Abstract
In the present study, as part of a more extensive longitudinal investigation of the in vivo anatomical markers of early and incipient AD in our laboratory, three groups of elderly participants were followed with yearly clinical evaluations and high resolution MRI scans over a 6-year period (baseline and 5 years of follow-up). At baseline, participants consisted of: (1) 35 old subjects with no cognitive impairment (controls); (2) 33 participants with amnestic mild cognitive impairment (MCI); and (3) 14 patients with very mild AD. 11 participants with amnestic MCI received a diagnosis of AD over the follow-up period and 9 controls declined in cognitive function. T1 weighted MRI scans were acquired using a 3D SPGR pulse sequence. At baseline, both the amnestic MCI and mild AD groups differed from the controls in hippocampal and entorhinal cortex volume, but not from each other. Longitudinal analyses showed that the rate of atrophy of the entorhinal cortex and hippocampus for the stable controls differed significantly from MCI participants who converted to AD and the AD groups. Furthermore, longitudinal decreases in hippocampal and entorhinal volume were related to longitudinal decline in declarative memory performance. These findings suggest that the rate of atrophy of mesial temporal lobe structures can differentiate healthy from pathological aging.
Keywords: MRI, Dementia, Mild cognitive impairment, Aging, Mesial temporal lobe, Imaging, Volumetric, Longitudinal
1. Introduction
Clinically, one of the hallmarks of Alzheimer’s disease (AD) during its initial stages is a disturbance of memory, especially characterized by difficulty in the acquisition of new information about events and things (declarative knowledge). The nature of this mnemonic dysfunction seems to be similar to that seen with bilateral lesions, dysfunction or disconnection of the hippocampal formation (HF) and related structures (Squire and Zola-Morgan, 1991; Young et al., 1997), thus implicating the pathophysiological disruption of this neural system in early AD. The entorhinal cortex (EC) and HF are part of the mesial temporal lobe memory system; the EC connects the neocortex with the HF via the perforant path, thereby providing the latter with multi-modal information. It is not surprising, therefore, that these mesial temporal lobe regions have received special attention in both post mortem and in vivo investigations on the pathophysiology of AD.
Structural magnetic resonance imaging (MRI) techniques provide a tool for examining alterations in brain anatomy in vivo and for tracking the anatomical sequence of alterations, as well as rate of change longitudinally. Atrophy of the HF has been well documented in patients with AD using both quantitative (deToledo-Morrell et al., 1997, 2000a,b, 2004; Dickerson et al., 2001; Du et al., 2001; Jack et al., 1992, 1997, 1998; Juottonen et al., 1999; Kesslak et al., 1991; Killiany et al., 1993; Köhler et al., 1998; Laakso et al., 1998; Petersen et al., 2000; Seab et al., 1988; Xu et al., 2000) and semi-quantitative (de Leon et al., 1997)MRI analyses. In particular, those studies that evaluated patients with very mild AD have shown that significant hippocampal atrophy can be detected very early in the disease process (deToledo-Morrell et al., 1997, 2000a,b, 2004; Killiany et al., 1993; Xu et al., 2000).
In addition to patients with mild AD, there is now increased interest in studying elderly individuals who are at high risk for developing AD (Apostolova et al., 2006; Bowen et al., 1997; Convit et al., 1997; de Leon et al., 1996; deToledo-Morrell et al., 2004; Devanand et al., 2007; Dickerson et al., 2001; Jack et al., 2005; Killiany et al., 2000, 2002; Stoub et al., 2005) in order to identify anatomical changes that precede a clinical diagnosis and to develop sensitive in vivo neurobiological markers of the disease. One such group consists of those patients who have cognitive complaints, cognitive impairment, or both, but who do not meet diagnostic criteria for dementia. Such groups are of special interest, since they provide valuable information on the transitional state between normal aging and dementia, although definitions and designations of this subgroup have varied (e.g., age associated memory impairment, Crook and Larabee, 1988; mild cognitive impairment, Petersen, 2000; Petersen et al., 1999).
Among the MRI studies that have examined the volumes of mesial temporal lobe structures in patients with mild cognitive impairment (MCI) or cognitive complaints, the majority have reported hippocampal atrophy (Convit et al., 1997; de Leon et al., 1996; Devanand et al., 2007; Dickerson et al., 2001; Du et al., 2001; Jack et al., 1999; Juottonen et al., 1999; Killiany et al., 2002; Xu et al., 2000), although a few have not (Laakso et al., 1998; Soininen et al., 1994).
Post mortem pathological studies have implicated the EC and the transentorhinal region as early sites of involvement in AD and in individuals with MCI (Braak and Braak, 1991, 1995; Braak et al., 1998; Gomez-Isla et al., 1996; Hyman et al., 1984; Kordower et al., 2001; Mufson et al., 1999; Van Hoesen et al., 1991). Furthermore, Braak and his colleagues have suggested that early AD related pathology may start in the EC and the transentorhinal region and then spread to the HF (Braak and Braak, 1991, 1995; Braak et al., 1998).
With the development of MRI-based protocols for segmenting the EC (Bobinski et al., 1999; Goncharova et al., 2001; Insausti et al., 1998), interest in quantifying the volume of this structure, in vivo, in patients with AD and in those at risk for developing AD has increased (Cardenas et al., 2002; deToledo-Morrell et al., 2000b, 2004; Devanand et al., 2007; Dickerson et al., 2001; Du et al., 2001, 2003, 2004; Juottonen et al., 1998, 1999; Killiany et al., 2002; Stoub et al., 2005; Xu et al., 2000). A number of studies have now demonstrated that there is significant atrophy of the EC in patients with AD (Bobinski et al., 1999; Cardenas et al., 2002; deToledo-Morrell et al., 2000b; Devanand et al., 2007; Dickerson et al., 2001; Du et al., 2001, 2003, 2004; Juottonen et al., 1998; Xu et al., 2000) and in those with incipient AD (deToledo-Morrell et al., 2000b, 2004; Devanand et al., 2007; Dickerson et al., 2001; Du et al., 2001; Killiany et al., 2000, 2002; Stoub et al., 2005; Xu et al., 2000).
