Abstract
Objective:
To assess whether markers of micro- and macro-structural brain abnormalities are associated with slower gait in older men and women independent of each other, and also independent of health-related conditions and of behavioral, cognitive and peripheral function.
Methods:
Magnetization transfer ratio [MTR], white matter hyperintensities [WMH], brain atrophy [BA] and brain infarcts [BI] were measured in 795 participants of the AGES-Reykjavik Study cohort (mean 75.6yrs, 58.9% women).
Results:
In women, lower MTR, higher WMH and BA, but not BI, remained associated with slower gait independent of each other and of other covariates. In men, WMH and BA, but not MTR or BI, remained associated with slower gait independently of each other. Only muscle strength, executive control function and depression test scores substantially attenuated these associations.
Interpretations:
MTR in older adults may be an important additional marker of brain abnormalities associated with slower gait. Studies to explore the relationship between brain micro- and macrostructural abnormalities with gait and the role of mediating factors are warranted.
Keywords: MTR, gait speed, community-dwelling seniors
1. Introduction
Slower walking pace is common in older adults and it is an important prognostic risk factor for occurrence of dementia, disability and mortality. (Buchman et al., 2007; Cesari et al., 2005; Guralnik et al., 1994; Marquis et al., 2002; Montero-Odasso et al., 2005) Emerging evidence suggests that slow walking in community–dwelling older adults is associated with disruptions in tracts connecting different parts of the brain, reflected by hyperintensities of the white matter, and also with lacunar infarcts and brain atrophy. (Benson et al., 2002; Camicioli et al., 1999; Carmelli et al., 2000; Rosano et al., 2007; Rosano et al., 2006; Rosano et al., 2005a; Starr et al., 2003) These are macrostructural brain abnormalities visible on standard Magnetic Resonance Images (MRI). However, standard MRI do not quantitatively measure the severity of tissue damage or account for brain parenchyma abnormalities at the microstructural level. In fact, microstructural abnormalities may accumulate in the ageing brain before they can be visually detected as macrostructural abnormalities on a conventional brain MRI.
Magnetization transfer imaging (MTI) has been used to uncover the presence of microstructural abnormalities that remain otherwise invisible on conventional MRI. (Inglese and Ge, 2004; Mehta et al., 1996; Rademacher et al., 1999; Spilt et al., 2005; Tanabe et al., 1997; Wolff and Balaban, 1989; Wong et al., 1995) MTI also detects differences in the degree of tissue destruction in macrostructural lesions in the white matter. (Rademacher et al., 1999; Wong et al., 1995) Specifically, peak height magnetization transfer ratio (peak height-MTR) is a MTI-derived summary measure that is sensitive to age-related and disease-related brain parenchyma abnormalities. (Rovaris et al., 2005) A lower brain peak height-MTR indicates loss of homogeneity of brain tissue (van Buchem and Tofts, 2000) and it is observed in brain parenchymal abnormalities that develop with ageing. (Benedetti et al., 2006)
The relationship of slower gait with structural brain abnormalities as measured by MTI has not been investigated in older men and women and neither has the relationship between MR markers of micro- and macrostructural brain abnormalities and slower gait. These are important questions because the progression of structural brain abnormalities can be delayed by interventions on cerebrovascular risk factors even late in life (Colcombe et al., 2006; Dufouil et al., 2005; Kramer et al., 1999; Miller et al., 1984) and addressing microstructural changes before they build up into macrostructural damage may have a substantial impact on the development of physical disability in older persons.
Here, we examine the association of gait speed to micro- and macrostructural brain abnormalities as detected using MTI, as well as conventional MRI to measure white matter hyperintensities (WMH), brain atrophy and brain infarcts. We test the hypotheses that the associations of peak height-MTR, WMH, brain atrophy and brain infarcts with slower gait remain independent of each other and also independent of other risk factors for slower gait, including health-related conditions (diabetes, burden of subclinical vascular disease and high body mass index), behavioral (physical activity) and peripheral (muscle strength) contributors of walking. We also hypothesize that the association between brain MRI markers and walking is partially explained by lower tests score of executive control function, working memory and depression test scores. The rationale for this hypothesis is that impairment in these domains, and in particular executive cognitive dysfunction, is associated with gait abnormalities (Inzitari et al., 2007; Penninx et al., 1998; Rosano et al., 2005b) as well as with brain structural abnormalities (Keener and Phillips, 2007; Lee et al., 2004; van Buchem et al., 1999; van der Flier et al., 2002). We test these hypotheses in a large and well established community-based sample of Caucasian-European men and women participating in the Age Gene/Environment Susceptibility – Reykjavik Study (AGES-RS). (Harris et al., 2007)
2. Methods
2.1 Study Population
This sample is drawn from the first 2,300 participants who were enrolled in AGES-Reykjavik Study (976 men, and 1,324 women, mean age 76 years). (Harris et al., 2007) AGES Reykjavik Study (AGES-RS) is a follow up of the original Reykjavik Study, started in 1967 by the Iceland Heart Association (IHA). AGES-RS was initiated in 2002 to investigate the contribution of genetic and environmental risk factors and their interactions to disorders of importance in old age. All participants signed an informed consent form and the study is approved by the Institutional Review Boards of the IHA and the National Institute on Aging. Baseline characteristics of the AGES-RS have been previously reported. (Harris et al., 2007)
2.2 Magnetization Resonance Imaging (MRI) Protocol
All eligible participants (n=2300) were asked to participate in an MRI exam. Between 2002 and 2003, the exam included Magnetization Transfer Imaging (MTI) which, because of clinic flow constraints, was offered to a 38% random sample.
