Abstract
Naturally occurring agents have always been appreciated for their medicinal value for both their chemopreventive and therapeutic effects against cancer. In fact, the majority of the drugs we use today, including the anti-cancer agents, were originally derived from natural compounds, either in their native form or modified to enhance their bioavailability or specificity. It is believed that for maximum effectiveness, it will useful to design novel target-based agents for chemoprevention as well as the treatment of cancer. Recent studies have shown that the serine/threonine kinase polo-like kinase (Plk) 1 is widely overexpressed in a variety of cancers and is being increasingly appreciated as a target for cancer management. Additionally, several chemopreventive agents have been shown to inhibit Plk1 in cancer cells. In this review, we will discuss if Plk1 could also be a target for designing novel strategies for cancer chemoprevention.
Keywords: cancer, chemoprevention, polo-like kinase
INTRODUCTION
Chemoprevention via naturally occurring non-toxic, often plant-based and dietary agents is being increasingly appreciated as a potential strategy for the management of cancer. Historically, naturally occurring agents have been very important for disease management and drug development, both for use in their native form and as templates to expand upon their chemical backbone in designing more specific, potent, or bioavailable derivatives. Over the past 20 years, up to 50% of all new drugs introduced were directly derived from plants or chemically modified natural products (1). In addition, 78% of antimicrobial compounds have also been derived from natural compounds (1). With the advancements in high-throughput drug and target analysis and the large diversity of plants and other organisms capable of producing bioactive compounds, the screening for natural compounds as novel drugs or model molecules for drug design will continue to play a major role. However, it is important to identify novel rationale- and mechanism-based targets that could be exploited to develop novel strategies for chemoprevention as well as treatment of cancer. Recent studies have suggested that Polo-like kinase (Plk) 1, which plays several crucial roles in cell cycle progression and cell division, may be a useful target for cancer chemoprevention.
Highly regulated progression through mitosis ensures genomic integrity from one cell division to the next. Improper checkpoint control throughout the cell cycle may result in uncontrolled proliferation, aneuploidy, and genetic instability culminating in neoplastic transformation. Considering the central role of phosphorylation in these checkpoints, primarily the DNA damage and spindle assembly checkpoints, it is not surprising that several mitotic kinases have been implicated in tumorigenesis (2–5). Plk1 has received significant attention as a critical regulator of cell division (6). Plk1, often over-expressed in a variety of cancers, has been shown to be essential for proper mitotic entry, progression and exit (7–10). Extensive efforts are being made towards exploiting Plk1 as a target for cancer management, particularly in developing small molecule inhibitors which could be utilized as anti-cancer drugs (7–10). Interestingly, some recent studies have demonstrated modulation of Plk1 by naturally occurring agents, suggesting that these agents, with limited or no toxicity, could be developed for chemoprevention of cancer. This review will discuss these studies and evaluate the potential and efficacy of these compounds as potential chemopreventive as well as therapeutic agents for cancer management.
ROLE OF PLK1 IN MITOSIS: WHY IT COULD BE AN IMPORTANT TARGET FOR CANCER MANAGEMENT
The founding member of the Plk family, polo, was originally identified in Drosophila melanogaster and thus named for the mono-polar phenotype (a single spindle pole surrounded by missegregated DNA) seen with polo mutants (11). Further research demonstrated that polo is an essential serine/threonine kinase required for proper mitosis (12). Evolutionarily conserved members have since been identified in Schizosaccharomyces pombe, Caenorhabditis elegans, Xenopus laevis, Mus musculus and humans. There are four defined mammalian Plks: Plk1, Plk2 (Snk), Plk3 (Fnk/Prk) and Plk4 (Sak). All contain a highly conserved N-catalytic domain and one (Plk4) or two (Plks 1–3) C-terminal polo box domains (PBD) (Fig. 1A). The PBDs have been shown to be involved in cellular localization, target binding, and cis acting regulation upon the catalytic domain (6).
Fig. 1.
Structure and function of Plk1. A Plk1 structure. Polo-like kinase 1 (Plk1) consists of a highly conserved kinase activity domain and two polo box domains involved in self regulation, target binding, and localization. The ATP binding site is located at lysine-83, and a phospho-activation site is located at threonine-210. Plk1 activity is increased upon phosphorylation at threonine-210 by Aurora A in conjunction with Bora. B Plk1 functions. Plk1 mRNA, protein and activity levels begin to rise in S-phase, peaking at the G2/M transition. These levels are relatively steady throughout mitosis but decline upon mitotic exit into G1. During cell cycle progression, Plk1 regulates multiple targets, both directly and indirectly, involved in the G2/M transition, spindle pole maturation (SPM), the spindle assembly checkpoint (SAC), and cytokinesis. (Cell cycle stages are not to scale.)