Recently, there has been a great deal of interest in longitudinal MRI studies in patients with AD compared to controls and in those at risk for AD (Cardenas et al., 2002; Convit et al., 1997; Du et al., 2004; Jack et al., 2000, 2004, 2005; Moffat et al., 2000; Stoub et al., 2005), in order to develop MRI markers that can be used as surrogate endpoints to assess and monitor the efficacy of pharmacological interventions. Longitudinal studies that measured the volumes of the hippocampus and entorhinal cortex have shown the annual rate of atrophy of both structures to be greater in patients with AD and in those at risk of AD compared to healthy elderly controls (Cardenas et al., 2002; Du et al., 2003, 2004; Jack et al., 2000, 2004, 2005). In addition, rate of mesial temporal lobe atrophy was found to be predictive of future cognitive decline among healthy elderly individuals without cognitive impairment (Kaye et al., 1997; Rodrigue and Raz, 2004; Rusinek et al., 2003). However, many of the longitudinal MRI studies cited above used scans from two time points separated by 1–5 years for determining rate of atrophy, rather than multiple serial scans.
The present investigation was undertaken to assess the rates of atrophy of the entorhinal cortex and hippocampus in participants with amnestic MCI who are at high risk of developing AD (Petersen et al., 1999) and in patients with very mild AD at entry into the study, compared to cognitively stable elderly controls. Rate of atrophy for regions of interest was derived from yearly scans spanning a period of 5 years (i.e., baseline and 5 years of follow-up). In addition, we investigated the relation of rates of atrophy in the EC and hippocampus to decline in declarative memory function assessed yearly over the 5-year follow-up period.
2. Materials and methods
2.1. Subjects
Baseline data reported here were obtained from the following three groups of participants: (1) 35 elderly normal control subjects with no cognitive impairment during the baseline evaluation (NCI); (2) 33 patients diagnosed with amnestic MCI at entry into the study; and (3) 14 patients diagnosed with mild probable AD. Participants were followed with yearly clinical evaluations and high resolution MRI scans for a 5-year period (baseline plus five follow-up scans). All participants were recruited from the Rush Alzheimer’s Disease Center (RADC; Chicago, IL) clinic, the Religious Order Study (ROS), a longitudinal, clinico-pathologic investigation of aging and AD in older nuns, priests and brothers (Bennett et al., 2002; Kordower et al., 2001; Mufson et al., 1999), or the Rush Memory and Aging Project (MAP), a separate longitudinal clinicopathologic investigation of aging and AD (Bennett et al., 2005). It is important to note that, individuals who came to the clinic for a work-up, but did not show any cognitive impairment were not recruited as controls; the latter were recruited from the community, the ROS or MAP.
Four of the 33 original individuals with MCI did not participate in follow-up imaging visits due to death, implantation of a pacemaker that prohibited further MRI examinations and relocation to another part of the country. Therefore, the longitudinal analyses included 78 subjects. Of the 35 subjects with NCI at baseline, 9 declined in cognitive status to MCI or AD over the 5-year follow-up period. Also, 11 of the 29 individuals with amnestic MCI followed longitudinally developed AD (MCI-AD). Therefore, the longitudinal analyses described below included the following 5 groups: 26 stable NCI (NCI-S), 9 declining NCI (NCI-D), 18 stable MCI (MCI-S), 11 MCI-AD and 14 very mild AD cases at entry.
2.2. Clinical work-up
All evaluations were carried out by the Rush Alzheimer’s Disease Center (RADC, Chicago, IL) as previously described (deToledo-Morrell et al., 1997; Wilson et al., 1996). Briefly, the evaluation, which was given to all participants in the study, incorporated the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD, Morris et al., 1989) procedures and included a medical history, neurological examination, neuropsychological testing, informant interview and blood tests.
The clinical diagnosis of probable AD followed NINCDS/ADRDA guidelines (McKhann et al., 1984); it required a history of cognitive decline and neuropsychological test evidence of impairment in at least two cognitive domains, one of which had to be memory.
Participants diagnosed with amnestic MCI underwent the same standard clinical evaluation as patients with AD, were found to have a deficit in memory only, but did not meet criteria for dementia, as previously described (Bennett et al., 2002). Exclusion criteria for both patients with mild probable AD and amnestic MCI were evidence of other neurologic, psychiatric or systemic conditions that could cause cognitive impairment (e.g., stroke, alcoholism, major depression, a history of temporal lobe epilepsy).
Selection as an elderly control subject required a normal neurological examination, normal cognition as determined by performance on neuropsychological tests, an MMSE (Folstein et al., 1975) score ≥27. Exclusion criteria for the controls were the same as those used for the AD and MCI groups.
Informed consent was obtained from all participants according to the rules of the Human Investigation Committee of Rush University Medical Center.
2.3. Acquisition and quantitation of MRI data
All MR images were acquired on a 1.5 T General Electric Signa scanner using the manufacturer’s 3D Fourier transform spoiled gradient recalled echo (SPGR) pulse sequence with the following parameters: 124 contiguous, 1.6mm thick images were acquired in the coronal plane, acquisition matrix = 256 × 192, field of view= 22 cm, TR/TE = 33.3/7 ms, flip angle = 35°, signals averaged = 1. All scans, both at entry into the study and longitudinally, were carried out with the same scanner.
The Analyze software package (Mayo Clinic Foundation, Rochester, MN) was used for determining the volumes of regions of interest, as well as for the co-registration of sequential scans. Co-registration modules within Analyze correct for possible differences in scaling due to variations in voxel dimensions that may be caused by software upgrades. Both the EC and the HF were manually segmented as described below. To correct for individual differences in brain size, entorhinal and hippocampal volumes were divided by total intracranial volume derived from sagittally formatted 5mm slices (i.e., normalized). To compute intracranial volume, the inner table of the cranium was traced in consecutive sagittal sections spanning the entire brain. At the level of the foramen magnum, a straight line was drawn from the inner surface of the clivus to the occipital bone. Normalized volume for brain regions of interest was determined using the formula: absolute volume in mm3/intracranial volume in mm3 × 1000. Intracranial volume derived from the baseline scan was used for normalizing regions of interest derived from the baseline, as well as follow-up scans.
EC volume was quantified with the use of a protocol developed and validated in our laboratory, technical details of which and validation procedures are presented in Goncharova et al. (2001). The advantage of this protocol is that EC volume is measured from the same oblique coronal sections most commonly used for hippocampal volumetry to avoid overestimation of one of these two adjacent structures at the expense of the other.
Briefly, both entorhinal and hippocampal volumes were computed separately for the right and left hemispheres from coronal slices reformatted to be perpendicular to the long axis of the HF. For the EC, tracing began with the first section in which the gyrus ambiens, amygdala and the white matter of the parahippocampal gyrus first appeared visible. The superomedial border in rostral sections was the sulcus semi-annularis and in caudal sections the subiculum. The shoulder of the collateral sulcus was used as the lateral border. The latter is a somewhat conservative criterion that allowed consistency in tracings and avoided the use of different lateral borders depending on individual differences in the depth of the collateral sulcus (see, for example, Insausti et al., 1998). The lateral border was constructed by drawing a straight line from the most inferior point of the white matter to the most inferior tip of the gray matter. The last section measured was three 1.6mm sections rostral to the image in which the lateral geniculate nucleus first appeared visible. In the majority of cases, tracings were carried out on 12–16 sections.