High resolution MR images were acquired on a 1.5T Signa Twinspeed system (General Electric Medical Systems, Waukesha, WI) with high performance gradients (amplitude 40mT/m and slew rate 150 T/m/s). All sequences gave full brain coverage. Number of slices varied among subjects to ensure full coverage and slices were angled parallel to the anterior commissure-posterior commissure line to give reproducible image views in the oblique-axial plane. The MRI protocol included the following sequences (3-mm thick interleaved slices, no skip): a PD/T2 - w fast spin echo (FSE) sequence (TE1, 22 ms; TE2, 90 ms; TR, 3220 ms; echo train length, 8; FA, 90°; FOV, 220 mm; matrix 256 × 256), a fluid attenuated inversion recovery (FLAIR) sequence (TE, 100 ms; TR, 8000 ms, Inversion time, 2000 ms, FA, 90°; FOV, 220 mm; matrix, 256 × 256), a diffusion weighted image [spin echo (SE) type echo planar images (EPI) (TE, 70 ms; TR, 14000 ms; FOV, 220 mm; matrix, 128 × 128; b values, 0 and 1000 s/mm2)] and a T2*-weighted gradient echo (GRE) type EPI sequence (TE, 50 ms; TR, 3050 ms; FA, 90°; FOV, 220 mm; matrix, 256 × 256). Images were acquired with a T1-w three dimensional spoiled gradient echo (3D-SPGR) sequence (TE, 8 ms; TR, 21 ms; FA, 30; FOV 240 mm; matrix 256 × 256, slice thickness 1.5 mm).
The MT images were obtained using a proton density (PD)-w gradient echo (GRE) pulse sequence (TE, 3.7 ms; TR, 960 ms) with (Ms images) and without (Mo images) the use of a saturation off-resonance (offset = 1200 Hz) pre-pulse (19 ms sinc shaped pulse) with FA of 25°, a matrix resolution of 256 × 192 and FOV, 220 mm. (van Buchem et al., 1996)
Magnetization transfer ratio (MTR)
The MTI sequences provide information to derive the magnetization transfer ratio (MTR), intracranial volume and brain parenchyma volume. MTI data were processed with SNIPER (Software for Neuro-Image Processing in Experimental Research, Leiden University Medical Center, Leiden, Netherlands), which has been described previously. (van Buchem et al., 1999) Briefly, following the segmentation of brain parenchyma and CSF, MTR values were calculated per voxel as the ratio of the Ms and Mo values. The data set of MTR values was displayed as an MTR histogram. Based on previous publications, (Rovaris et al., 2005; van Buchem et al., 1999; van der Flier et al., 2002) we selected peak height–MTR as the primary summary measure. Peak height-MTR was defined as the highest number of voxels with the same MTR value in the histogram. In normal brain tissue, a narrow and high peak height-MTR is observed, indicating that normal brain tissue has a relatively narrow range of MTR values. In diseases affecting brain tissue, however, the peak height-MTR lowers, which has been attributed to disease- and age-related loss of homogeneity of brain tissue. (Rovaris et al., 2005; van Buchem and Tofts, 2000)
Brain atrophy
The intracranial and brain parenchyma compartments were segmented automatically using SNIPER and were manually edited when necessary. Intracranial and brain volumes were obtained for each subject and the atrophy index was computed as (intracranial volume – brain volume) / intracranial volume.
White matter hyperintensities (WMH)
WMH were defined as periventricular or subcortical areas with increased signal intensity as compared to the surrounding white matter on FLAIR, T2- and PD-w images. The rating of subcortical WMH was based on a semiquantitative method (Debruyne et al., 2002) that is based on size (largest diameter) and number of WMH lesions. The periventricular WMH were rated semi-quantitatively and the total load was calculated as the sum of lesion size in each region (ventricles horns and walls).
Infarcts
Cerebral infarcts were identified as defects in brain parenchyma (that is areas isointense to cerebral spinal fluid (CSF) on all pulse sequences) with a maximal diameter of at least 4 mm, and without evidence of hemosiderin in their walls on T2*-weighted GRE-EPI images. Lesions with cortical involvement or located in the cerebellum were identified by a trained neuro-radiologist, who entered slice location of the infarcts into a data base accessed by three trained radiographers, who further characterized the lesions. These radiographers also identified and characterized sub-cortical lesions.
Five percent of all scans were re-read without knowledge of the prior reading at the Department of Radiology, Leiden University Medical Center, the Netherlands, to assess inter-rater reliability. The results of the analysis performed at this center were compared to those generated by the neuro-radiologist and radiographers. Average inter-rater reliability of subcortical WMH (present/absent and lesion size categories) was good (weighted kappa = 0.7), as was average inter-rater reliability of parenchymal defects (presence/absence) (weighted kappa = 0.7). Intra-rater reliability of subcortical WMH and parenchymal defects based on two ratings by each observer was high (weighted kappa = 0.9).
