Abstract
Metabolites offer an important unexplored complement to understanding the pluripotency of stem cells. Using mass spectrometry-based metabolomics, we show that embryonic stem cells are characterized by abundant metabolites with highly unsaturated structures whose levels decrease upon differentiation. By monitoring the reduced and oxidized glutathione ratio as well as ascorbic acid levels, we demonstrate that the stem cell redox status is regulated during differentiation. Based on the oxidative biochemistry of the unsaturated metabolites, we experimentally manipulated specific pathways in embryonic stem cells while monitoring the effects on differentiation. Inhibition of the eicosanoid signaling pathway promoted pluripotency and maintained levels of unsaturated fatty acids. In contrast, downstream oxidized metabolites (e.g., neuroprotectin D1) and substrates of pro-oxidative reactions (e.g., acyl-carnitines), promoted neuronal and cardiac differentiation. We postulate that the highly unsaturated metabolome sustained by stem cells makes them particularly attuned to differentiate in response to in vivo oxidative processes such as inflammation.
Introduction
Global gene-expression patterns of embryonic and adult stem cell populations have allowed for the identification of important genes in stem cell biology. Parallel studies of epigenetic regulation have led to the discovery of a bivalent histone modification motif in embryonic stem cells (ESCs) 1–2. At the proteomic level, studies have revealed protein expression patterns associated with stem cells 3. Despite these significant advances, the molecular framework that controls the balance between pluripotency and stem cell differentiation is still not fully understood. This may be partially due to the challenge of correlating gene and protein expression data with functional activity and cellular phenotype. Indeed, increases in mRNA levels do not always correlate with increases in protein levels 4–5. Additionally, because of a variety of post-translational modifications, once translated, a protein may not be functionally active.
A promising complementary approach to explore the stem cell phenotype is metabolomics, defined as the metabolic complement of functional genomics. Metabolomics enables the characterization of endogenous small molecules that are the products of biochemical reactions, revealing connections between different pathways that operate within a living cell 6–7. For the first time, pluripotent and differentiated cells from two germ layers (the ectoderm and mesoderm) were quantitatively characterized using an untargeted metabolomics approach with liquid chromatography (LC)-electrospray ionization (ESI) mass spectrometry (MS). The experimental comparison was performed on two different lines of murine embryonic stem cells (mESC) that have greater than 99% homogeneity (46C mESC and R1 mESC). Their differentiated progenies were analyzed when greater than 80% of the cells were neurons and greater than 70% were cardiomyocytes. Based on the mass spectrometry-based metabolomics results, we selectively inhibited enzymes in vitro that we predicted to mediate differentiation. Similarly, we supplemented stem cell media with naturally occurring metabolites involved in and produced by oxidative pathways to determine their effect on pluripotency and differentiation.
Taken together, our results show that the phenotype of ESCs is characterized by the presence of structurally unsaturated metabolites whose levels decrease upon differentiation. We suggest that this highly unsaturated metabolome is susceptible to pro-oxidative events that ultimately influence cell fate.
Results
Highly unsaturated metabolites in embryonic stem cells
Our mass spectrometry-based platform (Supplementary Fig. 1) involves LC-ESI-TOF-MS profiling followed by data analysis with an open-source software called XCMS 8. The relative abundance of metabolites in homogeneous populations of ESCs, neurons, and cardiomyocytes (as determined by characteristic morphology and marker expression, Supplementary Fig. 2) was quantified by comparing the integrated area of each feature, and assigning a fold value to indicate the level of differential regulation between populations (see full data in Supplementary Table 1).
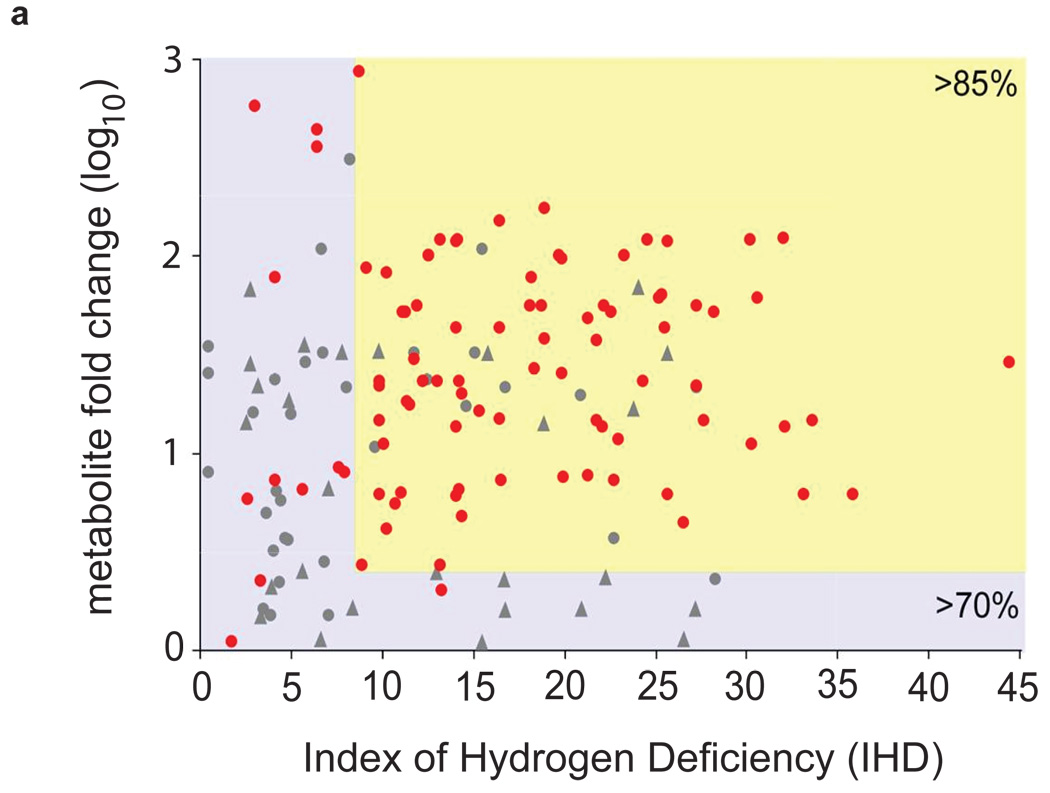
To identify a “metabolic signature” associated with the stem cell phenotype, we characterized differentially regulated (fold >2) metabolites that were statistically significant (p<0.01) in ESCs relative to neurons and cardiomyocytes. The high resolving power (~200,000) and high mass accuracy (< 1 ppm error) of FT-ICR MS, allowed for the determination of the molecular formula of more than 150 metabolites. Based on the chemical formulas, we were able to calculate the index of hydrogen deficiency for each metabolite (IHD), which we normalized by mass (Supplementary Fig. 1b). The IHD accounts for the degree of unsaturation of a specific molecule, determined by the number of ρ bonds and/or rings that the molecule contains. Figure 1a shows the distribution of IHD for the endogenous metabolites for which we determined molecular formulas, indicating a substantially higher degree of unsaturation in the stem cell metabolome relative to mature populations. Greater than 85% of the determined metabolite formulas in ESCs show a high degree of unsaturation (IHD > 8) and a high relative abundance (> 5 fold) in comparison to the mature populations. Most of the calculated molecular formulas for metabolites over 5 fold higher in mature populations compared to ESCs had an IHD < 8.