Transcription and translation of Plk1 is highly coordinated with cell cycle progression (Fig. 1B). Plk1 mRNA and protein levels begin to accumulate in S-phase and peak at the G2/M transition and then decline upon mitotic exit (13). Plk1 activation is achieved by an accumulation of Bora in G2 leading to Aurora A activation (14,15). Binding of Plk1 by Bora then opens up Plk1’s activation loop at threonine-210, allowing for Aurora A phosphorylation and Plk1 activation (14,15). Plk1 then plays multiple essential roles as the cell enters, progresses through and exits mitosis. At the G2/M transition, Plk1 activates the Cdk1/Cyclin B1 complex promoting mitotic entry both directly and indirectly. First, Plk1 directly regulates Cyclin B1 localization by phosphorylating Cyclin B1 and targeting it to the nucleus (16). Second, Plk1 decreases the inhibitory phosphorylations on Cdk1 by phosphorylating and inhibiting Myt1, which is one of the two kinases that inhibits Cdk1 through phosphorylation, with Wee1 being the other (9,17,18). Finally, Plk1 phosphorylates Cdc25C to promote its phosphatase activity on Cdk1, further amplifying Cdk1 activity and promoting mitotic progression (19).
Beyond the G2/M transition, Plk1 also plays a role in centrosome maturation by promoting increased recruitment of microtubules to the spindle pole bodies (Reviewed in (6)). Plk1 has been implicated in regulating the localization of a variety of centrosomal-associated proteins, including γ-tubulin ring complex, shugoshin 1, kizuna, cenexin, and NLP (6). Further, Plk1 also regulates the localization of Aurora A to the centrosomes for proper maturation (20–22). Plk1 regulates the spindle assembly checkpoint (SAC) possibly through its phosphorylation of BubR1 and NUDC (6, 23). However, this mechanism is not completely understood, and Plk1 activity is not essential for this checkpoint to occur in human cells. Finally, Plk1 regulates chromosome segregation, cytokinesis and mitotic exit. Emi1 is an inhibitor of the anaphase-promoting complex (APC), and upon Emi1 degradation, APC activity is increased, pushing the cell towards mitotic exit (9,24,25). Plk1 phosphorylates both Emi1, targeting it for ubiquitination and degradation by SCFβ-TrCP, and the anaphase promoting complex (APC) itself increasing APC activation (26–28). The APC then targets securin for ubiquitination and degradation. This, in turn, releases seperase to cleave the cohesins binding the sister chromatids together, thereby allowing anaphase to proceed (9,24). Increased APC activity also leads to Cdk1 and Cyclin B1 degradation, which is necessary for proper mitotic exit (6,9,24). At cytokinesis, Plk1 phosphorylates the HsCYK4 subunit of centralspindlin at the midzone (29). This phosphorylation promotes the recruitment of the Rho guanine nucleotide exchange factor ECT2, which in turn activates RhoA GTPase (29–31). RhoA is an activator of the cytokinetic actomyosin ring and contributes to the development of the cleavage furrow (29–31). With all these roles through mitosis, it is evident that Plk1 is a very important regulator and promoter of the cell cycle.
Dysregulation of Plk1 has been shown to result in the formation of abnormal centrosomes leading to chromosomal instability and aneuploidy, ultimately culminating in tumor development (32). Additionally, forced over-expression of Plk1 in normal cells results in a transformed phenotype and increased tumorigenicity of the cells (33). Therefore, it is not surprising that Plk1 expression is up-regulated in a variety of tumors. Elevated Plk1 levels have been found in breast cancer, colorectal cancer, endometrial carcinomas, esophageal carcinoma, head/neck squamous-cell carcinomas, melanoma, non-small-cell lung cancer, oropharyngeal carcinomas, ovarian cancer, pancreatic cancer, papillary carcinomas and prostate carcinomas (34–46). Further, it has been demonstrated that Plk1 expression is strongly correlated with poor prognosis and therefore could serve as a marker for cancer progression. However, it is possible that the over-expression of Plk1 in tumors in vivo is a consequence of the proliferative state of the tumor, and not a cause of it.