2.3 Mobility measures
Participants were asked to walk at usual pace over a 6 meter-course, marked by fluorescent orange traffic cones at the beginning and end of the course. The average time in seconds, converted into m/sec, of two trials was used in the analysis. Gait speed was also dichotomized at the cut-off of ≤1.0m/sec, which is a robust predictor of health events (Cesari et al., 2005; Montero-Odasso et al., 2005) and identifies older adults who are more likely to have underlying brain abnormalities as detected on MRI. (Rosano et al., 2005)
2.4 Covariates
In addition to age, sex and education, other potential covariates were those health-related conditions that are associated with slower gait speed. A questionnaire was used to assess self-reported medical history of osteoarthritis (hip and knee). Diabetes was assessed on the basis of self-report, a doctor's diagnosis of diabetes, use of oral hypoglycemic medications or insulin, or a fasting blood glucose ≥6.1 mmol/l. (Association, 2000) Coronary artery calcium (CAC) was quantified with computed tomographic images (Siemens Sensation 4 detector scanner, slice thickness; 2.5 mm, tube-voltage; 140 kilo-voltage, tube-current-time product; 50 milli-ampere-seconds and scan time 0.361 sec) and expressed in Agaston units. CAC was added to the model to adjust for subclinical atherosclerosis. Measures of height (cm), weight (kg) and of systolic blood pressure (mmHg) were obtained at the time of the clinic visit. Body mass index was computed [(Kg)/m2]. Other covariates included congestive heart failure, chronic pulmonary disease, TIA and stroke, assessed by self-report, and distance visual acuity and bilateral hearing loss at 25dB. (Harris et al., 2007)
Other important covariates of these analyses were those behavioral, peripheral and cognitive factors associated with the dependent and independent variables: physical activity, lower-extremity muscle strength, executive cognitive function and depression score. Regular leisure-time physical activity in the last 12 months and smoking history (ever/never) were assessed by questionnaire. (Kuo et al., 2006) Maximal isometric knee extension strength was measured using an adjustable dynamometric chair (Good Strength, Metitur, Palokka. Finland.). (Tiainen et al., 2004) For each person, the maximal measurement from three trials on the dominant leg was used. The executive cognitive function tests battery included the Digit Symbol Substitution Test (DSST), which has been used in many studies to measure processing speed, (Wechsler, 1955) the Figure Comparison (Salthouse and Babcock, 1991) and a modified Stroop Test (Stroop, 1935) Parts 1 and 2. All test results were normally distributed in the cohort and inter-rater reliability was excellent (Spearman correlations range from 0.96-0.99 for all the tests). We examined DSST separately, because we have shown that it is significantly associated with slower gait. (Inzitari et al., 2007; Rosano et al., 2005b) We also computed one composite score of processing speed using a previously validated construct (de Groot et al., 2001; Wilson et al., 2002) by converting raw scores on each test to standardized z-scores and averaging the z-scores across the tests in each composite. Depressive symptoms were measured using the 15-item Geriatric Depression Scale (GDS) (Sheik and Yesavage, 1986) where higher depressive symptomatology corresponded to greater GDS scores.
2.6 Statistical analysis
A total of 869 persons received MTI as part of their MRI. Of these, we excluded 60 participants who had cortical infarcts with a diameter >5.0 mm, because the WMH surrounding these defects have a different etiology (infarction based on occlusion of isolated, large end arteries) than the brain abnormalities we were interested in (presumably based on chronic hypoperfusion due to small vessel disease). To ensure internal validity, all analyses were repeated including these 60 participants. An additional 14 were excluded because of missing data in either time to walk or WMH load (n=14). Compared to those excluded from this analysis (n=74), those included were more likely to be female, less likely to have macrostructural brain abnormalities (prevalence of brain infarcts, WMH load, brain atrophy), had higher peak height-MTR, greater MMSE score and lower depression scores (p<0.05).
Time to walk at usual pace was used as a continuous measure (sec) in all regression analyses. Age- and sex-adjusted Pearson correlations of potential covariates to time to walk and peak height-MTR were used to identify the covariates that would be retained for the multivariable linear regression models.
Using age and gender-adjusted multivariable linear regression models, we tested the hypothesis that lower peak height-MTR, WMH, brain atrophy and brain infarcts were associated with slower walk time independently of each other. To adjust for brain volume we included the atrophy index in the model. Further, we tested the hypothesis that these associations remained independent of health-related conditions, and of physical activity, lower extremity muscle strength, cognition and depression. Each one of these covariates was entered into separate models first. If no substantial modification was observed in the regression coefficients of the main independent variables, then more than one covariate entered the model in one block. For example, the health-related conditions were first added to the model one at a time and if they did not substantially change the coefficient then they were entered together into the same block. Interactions between brain MRI abnormality and sex and among brain abnormalities were tested and analyses were conducted for the whole group and separately for women and men. All analyses were done using SPSS, version 15.0 (SPSS, Inc. Chicago, IL).
3. Results
Compared to women, men walked faster, had more years of education, were less likely to report knee/hip osteoarthritis, had a lower BMI, greater muscle strength and reported higher levels of physical activity (age-adjusted p<0.01 for all). Compared to women, men also had a greater burden of vascular diseases, as shown by higher CAC scores (Table 1), greater brain atrophy, and more brain infarcts (Table 2).