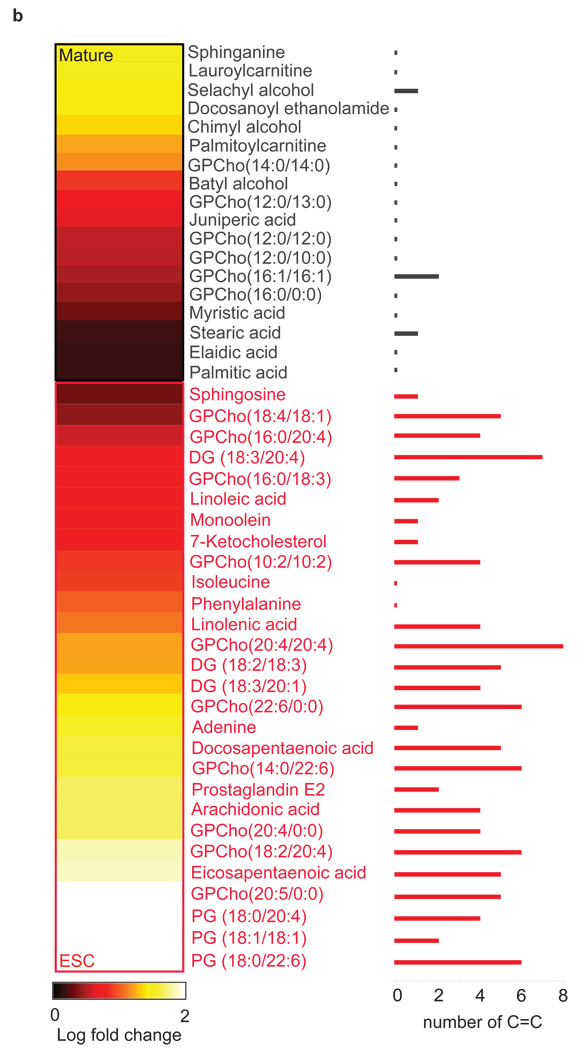
Figure 1. Embryonic stem cells are characterized by abundant metabolites with highly unsaturated structures.
(a) Degree of unsaturation in embryonic stem cells (ESCs) and mature populations. The plot shows the relative fold change of metabolites that are either (i) up-regulated in ESCs (red dots) or (ii) up-regulated in mature populations (gray dots and gray triangles to represent neurons and cardiomyocytes respectively) vs. the index of hydrogen deficiency (IHD) of each metabolite. Of the molecular formulas we characterized in ESCs, 85% have IHD values ≥ 8 and fold increases ≥ 5 (yellow-shaded area). Of the molecular formulas we characterized in mature populations, 70% have IHD values ≤ 8 and/or fold increases ≤ 5 (blue-shaded area). (b) Heatmap showing 46 metabolites whose structures were identified by tandem MS. Lighter colors (yellow and white) correspond to the largest fold changes, where fold is defined as the relative difference between the integrated peak area of each feature in ESCs relative to mature populations. Metabolite names shown in black are up-regulated in mature populations relative to ESCs (p<0.01). Metabolite names shown in red are up-regulated in ESCs relative to mature populations (p<0.01). The number of C-C double bonds for each metabolite is shown by the bar graph on the right.
Some of the most up-regulated metabolites identified by tandem MS/MS (Supplementary Fig. 1c) in ESCs relative to mature populations are secondary lipid messengers and inflammatory mediators (Fig. 1b) such as arachidonic acid, docosapentaenoic acid, eicosapentaenoic acid, linolenic acid, diacylglycerols (DG), glycerophosphocholines (GPCho) (Supplementary Table 2), glycerophosphoglycerols (PG), and eicosanoids such as prostaglandin E2. Sphingosine and 7-ketocholesterol, a sphingolipid and a cholesterol metabolite involved in signal transduction were also identified. Mature populations showed increased levels of saturated free fatty acids together with their acyl-carnitines (primarily in cardiomyocytes), which are fatty acyl-CoA (coenzyme A) conjugated to carnitine implicated in the transport into the mitochondria for β-oxidation. Neurons were unique in that they contained saturated fatty alcohols (namely chimyl, batyl (1) and selachyl alcohol) not observed in ESCs. Little is known about these fatty alcohols, and to our knowledge these molecules have not been previously described in mammalian neuronal cells. However, they have been associated with the inhibition of neurodegenerative diseases 9 and in bone marrow cell regeneration 10. Docosanoyl ethanolamide, a saturated N-acylethanolamide (endocannabinoid) that modulates voltage-dependent Ca(2+) channels 11 was found to be uniquely up-regulated in differentiated cardiomyocytes.
Overall, the structures of the differentially regulated metabolites indicate that the high degree of unsaturation reflected by the IHD in stem cells is primarily a result of carbon-carbon double (C=C) bonds (Fig. 1b). Consequently, since chemical unsaturations such as C=C are highly reactive under oxidative conditions (e.g., arachidonic acid is a metabolic precursor for the biosynthesis of over 100 structurally diverse metabolites inside the cell), we hypothesize that the abundance of these species in ESCs is important to maintaining ‘chemical plasticity’ and ultimately in mediating differentiation by regulation of redox status and activation of oxidative pathways.