Targeting Plk1 using multiple mechanisms in vitro has strengthened the potential of targeting Plk1 cancer management in vivo. Indeed, multiple studies have demonstrated significant reductions in tumor growth through targeting Plk1 using both in vivo siRNA delivery systems and novel small molecule inhibitors (47–54). Plks are especially attractive because they possess two potential targeting sites: the catalytic and polo box domains. The highly conserved nature of these sites presents an opportunity to design highly specific molecules for binding to these regions to deregulate Plk1 activity and/or localization. Some of these small molecule inhibitors have since begun advanced preclinical and early clinical trials to further evaluate their potential in humans. These include ZK-thiazolidinone, NMS-1, CYC-800, DAP-81, and LC-445 BI 2536, BI 6727, GSK461364, and HMN-214 (10). A limited number of studies have demonstrated a negative regulation of Plk1 by naturally occurring compounds, suggesting that targeting Plk1 could be used as an approach for cancer chemoprevention. These studies are described below.
NEGATIVE REGULATION OF PLK1 BY NATURAL AGENTS: PLK1 AS A TARGET FOR CANCER CHEMOPREVENTION?
Plk1 has attracted attention as a potential target for cancer management for two main reasons. First, its central role in mitosis coupled with its specific overexpression in cancer cells provides a technical advantage regarding its inhibition, primarily in cancer cells, thereby sparing normal cells. Second, the fact that Plk1 possesses two potential targeting sites, the enzymatic, kinase domain and the polo-box domains, increases the potential of discovering or designing highly specific inhibitors. Even more attractive would be a dual inhibitor that binds to both sites, thereby ensuring specificity to Plk1. An alternative, but potentially less desirable, mechanism for modulating Plk1 for cancer management would be through decreased transcription or protein levels. Being able to specifically decrease Plk1 presence could potentially be successful; however, the mechanism by which this could be achieved would very likely have off-target effects, as opposed to direct Plk1 binding and inhibition. Below, we evaluate these three mechanisms of Plk1 inhibition using naturally occurring agents, including certain well-known chemopreventive agents.
Plk1 Enzymatic Inhibitors
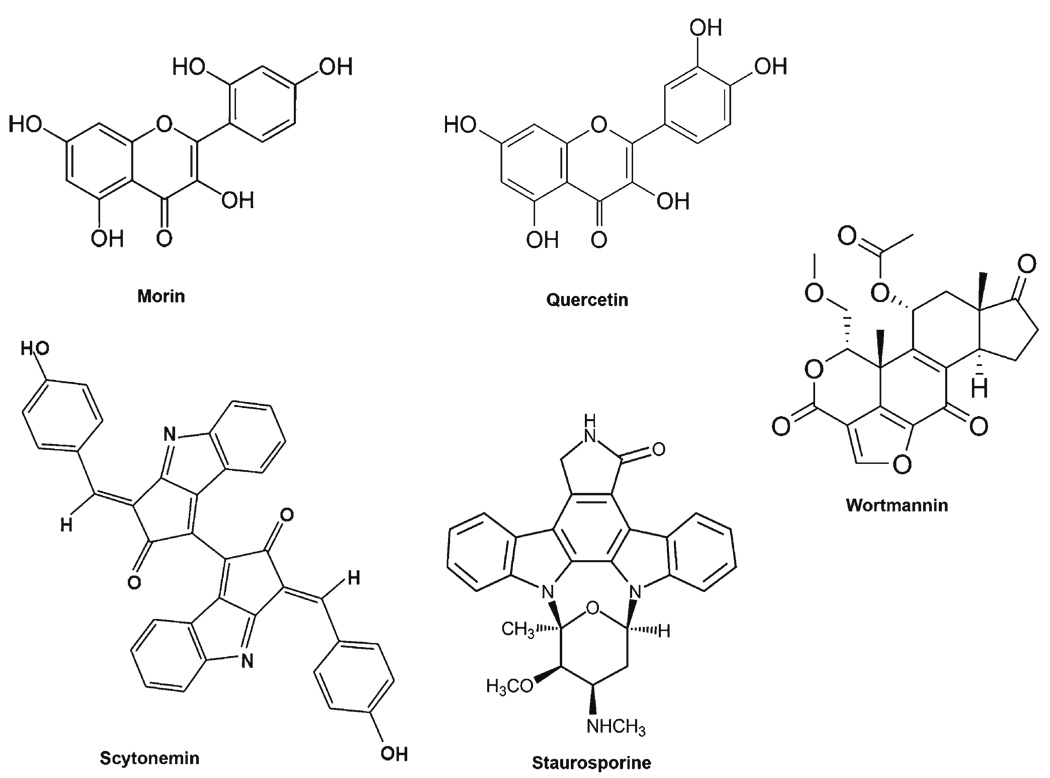
Scytonemin
The first natural compound shown to directly inhibit Plk1 was the marine pharmacophore, scytonemin (55). Scytonemin is a pigment isolated from over 300 species of cyanobacteria that possesses UV absorptive, anti-inflammatory and antiproliferative properties (Fig. 2) (56, 57). Stevenson et al. demonstrated that scytonemin was capable of inhibiting Plk1 activity through an ATP competitive manner with an IC50 of 2 µM. Treatment of Jurkat T cells with scytonemin resulted in a significant decrease in cell growth and an increase in apoptosis. However, scytonemin was also found to inhibit several other serine/threonine and tyrosine kinases, including Myt1, Chk1, and Cdk1, at IC50 values similar to that for Plk1. This broad kinase inhibitiory effect of scytonemin suggests that this compound decreases cell proliferation and increases apoptosis in a cell-cycle-independent manner. This does not appear to be in line with the specific Plk1 inhibition where a strong G2/M cell cycle arrest leads to apoptosis in cancer cells (36,50,51,54).