Table 1.
Population characteristics of the AGES-RS cohort (n=795) and for men and women.
| All N = 795 | Men N = 327 | Women N = 468 | |
|---|---|---|---|
| Outcome: | |||
| Time to walk, sec, mean (SD) | 6.4 (1.8) | 6.0 (1.4)† | 6.7 (2.0) |
| Demographics: | |||
| Age, yrs, mean (SD) | 75.6 (5.5) | 75.6 (5.4) | 75.6 (5.7) |
| Male, N (%) | 327 (41.1)* | - | - |
| Education, primary or more, N (%) | 794 (99.9) | 327 (100.0) | 467 (99.8) |
| Education, secondary or more, N (%) | 595 (74.8) | 271 (82.9)† | 324 (69.2) |
| Health-related conditions: | |||
| Osteoarthritis, hip or knee, N (%) | 95 (11.9) | 22 (6.7)† | 73 (15.6) |
| Diabetes or IFG, N (%) | 176 (22.1) | 82 (25.1) | 94 (20.1) |
| Coronary Arterial Calcium, Agaston score, median (75th percentile) | 230.2 (792.7) | 495.0 (1151.2)† | 98.1 (415.7) |
| Body Mass Index, kg/m2, mean (SD) | 26.7 (4.3) | 26.2 (3.5)† | 27.0 (4.8) |
| Other risk factors for slower gait: | |||
| Physical activity, ≥ 1-3 hrs/wk, N (%) | 256 (32.2) | 125 (38.2)† | 131 (28.0) |
| Muscle strength, Nwt, mean (SD) | 315.8 (114.5) | 404.9 (99.3)† | 254.6 (78.4) |
| DSST, number correct, mean (SD) | 29.4 (10.5) | 29.1 (10.1) | 29.6 (10.8) |
| GDS score, median (75th percentile) | 2.0 (3.0)* | 2.0 (3.0) | 2.0 (3.0) |
Age- and gender-adjusted differences of included vs. excluded (n=74) significant at p <0.05.
Age-adjusted differences of men vs. women significant at p<0.05.
IFG: impaired fasting glucose; DSST: Digit Symbol Substitution Test; GDS: Geriatric Depression Scale
Table 2.
Brain measures from MTI (peak height-MTR) and from conventional MRI sequences.
| All N = 795 |
Men N = 327 |
Women N = 468 |
|
|---|---|---|---|
| Peak height-MTR, voxels, mean (SD) | 41169.9 (4304.8)* | 41221.6 (4223.7) | 41133.9 (4364.7) |
| Subcortical WMH, cm3, median (75th percentile) | 1.5 (4.7)* | 0.9 (3.7)† | 1.9 (5.4) |
| Periventricular WMH score, median (75th percentile) | 6.00 (7.00) | 6.00 (7.00) | 6.00 (8.00) |
| Brain atrophy index, mean (SD)^ | 0.23 (.05)* | 0.25 (.04)† | 0.22 (.04) |
| Infarcts (cortical or subcortical) ≥ 1, N (%) | 85 (10.7)* | 51 (15.6)† | 34 (7.3) |
Age- and gender-adjusted differences of included vs. excluded (n=74) significant at p <0.05.
Age-adjusted differences of men vs. women significant at p<0.05.
Computed as: (intracranial volume − brain volume) / intracranial volume.
Peak height-MTR: Peak height of the MTR histogram; WMH: white matter hyperintensities
In the total cohort of this study, lower peak height-MTR, higher WMH and brain atrophy, but not brain infarcts, were associated with longer time to walk independent of age and of each other (See Supplementary Table 1). The interaction between peak height-MTR and sex in association with gait speed was significant (p=0.005). In gender stratified analyses (Table 3) the regression coefficient of peak height-MTR remained significant in both sexes after adjustment for WMH and infarcts (Table 3, Models 2 and 4), while it was substantially attenuated by more than 35% and it remained significant in women but not in men (Table 3, Model 3). Results were similar when the participants with cortical infarcts with a diameter >5.0 mm were included in the cohort.
Table 3.
Age-adjusted standardized betas (SE) and p-values of peak height-MTR, WMH volume, brain atrophy and brain infarcts in models predicting time to walk (sec) for men and women of the AGES-RS cohort. As expected, lower peak-height MTR and higher WMH load are associated with longer times to walk.
| Men (n=327) | |||||
|---|---|---|---|---|---|
| Standardized betas (SE) and p-values | |||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
| Peak height-MTR | − 0.20 (0.009) p=0.001 |
− 0.19 (0.009) p=0.001 |
− 0.12 (0.011) p=0.10 |
− 0.19 (0.009) p=0.001 |
− 0.10 (0.011) p=0.20 |
| WMH volume 1 | 0.13 (0.020) p=0.02 |
- | 0.12 (0.020) p=0.03 |
||
| Brain atrophy | 0.14 (2.416) p=0.05 |
0.14 (2.401) p=0.05 |
|||
| Brain infarcts | - | - | 0.06 (0.117) p=0.27 |
0.04 (0.117) p=0.50 |
|
| Women (n=468) | |||||
| Standardized betas (SE) and p-values | |||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
| Peak height-MTR | − 0.23 (0.010) p<0.001 |
− 0.23 (0.010) p<0.001 |
− 0.15 (0.012) p=0.01 |
− 0.22 (0.010) p<0.001 |
− 0.14 (0.012) p=0.01 |
| WMH volume 1 | 0.12 (0.019) p=0.004 |
- | 0.11 (0.019) p=0.01 |
||
| Brain atrophy | 0.14 (2.52) p=0.01 |
0.14 (2.50) p=0.01 |
|||
| Brain infarcts - | - | - | 0.09 (0.247) p=0.04 |
0.06 (0.249) p=0.20 |
|
Includes subcortical WMH. Adjustment for periventricular WMH (alone or in combination with subcortical WMH) yields similar results.