Redox status mediates embryonic stem cell differentiation
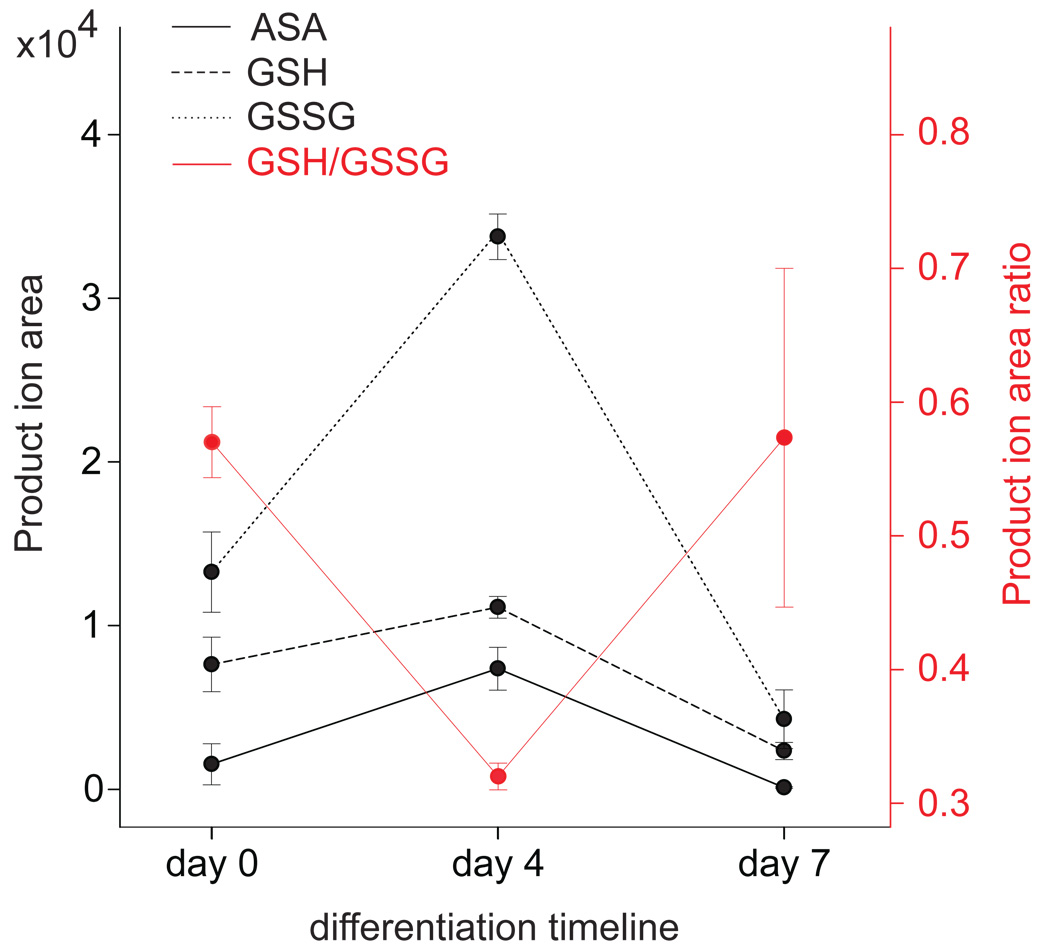
To confirm that the oxidation of the unsaturated metabolites we identified was associated with intracellular changes in redox status, we quantified the ratio of reduced and oxidized glutathione (GSH/GSSG) as a function of days of differentiation of ESCs using triple-quadrupole mass spectrometry and multiple reaction monitoring (MRM). Glutathione (GSH) is a reducing agent that protects the cell from oxidative damage, mainly through the catalyzed reduction of hydrogen peroxide to water by the enzyme glutathione peroxidase. When mammalian cells are exposed to increased oxidative stress the GSH/GSSG ratio decreases as GSH is converted to GSSG. Our results showed a significant decrease in the GSH/GSSG ratio at day 4 as a consequence of GSSG accumulation (Fig. 2). At day 7, the GSH/GSSG returned to the original ratio as observed at day 0. Note that the GSH/GSSG ratio at day 0 (0.58) as determined by the integrated peak areas of product ions using MRM corresponds to a concentration of GSH 90–100 fold higher than that of GSSG. This was confirmed with GSH and GSSG standards. Ascorbic acid (ASA) is another strong reducing agent that neutralizes reactive oxygen species (ROS) in cells. Interestingly, when ascorbic acid was compared with GSH and GSSG using MRM, we observed that the concentration of ASA increased at day 4, correlating with the lowest GSH/GSSG ratio. This indicates that the GSH/GSSG ratio and ascorbic acid levels are inversely related in response to ESC differentiation.
Figure 2. GSH/GSSG ratio and ascorbic acid levels are inversely related in response to ESC differentiation.
Ascorbic acid (ASA), reduced glutathione (GSH), and oxidized glutathione (GSSG) abundance, and GSH/GSSG ratio as a function of days of differentiation of mESCs. Values were determined by the integrated peak areas of product ions using triple quadrupole mass spectrometry and multiple reaction monitoring (see Supplementary Methods for details). Error bars represent mean values and standard deviation for three independently prepared replicates (4 million cells) of each time point.
Therefore our findings suggest that redox regulation, probably as a result of events associated with oxidative stress occurring during differentiation, is important to mediate embryonic stem cell fate.
Eicosanoid pathway inhibition promotes pluripotency
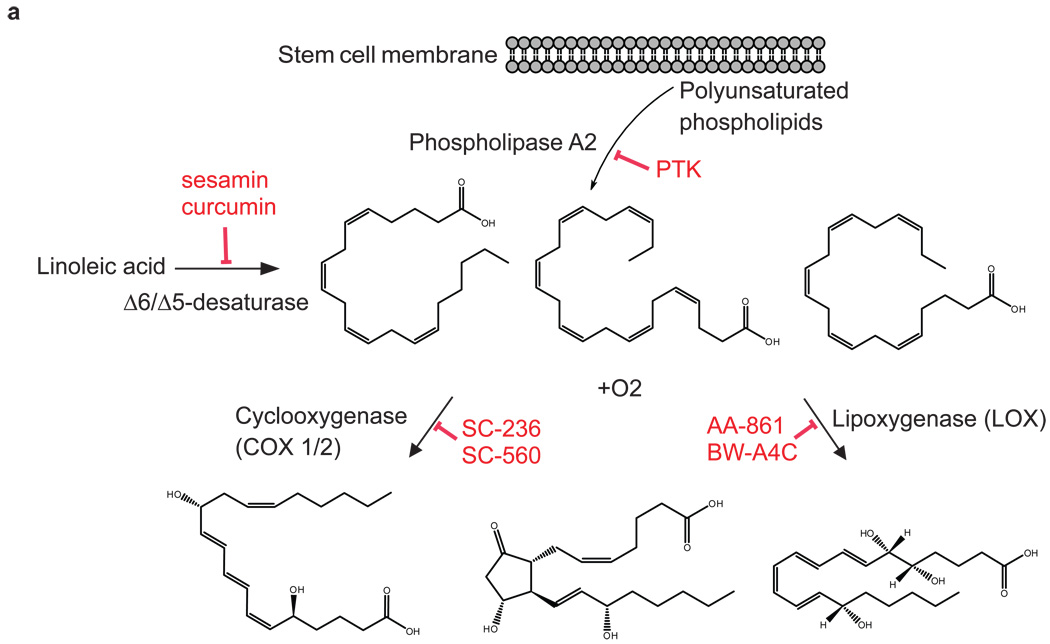
To test our hypothesis that activation of oxidative pathways is important for differentiation, we inhibited the eicosanoid signaling pathway (Fig. 3a), a well known pro-oxidative cascade in which the metabolic substrates (i.e., AA, DHA, EPA) are characteristic of the pluripotent state as revealed by our mass spectrometry-based metabolomics results.