The protocol and validation procedures used for quantifying hippocampal volume were published previously (deToledo-Morrell et al., 1997; Wilson et al., 1996). Tracings of the HF started with the first section where it could be clearly differentiated from the amygdala by the alveus and included the fimbria, dentate gyrus, the hippocampus proper and the subiculum. Tracings continued on all consecutive images until the slice before the full appearance of the fornix. In the majority of cases, hippocampal volume measurements were obtained from 22 to 26 sections.
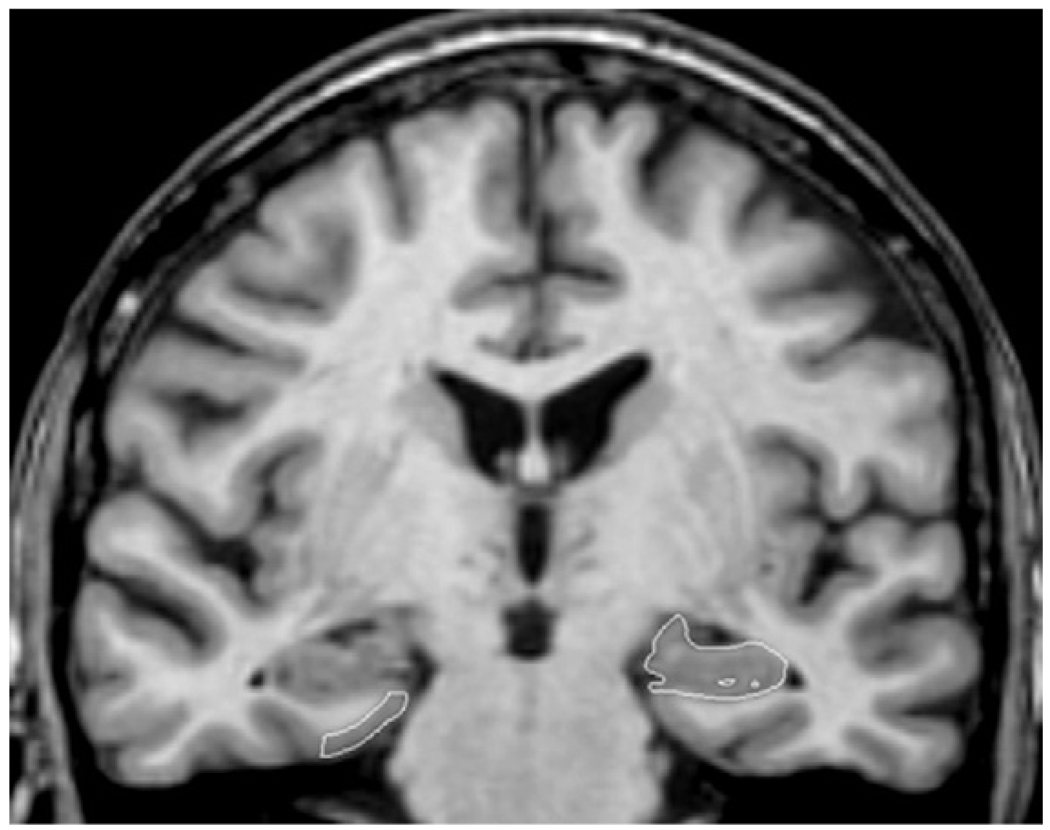
Fig. 1 shows sample tracings of both the EC and the HF on a single MR image. All tracings were carried out by TRS and EJR (who were trained to be within 95% of each other and of LdeT-M) and were checked, slice-by-slice, by LdeT-M. Inter and intra-rater correlation coefficients for TRS, based on a sample of 10, were 0.97 and 0.97, respectively, for the HF and 0.99 and 0.99, respectively, for the EC. The same correlations for EJR were 0.96 and 0.96, respectively, for the HF and 0.98 and 0.96, respectively, for the EC. Investigators involved in the MRI analyses were blinded to clinical information until all volumetric determinations were completed.
Fig. 1.
A single coronal slice illustrating the segmentation of the entorhinal cortex (right hand side of the image) and the hippocampal formation (left hand side of the image). The right hand side of the image corresponds to the left hemisphere and vice versa.
All baseline tracings were carried out first and information concerning slice location and angle were saved. All subsequent scans were co-registered to the baseline scan and analyzed in a random fashion so that the person carrying out tracings of regions of interest was blinded to the date of the scan. Follow-up scans were reformatted and cut according to the information saved from the baseline scan. Because of co-registration procedures used, any special positioning of the head in the scanner was not necessary.
2.4. Declarative memory testing
The declarative memory tests administered to all participants and used to define a memory deficit consisted of immediate and delayed recall of the East Boston Story (Albert et al., 1991) and of Story A from the Logical Memory of the Wechsler memory scale–Revised (Wechsler, 1987). An additional test involved the learning and retention of a 10-word list from the CERAD battery (Morris et al., 1989). The three scores for this test included Word List Memory (the total number of words immediately recalled after each of three consecutive presentations of the list), Word List Recall (the number of words recalled after a delay) and Word List Recognition (the number of words correctly recognized in a four-alternative, forced-choice format, administered after Word List Recall).
2.5. Statistical analyses
For cross-sectional analyses at baseline, group differences were assessed using separate one-way analyses of variance (ANOVA) followed by post hoc comparisons (Scheffé). Longitudinal MRI data were analyzed using separate mixed models for each anatomical region of interest with fixed effects for intercept and slope for each diagnostic group and random effects for intercept and slope within a given group (Brown and Prescott, 1999). The advantage of this model is that it keeps individual baseline values constant while allowing time to vary. Since within person linear terms explained over 99% of the variation in both entorhinal and hippocampal volumes, we did not employ models with slopes changing over time. Our modeling approach accommodates all missing data.
To assess the relationship between changes in mesial temporal lobe structures and memory performance, summary scores were calculated for combined performance on declarative memory tests by standardizing each of the seven declarative memory scores. We used the means and standard deviations of each test from the baseline visits of the first wave of 86 participants entered into our ongoing longitudinal project and averaged the standardized values to obtain a memory z-score. In addition, a summary score for combined entorhinal and hippocampal volumes was computed by averaging the standardized normalized total entorhinal and hippocampal volumes. The relation between changes in mesial temporal lobe structures and alterations in memory function over time were assessed using mixed models with memory function as response. These models included fixed effects for intercept and for coefficient of time for each diagnostic group and random effects for intercept and slope within a given group.