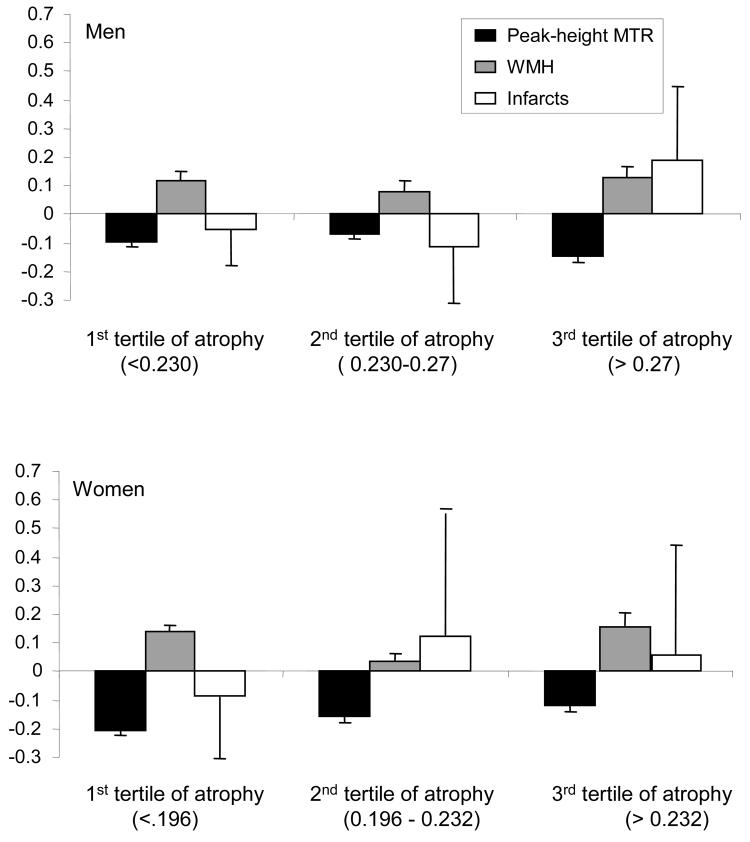
The interactions between peak height-MTR and brain atrophy in association with gait speed were significant (p=0.04) in men but not in women (p=0.2). Because men also had more atrophy than women (Table 1), in exploratory analyses we tested the hypothesis that the association of peak height-MTR and time to walk would become non-significant in women with very high brain atrophy. We did this by repeating the models of Table 3 after stratification by gender-specific tertiles of brain atrophy (Figure 1). Among men, trends were observed in the expected direction within each tertile. Among women in the lowest tertile of brain atrophy, peak height-MTR was associated with time to walk independent of WMH and of brain infarct (p=0.008). However, in the highest tertile of brain atrophy, the association of peak height-MTR and time to walk became not significant (p=0.12), a finding consistent with the associations observed in men. The association of WMH with time to walk was similar in the 1st compared to the 3rd tertile and it was borderline significant (p=0.05 in the 1st tertile and p=0.06 in the 3rd tertile).
Figure 1.
Standardized betas and standard errors (error bars) from multivariable regression models adjusted for age and stratified by gender-specific tertiles of brain atrophy. Outcome is time to walk at usual pace (sec). As expected, lower peak height-MTR and higher WMH load are associated with longer times to walk.
In further analyses, we tested the hypotheses that peak-height MTR, WMH and brain atrophy remained associated with time to walk independent of other covariates. After adjusting for health-related conditions and for physical activity, peak height-MTR, WMH and brain atrophy remained associated with slower gait in the total group (See supplementary Table 2) and in women (Table 4, Model 2 and 3). Adjustment for muscle strength attenuated the regression coefficients of WMH and of brain atrophy by more than 20% in men and women (Table 4, compare Model 1 vs. Model 4). Adjustment for DSST and for depression score also attenuated the regression coefficients of peak height-MTR and of WMH by 20% with a stronger effect in men compared to women (Table 4, compare Model 1 vs. Models 5 and 6).
Table 4.