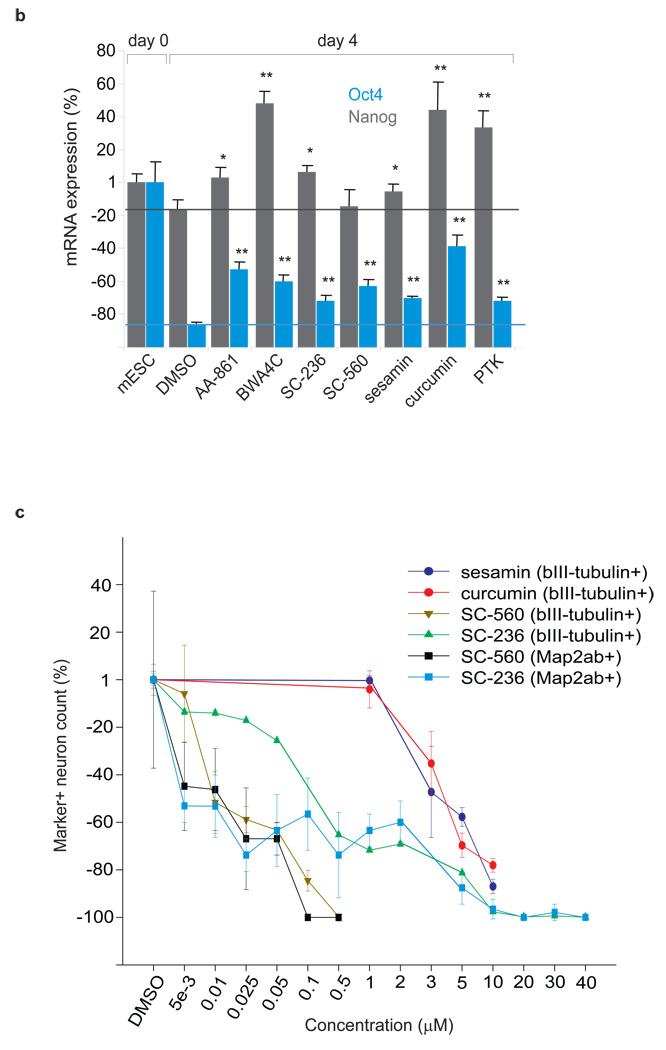
Figure 3. Inhibition of the eicosanoid signaling pathway promotes the pluripotent state of embryonic stem cells.
(a) The eicosanoid signaling pathway is mediated by the enzymes phospholipase A2 (PLA2), cyclooxygenase (COX), and lipooxygenase (LOX). PLA2 hydrolyzes phospholipids from the cellular membrane releasing AA, EPA or DHA, and lysophospholipids 38. In the presence of O2, COX and LOX oxidize C=C from AA, EPA, and DHA thereby producing important biological eicosanoid mediators that regulate the inflammatory response, such as thromboxanes, prostaglandins, and prostacyclins, producing reactive oxygen species (ROS) in the process 39. (b) Inhibition of PLA2, COX, LOX, and desaturase delays the loss of pluripotency at day 4, as indicated by the higher expression of pluripotent markers Oct4 (green) and Nanog (red) relative to the DMSO control (green and red lines as references). Sample labeled mESC corresponds to the levels of expression of Oct4 and Nanog at day 0 (taken for normalization). *indicates a p-value < 0.05. ** indicates a p-value < 0.001. (c) Fatty acid 5Δ and 6Δ desaturase inhibition by curcumin and sesamin, and COX inhibition by SC236 and SC560, significantly delay differentiation and neuron maturation. ESC cultures were treated with either curcumin, sesamin, COX inhibitors, or a DMSO control at various concentrations from days 1–10 of differentiation. Neuronal differentiation was evaluated with βIII-tubulin positive or Map2ab/βIII-tubulin double positive neuron counts at day 10 of differentiation. Four cell culture replicates were analyzed for each inhibitor concentration. Data points and error bars represent mean values and s.d.
Desaturase inhibitors (sesamin (2), a selective inhibitor of 5Δ desaturase; curcumin (3), an inhibitor of 5Δ and 6Δ desaturase), COX inhibitors (SC236 (4), a selective COX-2 inhibitor; SC560 (5), a selective COX-1 inhibitor), LOX inhibitors (AA-861 (6) and BW-A4C (7), selective 5-LOX inhibitors) and a PLA2 inhibitor (PTK, palmityl trifluoromethyl ketone (8)) were tested and each promoted the pluripotent state of mESCs when cultured in neuronal differentiation conditions. Pluripotency was measured by monitoring the expression of the mESCs markers Nanog and Oct4 (Fig. 3b) and analyzing colony morphology (Supplementary Fig. 3) at days 2 and 4 of treatment with the inhibitors. Specifically, selective inhibition of these enzymes led to 15–60% increased levels of Oct4 and Nanog expression relative to DMSO at day 4. Expression of Oct4 and Nanog in cells grown in the absence of DMSO was not different from those grown in DMSO control.
Inhibition of 5Δ and 6Δ desaturase with sesamin and curcumin resulted in a significant reduction in neuronal differentiation with increasing dosages. Sesamin curcumin, and DMSO vehicle were added to mESCs cultured in N2B27 media without LIF at day 1 and analyzed for neuronal differentiation at day 10 (Supplementary Fig. 4). βIII-tubulin positive neurons, a widely used maker for early neuronal differentiation 12, decreased with increasing concentrations of sesamin and curcumin, with no detectable reduction in total cell number over the treatment range of 0–10 µM as determined by DAPI staining (Fig. 3c and Supplementary Fig. 5). Compared to DMSO vehicle, 10 µM sesamin and 10 µM curcumin delayed neuronal differentiation as observed by 78 and 83% reductions in the number of βIII-tubulin positive neurons respectively. The number of βIII-tubulin positive neurons in the absence of DMSO was not different from the number in the DMSO control.
