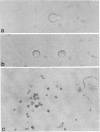
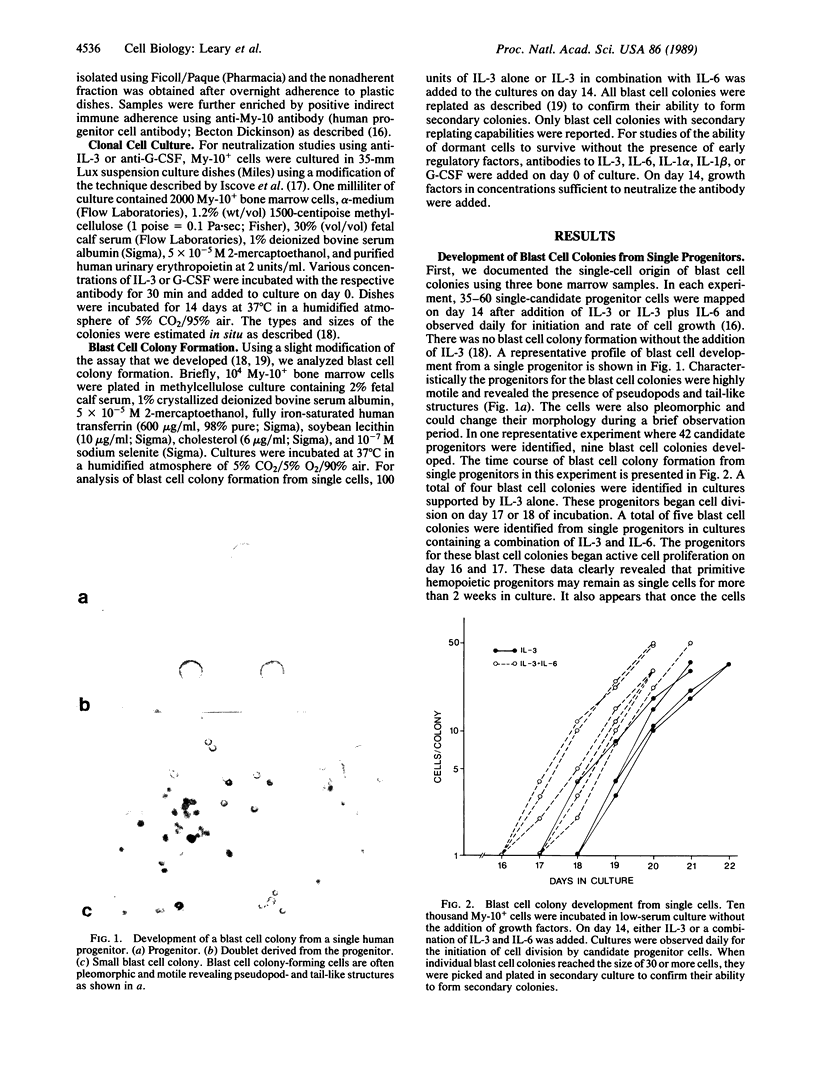
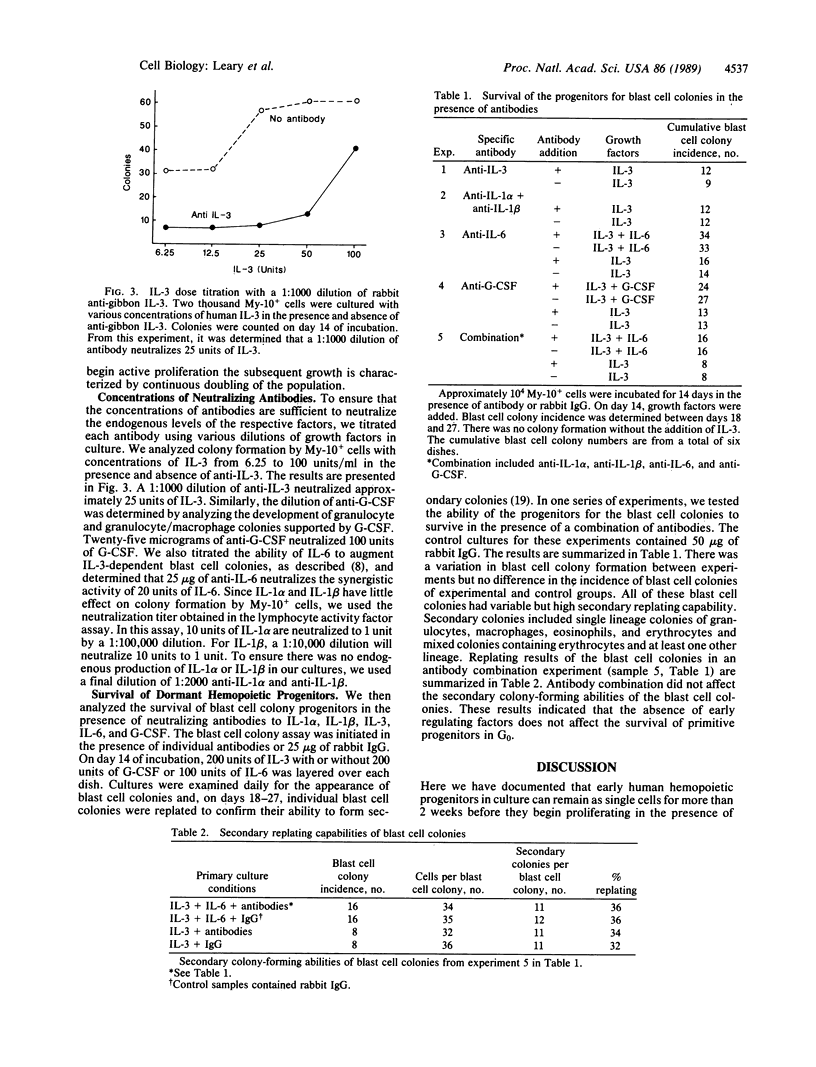
Although it is generally held that hemopoietic stem cells in steady-state marrow are dormant in the cell cycle, the direct proof for this concept has been lacking. In the present study, we have documented the development of human multipotential blast cell colonies from single cells by daily observation of the growth of candidate progenitors. The results clearly demonstrated that early hemopoietic progenitors may remain as single cells for more than 2 weeks of incubation. Once the progenitors began proliferation, the subsequent growth was characterized by steady cell doubling. Next, we tested the survival of blast cell colony progenitors in the presence of neutralizing antibodies prepared against early acting hemopoietic factors including interleukin (IL) 1 alpha, IL-1 beta, IL-3, IL-6, and granulocyte colony-stimulating factor. Cultures were initiated with individual antibodies, and, on day 14, IL-3 and the corresponding growth factor in concentrations that neutralize the antibodies were added. On days 18-27 of culture, blast cell colonies containing 25 or more cells were identified and replated for analysis of their ability to form secondary colonies. The cumulative frequency of the blast cell colonies in cultures containing antibody did not differ significantly from that of the control group containing rabbit IgG. A combination of anti-IL-1 alpha, anti-IL-1 beta, anti-IL-6, and anti-granulocyte colony-stimulating factor did not affect the survival of dormant blast cell colony-forming cells. These results indicate that survival of hemopoietic stem cells in the G0 period of the cell cycle is independent of early hemopoietic regulators.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER A. J., MCCULLOCH E. A., SIMINOVITCH L., TILL J. E. THE EFFECT OF DIFFERING DEMANDS FOR BLOOD CELL PRODUCTION ON DNA SYNTHESIS BY HEMOPOIETIC COLONY-FORMING CELLS OF MICE. Blood. 1965 Sep;26:296–308. [PubMed] [Google Scholar]