Standardized betas (SE) and p-values of peak height-MTR, WMH volume and brain atrophy in models predicting time to walk (sec) for men and women of the AGES-RS cohort. The brain MRI variables entered the model simultaneously. Brian infarcts did not enter these models because they were not significant in previous models (Table 3).
| Men (n=327) | ||||||
|---|---|---|---|---|---|---|
| Standardized betas (SE) and p-values | ||||||
|
Model 1 (adjusted for age) |
Model 2 (Model 1 further adjusted for health-relate conditions^) |
Model 3 (Model 1 + physical activity) |
Model 4 (Model 1 + Muscle strength) |
Model 5 (Model 1 + DSST) |
Model 6 (Model 1 + GDS) |
|
| Peak height-MTR | −0.11 (0.011) p=0.10 |
− 0.09 (0.011) p=0.20 |
− 0.09 (0.011) p=0.20 |
− 0.11 (0.010) p=0.10 |
− 0.04 (0.010) p=0.60 |
−0.07 (0.010) p=0.30 |
| WMH volume | 0.13 (0.020) p=0.02 |
0.14 (0.020) p=0.01 |
0.12 (0.020) p=0.02 |
0.11 (0.020) p=0.04 |
0.10 (0.020) p=0.06 |
0.09 (0.020) p=0.08 |
| Brain atrophy | 0.14 (2.400) p=0.05 |
0.12 (2.417) p=0.01 |
0.13 (2.389) p=0.06 |
0.10 (2.375) p=0.20 |
0.14 (2.320) p=0.04 |
0.11 (2.355) p=0.10 |
| Women (n=468) | ||||||
| Standardized betas (SE) and p-values | ||||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
| Peak height-MTR | −0.14 (0.012) p=0.01 |
− 0.12 (0.012) p=0.04 |
− 0.10 (0.012) p=0.06 |
− 0.13 (0.011) p=0.02 |
− 0.12 (0.012) p=0.04 |
−0.13 (0.012) p=0.02 |
| WMH volume | 0.12 (0.019) p=0.003 |
0.11 (0.020) p=0.01 |
0.11 (0.019) p=0.01 |
0.09 (0.019) p=0.02 |
0.11 (0.019) p=0.01 |
0.10 (0.019) p=0.01 |
| Brain atrophy | 0.15 (2.501) p=0.01 |
0.17 (2.528) p=0.003 |
0.16 (2.499) p=0.003 |
0.11 (2.429) p=0.04 |
0.15 (2.488) p=0.005 |
0.14 (2.473) p=0.01 |
Includes hip/knee osteoarthritis, diabetes or IFG, coronary artery calcium and body mass index.
4. Discussion
In this large community-based cohort, we found that micro- and macrostructural abnormalities as detected with MTI and conventional MR sequences, were associated with slower gait, independent of each other and of other important risk factors for impaired mobility, including health-related conditions, cardiovascular disease, osteoarthritis, low physical activity and high BMI.
MTI has been used to assess and quantify structural brain abnormalities in aging and in a variety of conditions, including multiple sclerosis (Mehta et al., 1996) and dementia, (van Es et al., 2006) but MTI markers of brain abnormalities have not been examined in relation to motor slowing in older adults. Our findings suggest there is tissue damage, not seen on conventional MRI, which contributes to slower walking in older adults. Compared to other studies examining peak height-MTR as a ratio or as a percent of total brain volume, the analytical approach of this study was to examine brain atrophy as a covariate in the model and to measure changes in the regression coefficients of the other MR markers in relationship to the outcome. This analytical approach revealed that brain atrophy attenuates the association of MTR to gait speed more substantially in men than in women (e.g. associations were non-significant in men, Table 3, Model 3). This suggests that in the presence of substantial brain atrophy, the effect of microstructural abnormalities is diminished. More loss of brain volume in men compared to women may reflect men's greater burden of vascular disease, reflected by greater brain atrophy, greater CAC scores and greater prevalence of brain infarcts.
We found lower muscle strength modified the association of peak height-MTR and WMH with time to walk. Lower-extremity muscle strength is associated with time to walk and in this study it was correlated with peak height-MTR (p<0.005). From a physiological point of view, muscle strength is in the pathway between the central nervous system and the actual motor performance. These findings suggest that brain structural abnormalities may lead to changes in the motor unit and results in lower strength and slower gait. Future studies on the pathogenesis of mobility impairment in older adults should include measures of central and peripheral nervous system function as well as of muscle strength and power.
Cognitive processing speed and depression also had a similar attenuation effect on the relationship between brain MRI markers and time to walk, although it was stronger in men than in women. The association of lower peak height-MTR with slower cognitive processing and greater depression scores is consistent with previous studies. (Lee et al., 2004; van Buchem et al., 1999; van der Flier et al., 2002) It has also been shown that changes in cognitive processing speed are associated with slower gait. (Haggard et al., 2000; Kerr et al., 1985; Kuo et al., 2006; Lindenberger et al., 2000; Maylor et al., 2001; Rosano et al., 2005b) Executive cognitive function, processing speed and visuospatial attention play a crucial role in the execution of motor tasks, including walking. (de Groot et al., 2001; Rosano et al., 2006; Wilson et al., 2002) Performing the DSST and walking may rely on similar or shared domains of brain functioning and underlying brain networks and impairment on these tasks may reflect similar brain structural changes. Although motor slowing is part of the diagnosis of depression, the association between depressive mood and slower gait has been less explored, and the underlying mechanisms that could link lower motivation levels with motor slowing are not known.