COX inhibitors were also administered to a separate culture at day 1 and cells were analyzed at day 10 of differentiation. SC236 (COX-2 IC50: 5–10 nM; COX-1 IC50: 17 µM) treated cultures showed a reduction in both βIII-tubulin positive and Map2ab positive neurons with increasing inhibitor concentrations (Fig. 3c and Supplementary Fig. 5). Map2ab (Microtubule Associated Protein 2ab) is a marker for mature neurons. 10 µM of SC236 exposure reduced βIII-tubulin positive and Map2ab positive neurons more than 90% relative to DMSO treated cultures. SC560 (COX-1 IC50: 0.009 µM; COX-2 IC50: 6.3 µM) treated cultures also displayed a dosage-dependent decrease in both βIII-tubulin positive and Map2ab positive neurons. Relative to the neuron counts of DMSO treated cultures, 0.5 µM SC560 treated cultures had a reduction greater than 95% of βIII-tubulin positive neurons and no Map2ab positive neurons could be seen above 0.5 µM of SC560 inhibitor. At the concentrations tested, both COX inhibitors reduced neuronal differentiation with no appreciable change in total cell number as determined by DAPI staining (Supplementary Fig. 6). The levels of the ω-6 and ω-3 fatty acids (AA, EPA, DHA, and linoleic acid), the metabolic substrates of the enzymes COX and LOX, were also analyzed at day 4 and day 7 of differentiation (Supplementary Fig. 7). Compared to the DMSO vehicle, cells treated with AA-861 (5-LOX inhibitor), and SC-236 (COX-2 inhibitor) showed an accumulation of unsaturated fatty acids at day 7.
Therefore, the results demonstrate that inhibition of the eicosanoid signaling pathway promotes the pluripotent state of ESCs, thereby delaying the loss of Nanog and Oct4 expression and maintaining the high levels of ω-6 and ω-3 fatty acids that are characteristic of ESCs. Accordingly, we hypothesized that specific downstream endogenous metabolites produced by and involved in oxidative pathways (e.g., hydrogenation and/or oxygenation) mediate stem cell differentiation.
Metabolites promote neurogenesis and cardiogenesis
To test the hypothesis that metabolites produced by and involved in oxidation metabolism mediate stem cell differentiation, mESCs were treated with eicosanoids (Fig. 3a) and metabolites that undergo mitochondrial β-oxidation (e.g., fatty acids and acyl-carnitines) (Fig. 4a) and allowed to differentiate in chemically defined neuronal 13 or cardiac permissive conditions.
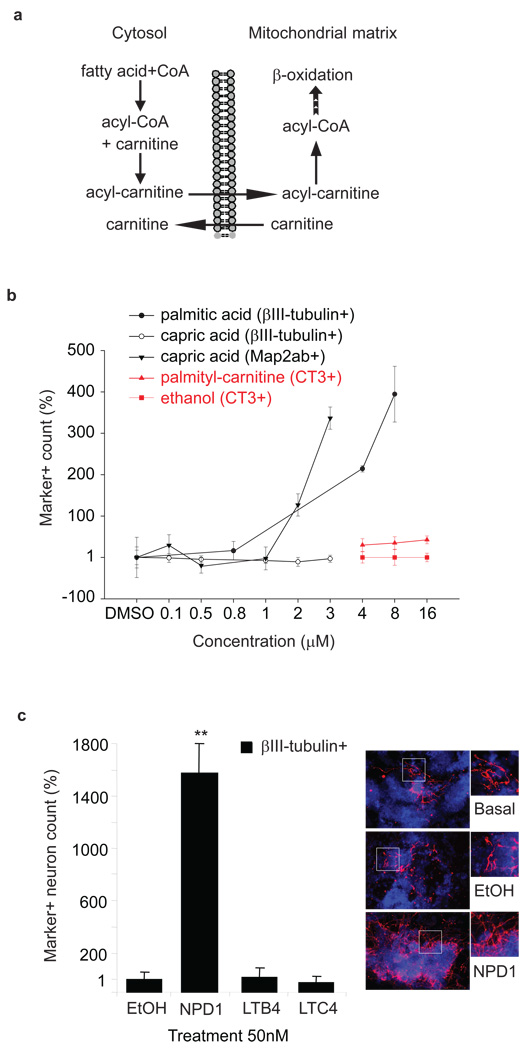
Figure 4. Metabolites that undergo mitochondrial β-oxidation, and neuroprotectin D1 promote cardiac and neuronal differentiation.
(a) Role of acyl-carnitines in fatty acid metabolism in the mitochondria. (b) Saturated fatty acids and acyl-carnitines enhance neuronal and cardiomyocyte differentiation, respectively. Map2ab/βIII-tubulin double positive neuron count increases with higher concentrations of palmitic acid (0.8 µM to 8µM) and capric acid (0.1 µM to 3 µM). An increase in CT-3 positive clusters was observed with increasing concentrations of palmityl-carnitine. Differentiation media was supplemented with 4, 8, or 16 µM concentrations of palmityl-carnitine for the first 9 days and the metabolite removed for the last day of differentiation. Cardiomyocyte differentiation was evaluated with CT3 staining, an antibody for the cardiac cell marker cardiotroponin. Marker positive counts in ES cells differentiated in the absence of DMSO or ethanol were not different from the DMSO or ethanol controls. (c) Neuroprotectin D1 (NPD1) enhances neuronal differentiation. Differentiation media was supplemented with 50 nM of NPD1, leukotriene B4 or C4 throughout differentiation. ** indicates p-value < 0.001 as compared to that of ethanol treated cells. Photographs show neuronal differentiation evaluated with βIII-tubulin (red) staining. Nuclear staining was performed with DAPI (blue). Four independently prepared cell culture replicates were analyzed for each metabolite concentration. Data points and error bars represent mean values and s.d.
For neuronal differentiation, the experiments were stopped at day 8 to 13 and differentiation was evaluated with βIII-tubulin and Map2ab staining at high magnification (10× or 20×). Random field counting of the number of neurons with βIII-tubulin positive and/or Map2ab positive staining was performed manually. The cells were determined to be neurons if they stained for βIII-tubulin and possessed correct neuron morphology (i.e., a round cell body with processes two times the length of the diameter of the cell body). Differentiation media was supplemented with 8 µM of one of the following: batyl alcohol, capric acid (9), lauric acid (10), myristic acid (11), caprylic acid (12), palmitic acid (13), or DMSO vehicle. The addition of each saturated metabolite resulted in an increase in the number of βIII-tubulin positive neurons as compared to the DMSO-treated control. Palmitic acid resulted in the greatest increase of βIII-tubulin positive neurons (400%), and capric acid gave the most substantial increase in the number of Map2ab positive neurons (> 300%) (Fig. 4b and Supplementary Fig. 8). It is also important to note that quantitative PCR revealed similar levels of Oct4 and Nanog mRNA transcripts in palmitic acid and capric acid treated cells relative to DMSO-treated cells, indicating that palmitic and capric acid had no significant effect on promoting ESC self-renewal (Supplementary Fig. 9).