- Hodgson G. S., Bradley T. R. Properties of haematopoietic stem cells surviving 5-fluorouracil treatment: evidence for a pre-CFU-S cell? Nature. 1979 Oct 4;281(5730):381–382. doi: 10.1038/281381a0. [DOI] [PubMed] [Google Scholar]
- Ikebuchi K., Clark S. C., Ihle J. N., Souza L. M., Ogawa M. Granulocyte colony-stimulating factor enhances interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1988 May;85(10):3445–3449. doi: 10.1073/pnas.85.10.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebuchi K., Ihle J. N., Hirai Y., Wong G. G., Clark S. C., Ogawa M. Synergistic factors for stem cell proliferation: further studies of the target stem cells and the mechanism of stimulation by interleukin-1, interleukin-6, and granulocyte colony-stimulating factor. Blood. 1988 Dec;72(6):2007–2014. [PubMed] [Google Scholar]
- Ikebuchi K., Wong G. G., Clark S. C., Ihle J. N., Hirai Y., Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9035–9039. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Kikumoto Y., Hong Y. M., Nishida T., Nakai S., Masui Y., Hirai Y. Purification and characterization of recombinant human interleukin-1 beta produced in Escherichia coli. Biochem Biophys Res Commun. 1987 Aug 31;147(1):315–321. doi: 10.1016/s0006-291x(87)80123-7. [DOI] [PubMed] [Google Scholar]
- Leary A. G., Ikebuchi K., Hirai Y., Wong G. G., Yang Y. C., Clark S. C., Ogawa M. Synergism between interleukin-6 and interleukin-3 in supporting proliferation of human hematopoietic stem cells: comparison with interleukin-1 alpha. Blood. 1988 Jun;71(6):1759–1763. [PubMed] [Google Scholar]
- Leary A. G., Ogawa M. Blast cell colony assay for umbilical cord blood and adult bone marrow progenitors. Blood. 1987 Mar;69(3):953–956. [PubMed] [Google Scholar]