One of the major strengths of this study is the rich imaging data, which included novel imaging sequences as MTI in addition to conventional sequences, as well as many quantitative markers of structural brain abnormalities. In addition, an extensive characterization of cognitive function and of other important risk factors for slower gait was available for a large number of older men and women. Although this cohort consists of Caucasian-Europeans, these findings are likely to generalize to other populations, since the health and functional characteristics of the participants of this study were similar to those of other cohorts of older adults. (Harris et al., 2007)
Supplementary Material
Acknowledgement
This study has been funded by the Icelandic Heart Association, by NIH contract N01-AG-12100 and is supported in part by the Intramural Research Program of the National Institute on Aging. Dr. Rosano is funded by 1K23 AG 028966-01, R03 AG 025076-01A1, 1 R01 AG029232-01, 1 P30 AG024827. There are no actual or potential conflicts of interest.
REFERENCES
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2000;27:S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- Benedetti B, Charil A, Rovaris M, Judica E, Valsasina P, Sormani MP, Filippi M. Influence of aging on brain gray and white matter changes assessed by conventional, MT, and DT MRI. Neurology. 2006;66:535–539. doi: 10.1212/01.wnl.0000198510.73363.c6. [DOI] [PubMed] [Google Scholar]
- Benson RR, Guttmann CR, Wei X, Warfield SK, Hall C, Schmidt JA, Kikinis R, Wolfson LI. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology. 2002;58:48–55. doi: 10.1212/wnl.58.1.48. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Change in motor function and risk of mortality in older persons. J Am Geriatr Soc. 2007;55:11–19. doi: 10.1111/j.1532-5415.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Moore MM, Sexton G, Howieson DB, Kaye JA. Age-related brain changes associated with motor function in healthy older people. J Am Geriatr Soc. 1999;47:330–334. doi: 10.1111/j.1532-5415.1999.tb02997.x. [DOI] [PubMed] [Google Scholar]
- Carmelli D, DeCarli C, Swan GE, Kelly-Hayes M, Wolf PA, Reed T, Guralnik JM. The joint effect of apolipoprotein E epsilon4 and MRI findings on lower-extremity function and decline in cognitive function. J Gerontol A Biol Sci Med Sci. 2000;55:M103–109. doi: 10.1093/gerona/55.2.m103. [DOI] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, Tylavsky FA, Brach JS, Satterfield S, Bauer DC, Visser M, Rubin SM, Harris TB, Pahor M. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and subjective cognitive dysfunction: the Rotterdam Scan Study. Neurology. 2001;56:1539–1545. doi: 10.1212/wnl.56.11.1539. [DOI] [PubMed] [Google Scholar]
- Debruyne JC, Van Laere KJ, Versijpt J, De Vos F, Keppens Eng J, Strijchmans K, Santens P, Achten E, Slegers G, Korf J, Kierckx RA, De Reuck JL. Semiquantification of the peripheral-type benzodiazepine ligand [11C]PK11195 in normal human brain and application in multiple sclerosis patients. Acta neurol belg. 2002;102:127–135. [PubMed] [Google Scholar]
- Dufouil C, Chalmers J, Coskun O, Besançon V, Bousser M, Guillon P, MacMahon S, Mazoyer B, Neal B, Woodward M, Tzourio-Mazoyer N, Tzourio C. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112:1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Haggard P, Cockburn J, Cock J, Fordham C, Wade D. Interference between gait and cognitive tasks in a rehabilitating neurological population. J Neurol Neurosurg Psychiatry. 2000;69:479–486. doi: 10.1136/jnnp.69.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese M, Ge Y. Quantitative MRI: hidden age-related changes in brain tissue. Top Magn Reson Imaging. 2004;15:355–363. doi: 10.1097/01.rmr.0000168069.12985.15. [DOI] [PubMed] [Google Scholar]
- Inzitari M, Newman AB, Yaffe K, Boudreau R, de Rekeneire N, Shorr R, Harris TB, Rosano C. Gait Speed Predicts Decline in Attention and Psychomotor Speed in Older Adults: The Health Aging and Body Composition Study. Neuroepidemiology. 2007;29:156–162. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keener MT, Phillips ML. Neuroimaging in bipolar disorder: A critical review of current findings Current Psychiatry Reports. 2007;9:512–520. doi: 10.1007/s11920-007-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B, Condon SM, McDonald LA. Cognitive spatial processing and the regulation of posture. J Exp Psychol Hum Percept Perform. 1985;11:617–622. doi: 10.1037//0096-1523.11.5.617. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MTM,E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau R, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Leveille SG, Yu YH, Milberg WP. Cognitive Function, Habitual Gait Speed, and Late-Life Disability in the National Health and Nutrition Examination Survey (NHANES) 1999-2002. Gerontology. 2006;53:102–110. doi: 10.1159/000096792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Kim TK, Park M, Ko S, Song IC, Cho IH. Age-related changes in conventional and magnetization transfer MR imaging in elderly people: comparison with neurocognitive performance. Korean J Radiol. 2004;5:96–101. doi: 10.3348/kjr.2004.5.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol Aging. 2000;15:417–436. doi: 10.1037//0882-7974.15.3.417. [DOI] [PubMed] [Google Scholar]