Based on our mass spectrometry-based metabolomics results and on the inhibition of COX and LOX enzymes, we suspected that eicosanoids (produced by transformation of ω-6 and ω-3 fatty acids) were important to ESC differentiation. Neuronal differentiation media was supplemented with 50 nM of one of the following inflammatory-mediator eicosanoids: neuroprotectin D1 (NPD1) (14), leukotriene B4 (LTB4) (15), and leukotriene C4 (LTC4) (16). The addition of neuroprotectin D1 resulted in a >1500% increase in the number of βIII-tubulin positive neurons (Fig. 4c) as compared to the ethanol-vehicle control. In contrast, leukotriene B4 and leukotrine C4 had no significant effect on neuronal differentiation.
To explore the generality of these findings, we conducted preliminary studies of human ESCs which demonstrated that the metabolites we identified in mouse neurons similarly have neural promoting activity in the human system. In particular, human ESCs were differentiated via embryoid body (EB) differentiation in chemically defined media. Neural differentiation was determined with Pax6 expression as well as neural rosette structure/morphology 14 (Supplementary Fig. 4). Human ESCs underwent EB differentiation and 50 nM neuroprotectin D1, 8 µM capric acid, 8 µM palmitic acid, or ethanol vehicle was added from day 4 through day 12 of differentiation. The addition of palmitic and capric acid resulted in an increase of neural rosette differentiation by 100% and 50% respectively (Supplementary Fig. 10), suggesting that the role of these metabolites with respect to differentiation is conserved in mouse and human stem cells.
Murine ESCs undergoing cardiac differentiation were also treated with different acyl-carnitines and allowed to differentiate in a chemically defined cardiomyocyte- inducing condition. The differentiation medium was supplemented with either 4, 8, or 16 µM of lauroyl- (17), caproyl- (18), and palmitoyl-carnitine (19), or ethanol vehicle for 9 days before being removed 24 hours prior to analysis. Cells were fixed and evaluated for cardiac differentiation with CT3 staining, an antibody for the cardiac cell marker cardiotroponin, at day 10. The addition of each acyl-carnitine resulted in an increase in the number of CT3 clusters as compared to ethanol-vehicle control. Palmitoyl-carnitine resulted in the greatest increase of CT3 cardiac progenitors at 10 day (> 40%) (Fig. 4b and Supplementary Fig. 11). Beating cell clusters (Supplementary Video 1) were visible in all treated wells at day 16 of differentiation. The addition of lauroyl-, caproyl-, and palmitoyl-carnitine in basal conditions alone without growth factors was not sufficient to increase CT3 expression at day 10 as compared to ethanol-vehicle treated cells.
These results suggest that certain endogenous metabolites are not merely final products of metabolic reactions, but rather are involved in driving stem cell differentiation. Further studies will determine the role of different eicosanoid signaling molecules and the detailed mechanism of this effect.
Discussion
Significant effort has focused on using synthetic small molecules to control stem cell development 15, but endogenous (i.e., naturally occurring) molecules have not been extensively examined. Our untargeted metabolomics analysis has revealed a unique metabolic signature in ESCs characterized by the presence of highly unsaturated, endogenous molecules. The high degree of unsaturation makes these metabolites reactive and susceptible to oxygenation and/or hydrogenation reactions, conferring them with what may be interpreted as ‘chemical plasticity’. Examples of such metabolites include polyunsaturated fatty acids such as AA, EPA, and DHA which are rapidly released by cell membranes in response to stress or altered homeostasis, making them available for oxidative metabolism by COX, LOX, and P450 enzymes.
Our findings suggest that the redox status of ESCs is regulated during the process of differentiation, as revealed by measuring the GSH/GSSG ratio and ascorbic acid levels. The inverse relationship between the GSH/GSSG ratio and the ASA level may indicate that ASA compensates for the accumulation of GSSG to maintain homeostasis during ESC differentiation. This is consistent with the closely linked antioxidant actions of glutathione and ASA previously observed in the liver of adult mice 16, where induction of glutathione deficiency was accompanied by a rapid increase in ASA level (contrary to other adult tissues or liver in newborn rats). Previous studies revealed the importance of ascorbic acid in promoting differentiation when present during the early stages of stem cells differentiation 17–18.
We show that inhibition of 5Δ and 6Δ desaturases by curcumin and sesamin delays differentiation, which are key enzymes involved in the synthesis of ‘plastic’ ω-3 and ω-6 polyunsaturated fatty acids. Interestingly, curcumin is also known to act as an antioxidant 19. Therefore, we cannot exclude the possibility that the increased levels of Nanog and Oct4 caused by curcumin are partially due to its regulation of the redox status.
Our experimental results also show that supplementation of ESC media with essential, naturally occurring metabolites associated with oxidative metabolism in the mitochondria 20, such as saturated fatty acids and acyl-carnitines in β-oxidation, leads to a significant increase in neuronal and cardiac differentiation. This is consistent with previous observations that increased production of mitochondrial proteins was associated with neurogenesis 21–23. Activation of oxidative enzymes such as NADPH oxidase, and subsequent reactive oxygen species (ROS) generation, is also a pre-requisite for cardiovascular differentiation of ESCs 24–25.
Overall, our results suggest that the activation of oxidation is a metabolic signature of stem-cell differentiation. Indeed, several independent lines of evidence demonstrated that stem cells contain lower levels of ROS than their more mature progeny 23,26–27 and that ROS accumulation and signaling was required for differentiation 23. This is consistent with previous observations that the intracellular oxidation state regulates the balance between self-renewal and differentiation 28. Specifically, signaling molecules that promote pluripotency make stem cells more reduced while those that promote differentiation make cells more oxidized. In addition, it is well known that hypoxia maintains the pluripotent and undifferentiated phenotype of stem/precursor cells both in vitro and in vivo 29–30. We speculate that redox regulation, together with hypoxic conditions, makes stem cells particularly attuned to differentiate in vivo in response to oxidative processes such as inflammation.
Finally, our results raise an intriguing possibility that specific endogenous inflammatory mediators might regulate the regenerative properties of stem cells. We found that inhibition of PLA2, COX and LOX promotes the pluripotent, undifferentiated state of ESCs. In support of our results, a high-throughput screening assay showed that anti-inflammatory drugs promote self-renewal in human ESCs 31. For the first time, we show that neuroprotectin D1 32, a DHA-derived lipid mediator that activates inflammation-resolution 33, accelerated neuronal differentiation. Pro-inflammatory lipid mediators such as leukotriene B4 and C4, in contrast, had no significant effect on mESC differentiation. Previous studies also revealed important roles of specific eicosanoids on stem cell fate, such as prostaglandin E2 34 or lipoxin A4 35. These data suggest that specific molecular responses to injury and inflammation may regulate the proliferation and differentiation of stem cells, and this may lead to the exploration of new avenues in understanding properties associated with regeneration. Further investigation will determine the mechanisms by which pro-inflammatory and pro-resolving endogenous metabolites activate proliferation and differentiation of quiescent stem/progenitor cells in response to tissue repair or wound healing.