3. Results
3.1. Demographic comparisons
Demographic and MMSE data at baseline for the three groups of participants are presented in Table 1. Separate one-way ANOVAs showed that the three groups did not differ in age or education. There was, however, a significant difference in MMSE scores [F(2,81) = 36.6, p < 0.001); both the very mild AD and amnestic MCI groups differed from the elderly controls (p < 0.001 for both), as well as from each other (p < 0.001) in MMSE.
Table 1.
Baseline demographic characteristics of participants
| Mild AD | MCI | NCI | |
|---|---|---|---|
| N | 14 | 33 | 35 |
| Gender | |||
| Male | 2 | 14 | 11 |
| Female | 12 | 19 | 24 |
| Age (in years) | |||
| (Mean ± S.D.) | 76 ± 5 | 81 ± 7 | 78 ± 5 |
| MMSE | |||
| (Mean ± S.D.) | 25.1 ± 1.4 | 26.8 ± 1.9* | 28.9 ± 1.0** |
| Education (in years) | |||
| (Mean ± S.D.) | 14 ± 3 | 16 ± 4 | 16 ± 3 |
Significantly different from NCI and AD (p < 0.001).
Significantly different from MCI and AD (p < 0.001).
3.2. Baseline comparisons of brain regions of interest
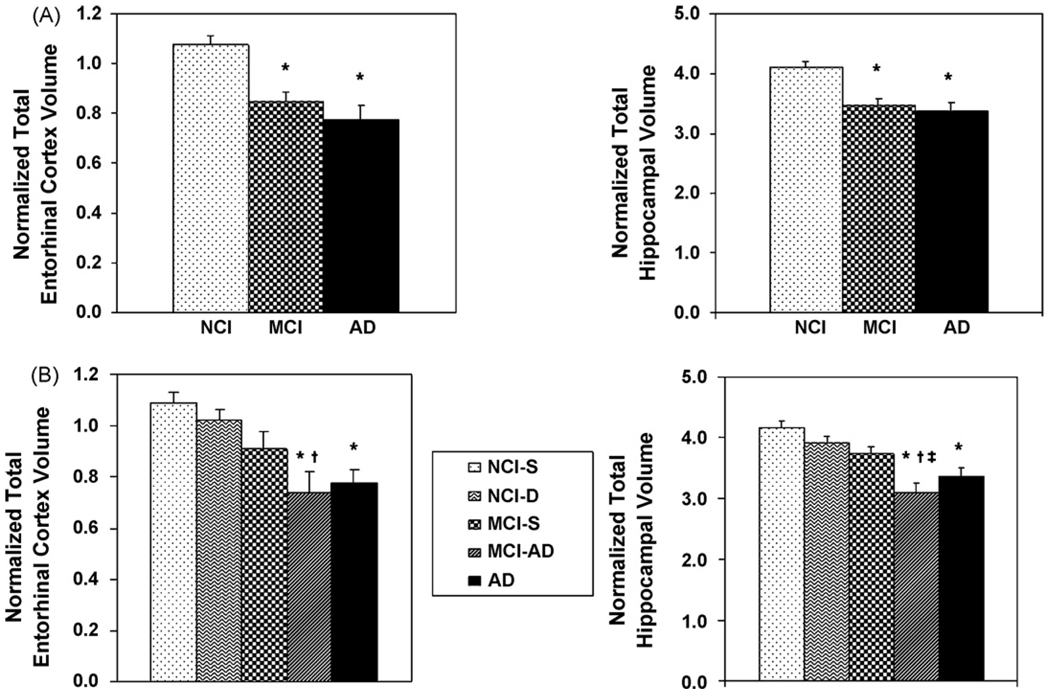
Mean normalized entorhinal and hippocampal volumes at baseline for the three groups of participants (control, amnestic MCI and mild AD) are plotted in Fig. 2A. Group differences in the volumes of the two regions of interest at baseline were assessed with separate one-way ANOVAs. These analyses showed a significant group effect for both regions of interest [F(2,81) = 13.53, p < 0.001 for the EC and F(2,81) = 15,97, p < 0.001 for the HF]. Post hoc comparisons indicated that participants with MCI and mild AD differed significantly from controls in entorhinal and hippocampal volume (p < 0.001 for both), but not from each other.
Fig. 2.
(A) Mean normalized entorhinal and hippocampal volumes (absolute volume in mm3/intracranial volume in mm3 × 1000) at baseline for elderly control subjects (NCI), patients with amnestic mild cognitive impairment (MCI) and those with very mild AD (A). Panels in (B) show baseline entorhinal an hippocampal volumes for control participants who remained stable during the follow-up period (NCI-S), controls who declined in cognitive function (NCI-D), MCI participants who remained stable (MCI-S), people with MCI who received a diagnosis of AD during the follow-up (MCI-AD), and the group that was diagnosed with mild AD at entry into the study. The values for each region of interest are based on the sum of right and left hemisphere volumes. Vertical bars represent the standard error of the mean. *Significantly different from NCI-S, p < 0.001; †significantly different from NCI-D, p = 0.03 for EC and p = 0.005 for hippocampus; ‡significantly different from MCI-S, p = 0.013.
Mean normalized EC and HF volumes at baseline for people with MCI who developed AD during the follow-up period (MCI-AD), MCI participants who remained stable over time (MCI-S) and patients with mild AD compared to stable and declining controls (NCI-S and NCI-D, respectively) are presented in Fig. 2B. The baseline demographic data for these five groups are shown in Table 2. It should be noted that among the MCI participants who developed AD, 5 converted to AD during the first follow-up year, 2 during the second, 2 during the third and 1 each in the fourth and fifth year of follow-up. Differences in the volumes of the EC and HF at baseline among the five longitudinally determined groups were assessed with separate one-way ANOVAs. Once again, these analyses showed a significant group effect for both regions of interest [F(4,77) = 8.47, p < 0.001 for the EC and F(4,77) = 11.61, p < 0.001 for the hippocampus]. With respect to the EC, post hoc comparisons demonstrated that both the MCI cases who converted to AD over time and people who entered the study with a diagnosis of mild AD differed from the stable controls (p < 0.001 for both), but the stable MCI and MCI-AD cases did not differ from each other. In contrast, although both the MCI-AD and mild AD groups differed from the stable controls in baseline hippocampal volume, unlike the entorhinal cortex, the difference between the stable MCI and the MCI-AD groups also reached significance (p = 0.013). These findings suggest that entorhinal pathology, as defined by atrophy may already be present in the stable MCI cases; its further spread to the hippocampus and other cortical regions may be necessary for conversion to AD.