- Leary A. G., Ogawa M., Strauss L. C., Civin C. I. Single cell origin of multilineage colonies in culture. Evidence that differentiation of multipotent progenitors and restriction of proliferative potential of monopotent progenitors are stochastic processes. J Clin Invest. 1984 Dec;74(6):2193–2197. doi: 10.1172/JCI111645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary A. G., Yang Y. C., Clark S. C., Gasson J. C., Golde D. W., Ogawa M. Recombinant gibbon interleukin 3 supports formation of human multilineage colonies and blast cell colonies in culture: comparison with recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1987 Nov;70(5):1343–1348. [PubMed] [Google Scholar]
- Mochizuki D. Y., Eisenman J. R., Conlon P. J., Larsen A. D., Tushinski R. J. Interleukin 1 regulates hematopoietic activity, a role previously ascribed to hemopoietin 1. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5267–5271. doi: 10.1073/pnas.84.15.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. A., Warren D. J. Synergy of interleukin 1 and granulocyte colony-stimulating factor: in vivo stimulation of stem-cell recovery and hematopoietic regeneration following 5-fluorouracil treatment of mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7134–7138. doi: 10.1073/pnas.84.20.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata T., Ogawa M. Identification in culture of a class of hemopoietic colony-forming units with extensive capability to self-renew and generate multipotential hemopoietic colonies. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3843–3847. doi: 10.1073/pnas.79.12.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Bartocci A., Patinkin D., Rosendaal M., Bradley T. R. Regulation of very primitive, multipotent, hemopoietic cells by hemopoietin-1. Cell. 1986 Jun 6;45(5):667–674. doi: 10.1016/0092-8674(86)90781-6. [DOI] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M., Ihle J. N. Permissive role of interleukin 3 (IL-3) in proliferation and differentiation of multipotential hemopoietic progenitors in culture. J Cell Physiol. 1985 Aug;124(2):182–190. doi: 10.1002/jcp.1041240203. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]