Methods
Metabolites and small molecules
Sphinganine, GPCho, sphigosine, DG and PG (Avanti Polar Lipids, Inc). Fatty acids, acyl-carnitines, amino acids, sesamin, curcumin and BW-A4C (Sigma-Aldrich). Docosanoyl ethanolamide, 7-ketocholesterol, prostaglandin E2, neuroprotectin D1, leukotriene B4 and C4, palmityl trifluoromethyl ketone and SC-560 (Cayman Chemical Company). SC-236 (Calbiochem). AA-861 (Biomol International). All chemicals were at a purity ≥94%.
Murine embryonic stem cell culture
R1 and 46C mESCs were routinely cultured on 0.1% gelatin-coated tissue culture plates with irradiated CF-1 MEF feeder cells in growth media (GM). Cells were passaged at the ratio of 1 to 10 every 3 days using 0.05% Trypsin EDTA (GIBCO). Before in vitro differentiation, cells were first cultured for one passage in either GM or chemically defined media (CDM). See supplementary methods for GM and CDM composition.
Human embryonic stem cell culture
Hues9 human ESC lines was routinely cultured on irradiated CF-1 MEF feeder cells in ESC growth media: DMEM/F-12 Media supplied with 20% KnockOut Serum Replacement (GIBCO), 2 mM L-glutamine, 1.1 mM β-mercaptoethanol, 1 mM non-essential amino acids, and 10 ng/mL bFGF (Invitrogen). Cells were passaged at the ratio of 1 to 6 every ~7 days by using 1mg/mL dispase (GIBCO) solution.
Murine neuronal differentiation
Neuronal differentiation is defined as the differentiation of embryonic stem cells into cells with (i.) neuron morphology, namely a small round cell body consisting of multiple processes and (ii.) clear neuron-specific class III β-tubulin staining with Tuj1 antibody. Feeder free mES cell culture were washed once with PBS and enzymatically disperse with trypsin EDTA (GIBCO). Cells were replated on 0.1% gelatin-coated plates at 1.5 × 104/cm2, and allowed to attach for 12–15 hr in GM or CDM. Cells were washed once with PBS and the media was replaced with chemically defined N2B27 media 13. The N2B27 medium was refreshed every day for 6–7 days. Cells were allowed to differentiate in N2B27 media alone or with 20ng/mL bFGF (invitrogen), 100 ng/ml FGF8 (R&D Systems), and 400ng/ml sonic hedgehog (R&D Systems) or 1µM of hedgehog agonist purmorphamine for an additional 5–8 days. Cells were collected for analysis upon achieving >80% of the culture possessing βIII-tubulin expression with neuronal morphology, approximately at day 13. See supplementary methods for composition of neuronal differentiation basal media.
Human ESC differentiation
Human neural differentiation was accomplished via embryoid body formation. Hues9 human ESCs were lifted from feeder culture with 1mg/mL Dispase (GIBCO) solution and cultured overnight in low attachment tissue culture plates. Human embryoid bodies were cultured in human growth media for four days. On day five human growth media was replaced with neuron induction media 14,36 which is 100 mL F-12 media, 300 mL DMEM media, 1× N2 supplement, 1 mM non-essential amino acids, 500uL Heparin solution (2mg/mL) (GIBCO) and 20 ng/mL bFGF (Invitrogen). At day seven Embryoid bodies were seeded on Geltrex (GIBCO) coated plates and allowed to mature into neural progenitor structures termed rosettes.
Neuronal differentiation
Experiments were stopped at day 8 to 13 of differentiation and neuronal differentiation was evaluated with βIII-tubulin and Map2ab staining at high magnification (10× or 20×). Random field counting of βIII-tubulin positive and/or Map2ab positive neurons was performed and statistical significance was evaluated with one-tail Student’s t-test using the Excel software. Metabolites and inhibitors were not found to be autofluorescent.
Cardiac differentiation
Feeder free mES cell cultures were washed once with PBS and enzymatically disperse with Trypsin EDTA (GIBCO). Cells were plated on Matrigel (Invitrogen) coated plates at 2 × 104/cm2 in GM. After overnight attachment cells were washed once with PBS, and GM replaced with cardiac differentiation basal media (BM) containing 20ng/mL BMP4 (Stemgent) and 4 µM BIO (Stemgent). The BM with BMP4 and BIO was refreshed every other day for 6 days. Cells were then treated with BM supplemented with 100 ng/ml DKK1 (R&D Systems). The BM with DKK1 was refreshed every other day for 6 days. DKK1 was then removed and cells were allowed to mature in BM alone until beating was observed.
Phospholipase A2 (PLA2), cyclooxygenase (COX), lipoxygenase (LOX) and 5Δ and 6Δ desaturase inhibition
COX was inhibited with SC236 or SC560. COX inhibitors were resuspended in DMSO solution and used at a range of 0.005 µM to 10 µM. Fatty acid desaturases were inhibited with sesamin or curcumin. Desaturase inhibitors were resuspended in DMSO solution and used at a range of 0.01 µM to 10 µM. LOX inhibitors, AA861 and BW-A4C, were resuspended in DMSO solution and used with at a range of 0.2 µM to 10 µM. PLA2 inhibitor, palmityl trifluoromethyl ketone, was resuspended in DMSO solution and used at a 3 µM. DMSO control (1 µL/mL of media) has no effect on stem cell fate.
GSH/GSSG ratio and ascorbic acid
Metabolite extraction was performed as described elsewhere 37, with some modifications as detailed here. Cells (4 million) at each time point (n=3) were frozen in liquid N2, and stored at −80 °C until analysis. The cell pellet was homogenized with 300 µL of cold (4 °C) extraction solution (5% (w/v) meta-phosphoric acid (MPA) and 1mM EDTA in 0.1% formic acid). Samples were then vortexed-mixed for 30 sec and submerged for 1 min in liquid N2. Samples were then thawed in ice. This process was repeated three times and samples were centrifuged at 13,000g for 15 min at 4 °C.