Table 2.
Baseline demographic characteristics of longitudinally determined groups
| Mild AD | MCI-AD | MCI-S | NCI-D | NCI-S | |
|---|---|---|---|---|---|
| N | 14 | 11 | 18 | 9 | 26 |
| Gender | |||||
| Male | 2 | 3 | 8 | 6 | 5 |
| Female | 12 | 8 | 10 | 3 | 21 |
| Age (in years) | |||||
| (Mean ± S.D.) | 76 ± 5 | 80 ± 8 | 82 ± 7 | 80 ± 4 | 78 ± 6 |
| MMSE | |||||
| (Mean ± S.D.) | 25.1 ± 1.4 | 25.8 ± 1.5 | 27.4 ± 2.0* | 28.6 ± 0.9 | 29.0 ± 1.0** |
| Education (in years) | |||||
| (Mean ± SD) | 14 ± 3 | 17 ± 2 | 15 ± 4 | 15 ± 3 | 16 ± 3 |
Significantly different from MCI who converted to AD and those with AD.
Significantly different from all groups except the declining NCI.
3.3. Longitudinal comparisons of brain regions of interest
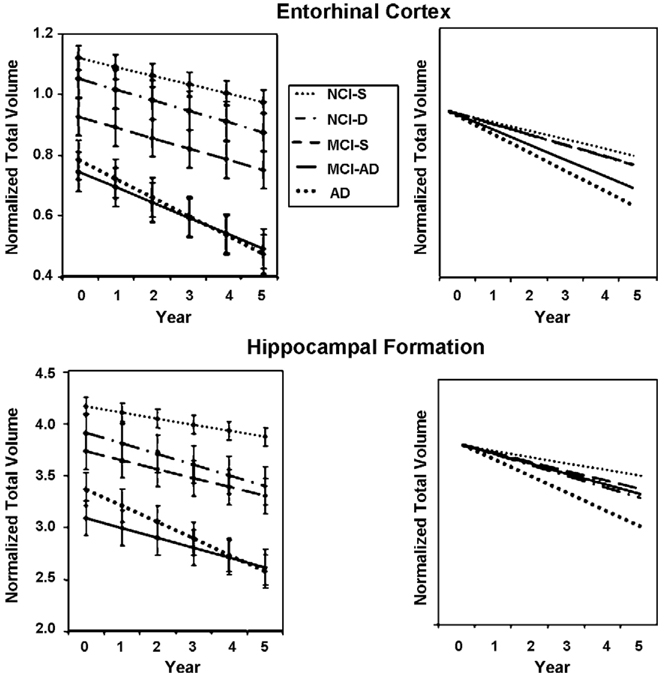
Mean normalized entorhinal and hippocampal volumes at baseline and over the 5 years of follow-up for the longitudinally determined five groups are presented in Fig. 3. The results of mixed models used to assess the rate of atrophy of the entorhinal cortex revealed that the slopes of decline differed significantly among all groups [F(4,262) = 18.08, p < 0.0001]. Post hoc comparisons demonstrated that the slope of decline for the MCI-AD and mild AD groups differed significantly from the stable control group (p < 0.001 for both), but not from each other. In addition, the slopes for the declining NCI and the stable MCI groups differed significantly from the slope for the AD group (p < 0.0001 both comparisons). However, the stable MCI and stable NCI groups did not differ in slope (p = 0.34).
Fig. 3.
Mean normalized entorhinal cortex and hippocampal volumes at baseline and over the 5 years of follow-up for the five groups described in Fig. 2B (left hand panels). The slopes shown are modeled linear slopes. Vertical bars represent the standard error of the mean. In the right hand panels, the same baseline starting point was used for all groups to better appreciate differences in slope.
The slopes of decline for the hippocampus also differed significantly among all groups [F(4,262) = 24.46, p < 0.0001]. Furthermore, the slopes of decline for the NCI-D, MCI-S, MCI-AD and AD groups were significantly different from that of the stable control group (p < 0.0001, p = 0.0058, p = 0.0011, and p < 0.0001, respectively). In addition, the slope of decline for the AD group was significantly different from the slopes for the other four groups (p < 0.001 for all comparisons).
3.4. Relation between longitudinal changes in memory function and in mesial temporal lobe structures
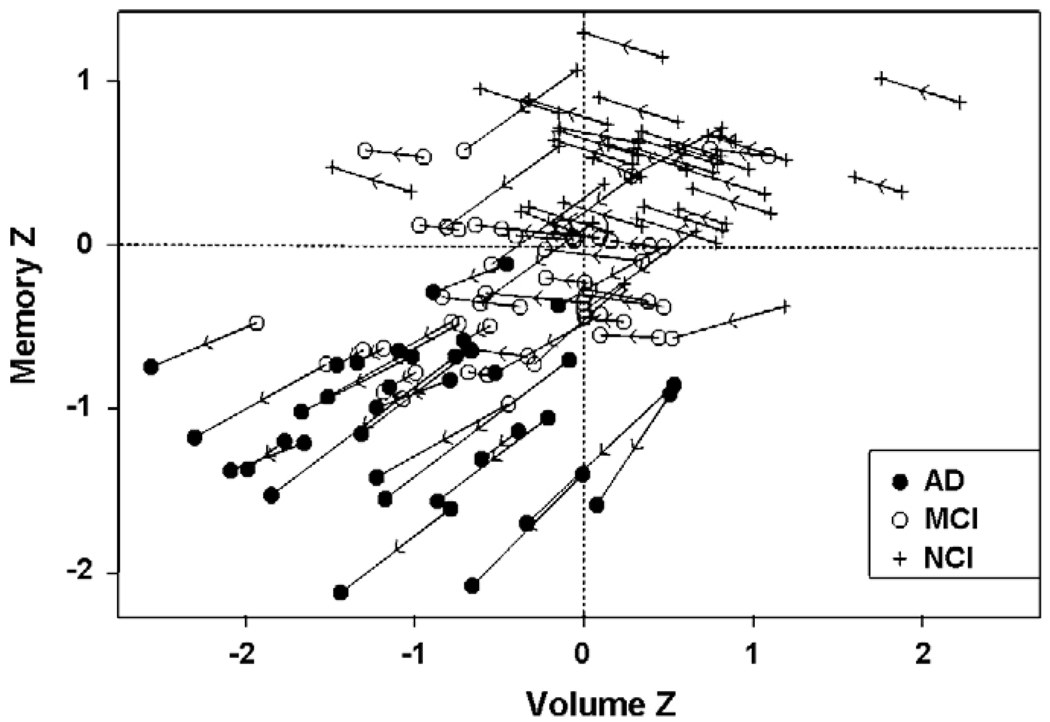
The relation between rate of change in combined entorhinal and hippocampal volume and rate of change in memory function over time is shown in Fig. 4. Data on rates of atrophy for the entorhinal cortex and hippocampus, as well as the rate of memory decline, are presented in Table 3. As indicated above, mixed models were fit to the data with fixed effects for intercept and for coefficient of time for each diagnostic group and random effects for intercept and slope within a given group. These models demonstrated that rate of change in memory function varied significantly with EC +HF volume and its slope of decline [t(262) = 2.42, p < 0.0143 and t(262) = 7.70, p < 0.001, respectively]. When EC and HF volumes were entered into the model separately, results demonstrated that baseline EC volume and its slope of decline were independent predictors of memory z-scores [t(262) = 2.71, p < 0.0072 and t(262) = 8.49, p < 0.0001, respectively]. Baseline HF volume and its slope of decline were also independent predictors of declarative memory function [t(262) = 3.29, p < 0.001 and t(262) = 7.41, p < 0.0001, respectively].