- Marquis S, Moore MM, Howieson DB, Sexton G, Payami H, Kaye JA, Camicioli R. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002;59:601–606. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Allison S, Wing AM. Effects of spatial and nonspatial cognitive activity on postural stability. Br J Psychol. 2001;92(Part 2):319–338. [PubMed] [Google Scholar]
- Mehta RC, Pike GB, Enzmann DR. Measure of magnetization transfer in multiple sclerosis demyelinating plaques, white matter ischemic lesions, and edema. Am J Neuroradiol. 1996;17:1051–1055. [PMC free article] [PubMed] [Google Scholar]
- Miller RE, Shapiro AP, King HE, Ginchereau EH, Hosutt JA. Effect of antihypertensive treatment on the behavioral consequences of elevated blood pressure. Hypertension. 1984;6:202–208. [PubMed] [Google Scholar]
- Montero-Odasso M, Schapira M, Soriano ER, Varela M, Kaplan R, Camera LA, Mayorga LM. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60:1304–1309. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279:1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Engelbrecht V, Burgel U, Freund H, Zilles K. Measuring in vivo myelination of human white matter fiber tracts with magnetization transfer MR. Neuroimage. 1999;9:393–406. doi: 10.1006/nimg.1998.0416. [DOI] [PubMed] [Google Scholar]
- Rosano C, Aizenstein H, Studenski S, Newman AB. A Regions-of-Interest Volumetric Analysis of Mobility LImitations in Community-Dwelling Older Adults Journal of Gerontology: Medical Sciences. 2007;62A:1048–1055. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]
- Rosano C, Brach J, Longstreth WT, Jr, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26:52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005a;53:649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- Rosano C, Simonsick EM, Harris TB, Kritchevsky SB, Brach J, Visser M, Yaffe K, Newman AB. Association between physical and cognitive function in healthy elderly: the health, aging and body composition study. Neuroepidemiology. 2005b;24:8–14. doi: 10.1159/000081043. [DOI] [PubMed] [Google Scholar]
- Rovaris M, Gallo A, Valsasina P, Benedetti B, Caputo D, Ghezzi A, Montanari E, Sormani MP, Bertolotto A, Mancardi G, Bergamaschi R, Martinelli V, Comi G, Filippi M. Short-term accrual of gray matter pathology in patients with progressive multiple sclerosis: an in vivo study using diffusion tensor MRI. Neuroimage. 2005;24:1139–1146. doi: 10.1016/j.neuroimage.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in executive function. Developmental Psychology. 1991;27:763–776. [Google Scholar]
- Sheik JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontologist. 1986;5:165–173. [Google Scholar]
- Spilt A, Geeraedts T, de Craen AJ, Westendorp RG, Blauw GJ, van Buchem MA. Age-related changes in normal-appearing brain tissue and white matter hyperintensities: more of the same or something else? Am J Neuroradiol. 2005;26:725–729. [PMC free article] [PubMed] [Google Scholar]
- Starr JM, Leaper SA, Murray AD, Lemmon HA, Staff RT, Deary IJ, Whalley LJ. Brain white matter lesions detected by magnetic resonance [correction of resosnance] imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003;74:94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Tanabe JL, Ezekiel F, Jagust WJ, Schuff N, Fein G. Volumetric method for evaluating magnetization transfer ratio of tissue categories: application to areas of white matter signal hyperintensity in the elderly. Radiology. 1997;204:570–575. doi: 10.1148/radiology.204.2.9240555. [DOI] [PubMed] [Google Scholar]
- Tiainen K, Sipila S, Alen M, Heikkinen E, Kaprio J, Koskenvuo M, Tolvanen A, Pajala S, Rantanen T. Heritability of maximal isometric muscle strength in older female twins. J Appl Physiol. 2004;96:173–180. doi: 10.1152/japplphysiol.00200.2003. [DOI] [PubMed] [Google Scholar]
- van Buchem MA, McGowan JC, Grossman RI. Magnetization transfer histogram methodology: its clinical and neuropsychological correlates. Neurology. 1999;53:S23–28. [PubMed] [Google Scholar]
- van Buchem MA, McGowan JC, Kolson DL, Polansky M, Grossman RI. Quantitative volumetric magnetization transfer analysis in multiple sclerosis: estimation of macroscopic and microscopic disease burden. Magn Reson Med. 1996;36:632–636. doi: 10.1002/mrm.1910360420. [DOI] [PubMed] [Google Scholar]
- van Buchem MA, Tofts PS. Magnetization transfer imaging. Neuroimaging Clin N Am. 2000;10:771–788. [PubMed] [Google Scholar]
- van der Flier WM, van den Heuvel DM, Weverling-Rijnsburger AW, Bollen EL, Westendorp RG, van Buchem MA, Middelkoop HA. Magnetization transfer imaging in normal aging, mild cognitive impairment, and Alzheimer's disease. Ann Neurol. 2002;52:62–67. doi: 10.1002/ana.10244. [DOI] [PubMed] [Google Scholar]
- van Es AC, van der Flier WM, Admiraal-Behloul F, Olofsen H, Bollen EL, Middelkoop HA, Weverling-Rijnsburger AW, Westendorp RG, van Buchem MA. Magnetization transfer imaging of gray and white matter in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2006;27:1757–1762. doi: 10.1016/j.neurobiolaging.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual. Psychological; New York: 1955. Wechsler Adult Intelligence Scale. [Google Scholar]
- Wilson RS, Mendes, De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989;10:135–144. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- Wong KT, Grossman RI, Boorstein JM, Lexa FJ, McGowan JC. Magnetization transfer imaging of periventricular hyperintense white matter in the elderly. Am J Neuroradiol. 1995;16:253–258. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.