Supplementary Material
Acknowledgements
We thank Drs. Gudmundur G. Haraldsson and Carlos D. Magnusson for providing us with selachyl alcohol. Drs. Cynthia T. McMurray and John W. Trauger for helpful comments. Simon Hilcove, Sherman Ku and Debbie Watry for technical assistance. We gratefully acknowledge financial support from the California Institute for Regenerative Medicine, Department of Energy, National Science Foundation, National Cancer Institute, and the National Institutes of Health. Antonio Sánchez thanks Fundación Ramón Areces for his postdoctoral fellowship.
Footnotes
Author Contributions. O.Y. and J.C. contributed equally to this work. O.Y. and J.C. designed and performed experiments, analyzed data, and wrote the manuscript. G.J.P. analyzed data and wrote the manuscript. D.W. and P.B. analyzed data. A.S.R. performed organic synthesis. S.A.T. and C.D. performed experiments. S.D. and G.S. oversaw the project, assisted in data analysis, wrote the manuscript, and approved all intellectual content.
Competing financial interests statement. The authors declare no competing financial interests.
Additional information. Supplementary information and chemical compound information is available online at http://www.nature.com/naturechemicalbiology/. Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/. Correspondence and requests for materials should be addressed to S.D. or G.S.
References
- 1.Azuara V, et al. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–538. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein BE, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 3.Graumann J, et al. Stable isotope labeling by amino acids in cell culture (SILAC) and proteome quantitation of mouse embryonic stem cells to a depth of 5,111 proteins. Mol Cell Proteomics. 2008;7:672–683. doi: 10.1074/mcp.M700460-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Foss EJ, et al. Genetic basis of proteome variation in yeast. Nat Genet. 2007;39:1369–1375. doi: 10.1038/ng.2007.22. [DOI] [PubMed] [Google Scholar]
- 5.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon YK, et al. A domino effect in antifolate drug action in Escherichia coli. Nat Chem Biol. 2008;4:602–608. doi: 10.1038/nchembio.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sreekumar A, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 8.Smith CA, Want EJ, O'Maille G, Abagyan R, Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78:779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 9.Pruss TP. Treatment for the inhibition of neuro-degenerative disease states United States patent. 1998 5731354. [Google Scholar]
- 10.Brohult A. Effects of alkoxyglycerols and especially selachyl alcohol on the bone marrow in connexion with irradiation treatment and in leukaemia therapy. Nature. 1958;181:1484–1485. doi: 10.1038/1811484b0. [DOI] [PubMed] [Google Scholar]
- 11.Oz M, Alptekin A, Tchugunova Y, Dinc M. Effects of saturated long-chain N-acylethanolamines on voltage-dependent Ca2+ fluxes in rabbit T-tubule membranes. Arch Biochem Biophys. 2005;434:344–351. doi: 10.1016/j.abb.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 13.Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 14.Yan Y, et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Shi Y, Ding S. A chemical approach to stem-cell biology and regenerative medicine. Nature. 2008;453:338–344. doi: 10.1038/nature07042. [DOI] [PubMed] [Google Scholar]
- 16.Martensson J, Meister A. Glutathione deficiency increases hepatic ascorbic acid synthesis in adult mice. Proc Natl Acad Sci U S A. 1992;89:11566–11568. doi: 10.1073/pnas.89.23.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuneto M, Yamazaki H, Yoshino M, Yamada T, Hayashi S. Ascorbic acid promotes osteoclastogenesis from embryonic stem cells. Biochem Biophys Res Commun. 2005;335:1239–1246. doi: 10.1016/j.bbrc.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Sato H, et al. Collagen synthesis is required for ascorbic acid-enhanced differentiation of mouse embryonic stem cells into cardiomyocytes. Biochem Biophys Res Commun. 2006;342:107–112. doi: 10.1016/j.bbrc.2006.01.116. [DOI] [PubMed] [Google Scholar]
- 19.Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv Exp Med Biol. 2007;595:197–212. doi: 10.1007/978-0-387-46401-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Idell-Wenger JA, Grotyohann LW, Neely JR. Regulation of fatty acid utilization in heart. Role of the carnitine-acetyl-CoA transferase and carnitine-acetyl carnitine translocase system. J Mol Cell Cardiol. 1982;14:413–417. doi: 10.1016/0022-2828(82)90172-9. [DOI] [PubMed] [Google Scholar]
- 21.Cordeau-Lossouarn L, Vayssiere JL, Larcher JC, Gros F, Croizat B. Mitochondrial maturation during neuronal differentiation in vivo and in vitro. Biol Cell. 1991;71:57–65. doi: 10.1016/0248-4900(91)90051-n. [DOI] [PubMed] [Google Scholar]
- 22.Facucho-Oliveira JM, Alderson J, Spikings EC, Egginton S, St John JC. Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J Cell Sci. 2007;120:4025–4034. doi: 10.1242/jcs.016972. [DOI] [PubMed] [Google Scholar]
- 23.Tsatmali M, Walcott EC, Crossin KL. Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Res. 2005;1040:137–150. doi: 10.1016/j.brainres.2005.01.087. [DOI] [PubMed] [Google Scholar]
- 24.Buggisch M, et al. Stimulation of ES-cell-derived cardiomyogenesis and neonatal cardiac cell proliferation by reactive oxygen species and NADPH oxidase. J Cell Sci. 2007;120:885–894. doi: 10.1242/jcs.03386. [DOI] [PubMed] [Google Scholar]
- 25.Li J, et al. The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell. 2006;17:3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diehn M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009 doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 28.Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A. 2000;97:10032–10037. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281:30678–30683. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- 31.Desbordes SC, et al. High-throughput screening assay for the identification of compounds regulating self-renewal and differentiation in human embryonic stem cells. Cell Stem Cell. 2008;2:602–612. doi: 10.1016/j.stem.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serhan CN, et al. Resolution of inflammation: state of the art, definitions and terms. Faseb J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT, Zon LI. Genetic Interaction of PGE2 and Wnt Signaling Regulates Developmental Specification of Stem Cells and Regeneration. Cell. 2009;20:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada K, et al. Leukotriene B4 and lipoxin A4 are regulatory signals for neural stem cell proliferation and differentiation. Faseb J. 2006;20:1785–1792. doi: 10.1096/fj.06-5809com. [DOI] [PubMed] [Google Scholar]
- 36.Li XJ, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 37.Rellan-Alvarez R, Hernandez LE, Abadia J, Alvarez-Fernandez A. Direct and simultaneous determination of reduced and oxidized glutathione and homoglutathione by liquid chromatography-electrospray/mass spectrometry in plant tissue extracts. Anal Biochem. 2006;356:254–264. doi: 10.1016/j.ab.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 38.Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J Neurochem. 2007;101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 39.Schneider C, Pratt DA, Porter NA, Brash AR. Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem Biol. 2007;14:473–488. doi: 10.1016/j.chembiol.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.