Fig. 4.
The relation between memory z-scores (vertical axis) and z-scores based on normalized entorhinal cortex and hippocampal volumes (horizontal axis) plotted for each individual participant over time. The arrowhead shows the direction of time, and points from the baseline exam towards the last exam for any given participant. The diagnosis at the first and the last exam are shown as symbols indicated in the legend of the figure.
Table 3.
Rates of atrophy and rate of memory decline for longitudinally determined groups
| Mild AD | MCI-AD | MCI-S | NCI-D | NCI-S | |
|---|---|---|---|---|---|
| N | 14 | 11 | 18 | 9 | 26 |
| Hippocampus | |||||
| Rate of atrophy (mean ± S.E.) | −0.1583 ± 0.004 | −0.0947 ± 0.004 | −0.0856 ± 0.003 | −0.1030 ± 0.004 | −0.0592 ± 0.002 |
| Entorhinal cortex | |||||
| Rate of atrophy (mean ± S.E.) | −0.0579 ± 0.012 | −0.0473 ± 0.011 | −0.0327 ± 0.009 | −0.0329 ± 0.010 | −0.0272 ± 0.004 |
| Memory z-score | |||||
| Rate of decline (mean ± S.E.) | −0.1694 ± 0.028 | −0.0903 ± 0.029 | 0.0149 ± 0.024 | −0.0970 ± 0.026 | 0.0295 ± 0.012 |
4. Discussion
The major aim of the present study was to examine annual rates of mesial temporal lobe atrophy in relation to annual change in memory function in patients diagnosed with amnestic MCI and very mild AD at baseline, compared to elderly controls with no cognitive impairment. Since during the 5-year period of the study some of the controls declined in cognitive status and some participants with amnestic MCI developed AD, we were further able to examine, for the first time, rates of atrophy in MCI converters, MCI participants who remained stable and declining controls separately.
Our results demonstrate that at baseline, both participants with mild AD and those with amnestic MCI differed in entorhinal and hippocampal volume from controls, but not from each other. These in vivo findings support the notion that AD-related pathology already exists in people who receive a diagnosis of amnestic MCI and are in line with recent arguments that MCI may represent early stage AD (Morris, 2006; Morris and Cummings, 2005). This notion is further supported by baseline MRI findings in our longitudinally determined subgroups (see Fig. 2B) which demonstrate that the initial entorhinal cortex volume in participants with very mild AD at entry and in those with amnestic MCI who developed AD over the follow-up period, was very similar and did not differ significantly. Since the EC is one of the early sites of pathological involvement in AD, these results suggest that even the initial scan can be indicative of who is at risk of developing AD in the future (see also deToledo-Morrell et al., 2004 and Stoub et al., 2005).
Although the results reported here for EC volume at baseline are very similar to those of a previous publication from our laboratory (Dickerson et al., 2001) carried out in older non-demented people with cognitive complaints, the findings on hippocampal volume are somewhat different. In our previous study, cognitive complainers showed entorhinal atrophy compared to controls, but did not differ from patients with very mild AD in EC volume. In contrast, hippocampal volume in these non-demented participants was significantly smaller than controls, but larger than that of patients with mild AD. It is important to note that some of the participants with cognitive complaints in the previous study tested within normal limits on neuropsychological tests which included declarative memory. The amnestic MCI participants in the present study all had documented memory deficits. It is not surprising, therefore, that they did not differ in either entorhinal or hippocampal volume from patients with mild AD, since they were probably at a later stage in the disease process.
Our longitudinal findings regarding entorhinal and hippocampal rate of atrophy support and extend previous findings in the field. In line with previous investigations (Cardenas et al., 2002; Du et al., 2003, 2004; Jack et al., 1998), we found that the rate of atrophy of both the entorhinal cortex and hippocampus in patients with mild AD at entry into the study was significantly steeper compared to stable controls. Furthermore, based on longitudinally determined clinical decline, we were able to demonstrate that participants with amnestic MCI who received a diagnosis of AD over time, not only had smaller entorhinal and hippocampal volumes at baseline compared to stable controls, but also showed faster rates of atrophy of both structures. Most importantly, these “converters to AD” resembled patients with mild AD with respect to both baseline volumes and rates of atrophy of the entorhinal cortex. With respect to the hippocampus, however, the slope of decline for the AD group was significantly different than that of the MCI-AD. It may very well be that the entorhinal cortex, which is a small structure and which is pathologically involved very early in the disease process, is so affected by the time people have frank AD that it is difficult to measure increased rate of atrophy in the AD group.
The relation between declarative memory function and hippocampal volume in patients with AD has been well documented (deToledo-Morrell et al., 2000a, 2000b; Grundman et al., 2003; Köhler et al., 1998; Mori et al., 1997;Wilson et al., 1996). However, these studies have, in general, been cross-sectional in nature. In the present paper we present results that tracked yearly hippocampal and entorhinal volume change in relation to memory function determined longitudinally not only in participants with AD, but also in people who entered the study as amnestic MCI or controls with no cognitive impairment. These results clearly demonstrate a very strong relation between changes in memory function and hippocampal volume, as well as entorhinal cortex volume. Inspection of Fig. 4 indicates that stable controls showed some slight improvement in memory function over time. This is not surprising since there is some “learning to learn” effect during cognitive testing. What is of importance, however, is the fact that those declining in cognitive function did not benefit from this effect.
In summary, the findings presented in this paper suggest that atrophy in critical brain regions known to be pathologically involved very early in AD can differentiate healthy from pathological aging. Although even the baseline entorhinal and hippocampal volumes were indicative of MCI participants who would in the future receive a diagnosis of AD, longitudinal rate of atrophy measures seemed to be better at discriminating declining groups from stable controls. Thus, in vivo MRI-derived anatomical changes that are predictive of AD, as described in the present paper, could aid in the development and application of preventative therapeutic strategies.
Acknowledgements
This research was supported by grants P01 AG09466, P30 AG10161 and R01 AG17917 from the National Institute on Aging, National Institutes of Health. We thank Barbara Eubler and Carolyn Ferrari for help with the recruitment and follow-up of participants.
Footnotes
Conflict of interest
The authors do not have any actual or potential conflicts of interest.
References
- Albert M, Smith L, Scherr P, Taylor J, Evans D, Funkenstein H. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int. J. Neurosci. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, Thompson PM. Conversion of mild cognitive impairment to Alzheimer’s disease predicted by hippocampal atrophy maps. Arch. Neurol. 2006;63:693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon CF, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- Bobinski M, de Leon MJ, Convit A, De Santi S, Wegiel J, Tarshish CY, Louis LAS, Wisniewski HM. MRI of entorhinal cortex in mild Alzheimer’s disease. Lancet. 1999;353:38–40. doi: 10.1016/s0140-6736(05)74869-8. [DOI] [PubMed] [Google Scholar]
- Bowen J, Teri L, Kukull W, McCormick W, Mc Curry SM, Larson EB. Progression to dementia in patients with isolated memory loss. Lancet. 1997;349:763–765. doi: 10.1016/S0140-6736(96)08256-6. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer related changes. Acta. Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging. 1995;16:271–288. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J, Bratzke H. Evolution of Alzheimer’s disease related cortical lesions. J. Neural Transm. Suppl. 1998;54:97–106. doi: 10.1007/978-3-7091-7508-8_9. [DOI] [PubMed] [Google Scholar]
- Brown H, Prescott R. Applied Mixed Models in Medicine. Chichester, England: John Wiley & Sons; 1999. [Google Scholar]
- Cardenas VA, Du AT, Hardin D, Ezekiel F, Weber P, Jagust WJ, Chui HC, Schuff N, Weiner MW. Comparison of methods for measuring longitudinal brain change in cognitive impairment and dementia. Neurobiol. Aging. 2002;23:1–8. doi: 10.1016/s0197-4580(02)00130-6. [DOI] [PubMed] [Google Scholar]
- Crook T, Larabee GT. Age-associated memory impairment: diagnostic criteria and treatment strategies. Psychopharm. Bull. 1988;24:509–514. [PubMed] [Google Scholar]
- Convit A, de Leon MJ, Tarshish C, De Santi S, Tsui W, Rusinek H, George A. Specific hippocampal volume reductions in individuals at risk for Alzheimer’s disease. Neurobiol. Aging. 1997;18:131–138. doi: 10.1016/s0197-4580(97)00001-8. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, George AE, Golomb J, De Santi S, Tarshish C, Rusinek H, Bobinski M, Ince C, Miller D, Wisniewski H. In vivo structural studies of the hippocampus in normal aging and in incipient Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1996;777:1–13. doi: 10.1111/j.1749-6632.1996.tb34395.x. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, George AE, Golomb J, Tarshish C, Convit A, Kluger A, De Santi S, McRae T, Ferris SH, Reisberg B, Ince C, Rusinek H, Bobinski M, Quinn B, Miller DC, Wisniewski HM. Frequency of hippocampal formation atrophy in normal aging and Alzheimer’s disease. Neurobiol. Aging. 1997;8:1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Sullivan MP, Morrell F, Wilson RS, Bennett DA, Spencer S. Alzheimer’s disease: in vivo detection of differential vulnerability of brain regions. Neurobiol. Aging. 1997;18:463–468. doi: 10.1016/s0197-4580(97)00114-0. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Dickerson B, Sullivan MP, Spanovic C, Wilson RS, Bennett D. Hemispheric differences in hippocampal volume predict verbal and spatial memory performance in patients with Alzheimer’s disease. Hippocampus. 2000a;10:136–142. doi: 10.1002/(SICI)1098-1063(2000)10:2<136::AID-HIPO2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Goncharova I, Dickerson B, Wilson RS, Bennett DA. From healthy aging to early Alzheimer’s disease. In vivo detection of entorhinal cortex atrophy. Ann. N. Y. Acad. Sci. 2000b;911:240–753. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Stoub TR, Bulgakova M, Wilson RS, Bennett DA, Leurgans S, Wu J, Turner DA. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol. Aging. 2004;25:1197–1203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Pradhaban G, Liu X, Khadji A, De Santi S, Segal S, Rusinek H, Pelton GH, Honig LS, Mayeux R, Stern Y, Talbert MH, de Leon MJ. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer’s disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, Beckett LA, deToledo-Morell L. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol. Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, Yaffe K, Kramer JH, Reed B, Norman D, Chui HC, Weiner MW. MRI of entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Zhu XP, Jagust WJ, Miller BL, Reed BR, Kramer JH, Mungas D, Yaffe K, Chui HC, Weiner MW. Atrophy rates of entorhinal cortex in AD and normal aging. Neurology. 2003;60:481–486. doi: 10.1212/01.wnl.0000044400.11317.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Kramer JH, Ganzer S, Zhu XP, Jagust WJ, Miller BL, Reed BR, Mungas D, Yaffe K, Chui HC, Weiner MW. Higher atrophy rate of entorhinal cortex than hippocampus in AD. Neurology. 2004;62:422–427. doi: 10.1212/01.wnl.0000106462.72282.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the mental status of patients for the clinician. J. Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J. Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharova II, Dickerson BC, Stoub TR, deToledo-Morrell L. MRI of entorhinal cortex: a reliable protocol for volumetric measurement. Neurobiol. Aging. 2001;22:737–745. doi: 10.1016/s0197-4580(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Grundman M, et al. Hippocampal volume is associated with memory but not non-memory cognitive performance in patients with mild cognitive impairment. J. Mol. Neurosci. 2003;20:241–248. doi: 10.1385/jmn:20:3:241. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkanen A. MR volumetric analysis of the human entorhinal, perirhinal and temporopolar cortices. Am. J. Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, O’Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Kokmen E. Rate of medial temporal lobe atrophy in typical aging and Alzheimer’s disease. Neurology. 1998;51:993–999. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Tangalos EG, Kokmen E. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Shiung MM, Gunter JL, O’Brien PC, Weigand SD, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Cha RH, Tangalos EG, Petersen RC. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Shiung MM, Weigand SD, O’Brien PC, Gunter JL, Boeve BF, Knopman DS, Smith GE, Ivnik RJ, Tangalos EG, Petersen RC. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juottonen K, Laakso MP, Insausti R, Lehtovirta M, Pitkanen A, Soininen H. Volumes of the entorhinal and perirhinal cortices in Alzheimer’s disease. Neurobiol. Aging. 1998;19:15–22. doi: 10.1016/s0197-4580(98)00007-4. [DOI] [PubMed] [Google Scholar]
- Juottonen K, Laakso MP, Partanen K, Soininen H. Comparative MR analysis of the entorhinal cortex and hippocampus in diagnosing Alzheimer’s disease. Am. J. Neuroradiol. 1999;20:139–144. [PubMed] [Google Scholar]
- Kaye JA, Swihart T, Howieson D, Dame A, Moore MM, Karnos T, Camicioli R, Ball M, Oken B, Sexton G. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- Kesslak JP, Nalcioglu O, Cotman CW. Quantification of magnetic scans for hippocampal and parahippocampal atrophy in Alzheimer’s disease. Neurology. 1991;41:51–54. doi: 10.1212/wnl.41.1.51. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Moss MB, Albert MS, Sandor T, Tieman J, Jolesz F. Temporal lobe regions on magnetic resonance imaging identify patients with early Alzheimer’s disease. Arch. Neurol. 1993;50:949–954. doi: 10.1001/archneur.1993.00540090052010. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman BT, Albert MS. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann. Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- Killiany RJ, Hyman BT, Gomez-Isla T, Moss MB, Kikinis R, Jolesz F, Tanzi R, Jones K, Albert MS. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- Köhler S, Black SE, Sinden M, Szekely C, Kidron D, Parker JL, Foster JK, Moscovitch M, Winocour G, Szalai JP, Bronskill MJ. Memory impairments associated with hippocampal versus parahippocampal gyrus atrophy: an MRI volumetric study in Alzheimer’s disease. Neuropsychologia. 1998;36:901–914. doi: 10.1016/s0028-3932(98)00017-7. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, Mufson EJ. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann. Neurol. 2001;49:202–213. [PubMed] [Google Scholar]
- Laakso MP, Soininen H, Partanen K, Lehtovirta M, Hallikainen M, Haninen T, Helkala EL, Vainio P, Riekkinen PJ. MRI of the hippocampus in Alzheimer’s disease: sensitivity, specificity, and analysis of incorrectly classified subjects. Neurobiol. Aging. 1998;19:23–31. doi: 10.1016/s0197-4580(98)00006-2. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS/ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Szekely CA, Zonderman AB, Kabani NJ, Resnick SM. Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology. 2000;55:134–136. doi: 10.1212/wnl.55.1.134. [DOI] [PubMed] [Google Scholar]
- Mori E, Yoneda Y, Yamashita H, Hirono N, Ikeda M, Yamadori A. Medial temporal structures relate to memory impairment in Alzheimer’s disease: an MRI volumetric study. J. Neurol. Neurosurg. Psychiatry. 1997;63:214–221. doi: 10.1136/jnnp.63.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. Mild cognitive impairment is early stage Alzheimer’s disease: time to revise diagnostic criteria. Arch. Neurol. 2006;63:15–16. doi: 10.1001/archneur.63.1.15. [DOI] [PubMed] [Google Scholar]
- Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer’s disease. J. Alzheimers Dis. 2005;7:235–239. doi: 10.3233/jad-2005-7306. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellitis ED, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Chin EY, Cochran EJ, Beckett LA, Bennett DA, Kordower JH. Entorhinal cortex beta amyloid load in individuals with mild cognitive impairment. Exp. Neurol. 1999;158:469–490. doi: 10.1006/exnr.1999.7086. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Aging, mild cognitive impairment and Alzheimer’s disease. Dementia. 2000;18:789–805. doi: 10.1016/s0733-8619(05)70226-7. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Jack CR, Xu YC, Waring SC, O’Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Boeve BF, Kokmen E. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54:581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. J. Neurosci. 2004;24:956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinek H, De Santi S, Frid D, Tsui WH, Tarshish CY, Convit A, de Leon MJ. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology. 2003;229:691–696. doi: 10.1148/radiol.2293021299. [DOI] [PubMed] [Google Scholar]
- Seab JP, Jagust ST, Wong ST, Roos MS, Reed BR, Budinger TF. Quantitative NMR measurements of hippocampal atrophy in Alzheimer’s disease. Magn. Reson. Med. 1988;8:200–208. doi: 10.1002/mrm.1910080210. [DOI] [PubMed] [Google Scholar]
- Soininen HS, Partanen K, Pitkanen A, Vainio P, Hanninen T, Hallikainen M, Koivisto K, Riekkinen PJ. Volumetric MRI analysis of the amygdala and hippocampus in subjects with age-associated memory impairment: correlation to visual and verbal memory. Neurology. 1994;44:1660–1668. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stoub TR, Bulgakova M, Leurgans S, Bennett DA, Fleischman D, Turner DA, deToledo-Morrell L. MRI predictors of risk of incident Alzheimer’s disease: a longitudinal study. Neurology. 2005;64:1520–1524. doi: 10.1212/01.WNL.0000160089.43264.1A. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Hyman BT, Damasio AR. Entorhinal cortex pathology in Alzheimer’s disease. Hippocampus. 1991;1:1–8. doi: 10.1002/hipo.450010102. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale—Revised Manual. San Antonio: Psychological Corp; 1987. [Google Scholar]
- Wilson RS, Sullivan MP, deToledo-Morrell L, Stebbins GT, Bennett DA, Morrell F. Association of memory and cognition in Alzheimer’s disease with volumetric estimates of temporal lobe structures. Neuropsychology. 1996;10:459–463. [Google Scholar]
- Xu Y, Jack CR, O’Brien PC, Kokmen E, Smith GE, Ivnik RJ, Boeve BF, Tangalos RG, Petersen RC. Usefulness of MRI measures of entorhinal cortex versus hippocampus in AD. Neurology. 2000;54:1760–1767. doi: 10.1212/wnl.54.9.1760. [DOI] [PubMed] [Google Scholar]
- Young B, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. J. Neurosci. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]