Fig. 2.
Plk1 kinase activity inhibitors. These compounds have been shown to directly inhibit Plk1 kinase activity. However, many have off-target effects on multiple, closely related targets.
Wortmannin
Wortmannin, a steroidal furanoid purified from the broth of Penicillium wortmanni Klocker, has long been used as an inhibitor of PI3K (Fig. 2) (58,59). However, like scytonemin and many other natural kinase inhibitors, it shows promiscuity towards other kinases, including Plk1 (60, 61). Using a tetrame-thylrhodamine-wortmannin conjugate, AX7503, as an activity-based probe, the authors show that in addition to PI3K, wortmannin also binds to and inhibits the kinase activities of Plk1 and Plk3, both in vitro and in vivo, through binding to their respective ATP binding sites (60,61). Whereas wortmannin has an in vitro IC50 of 4.2 nM for PI3K, its IC50 for Plk1 and Plk3 were six- and ten-fold higher at 24 and 48 nM, respectively (60–62). However, wortmannin still shows affinity for Plk1 (IC50 > 100 nM) and Plk3 (IC50 = 220 nM) in vivo at doses typically used for PI3K inhibition, indicating wortmannin is potently inhibiting the activity of multiple kinases in addition to its most specific defined target, PI3K. Therefore, the clinical use of wortmannin as a specific kinase inhibitor may not be possible, but may be of value as a pan-kinase inhibitor targeting multiple essential kinases involved in cell proliferation.
Staurosporine, Morin and Quercetin
McInnes and colleagues evaluated a large sample of compounds for their ability to inhibit Plk1 activity and their structural relationships (8). Of interest for this review, the first compound they looked at is the antibiotic staurosporine, originally isolated from a bacterium (Streptomyces staurosporeus), which shows similar characteristics to that of wortmannin (Fig. 2) (8). Staurosporine is a very non-specific kinase inhibitor that shows activity towards Plk1 (IC50 = 0.8 µM). The authors also found that the natural flavanoids morin and quercetin, two compounds found frequently in a variety of fruits and vegetables, inhibited Plk1 activity with IC50 values of 12.6 and 64 µM, respectively (Fig. 2). It is interesting to note that these two flavanoids in particular inhibited Plk1 where others, including myricetin, luteolin, and kaemperfol, did not, with IC50 values greater than 100 µM (8). Interestingly, the flavanoids that are inactive against Plk1 are potent inhibitors of PI3K, and vice versa. Further, all these flavanoids contain the same chromenone backbone, but differ in only their hydroxylation pattern. Though the IC50 values of morin and quercetin are much higher than the marine- and bacterial-originating compounds of scytonemin, staurosporine and wortmannin, these compounds may be of greater chemo-preventive interest due to their availability in plants. It should also be noted that the data with kaemperfol are inconsistent with data reported by Kang et al. that a dose of 50 µM inhibits Plk1 in MCF-7 cells (63). Thus, kaemperfol may reduce Plk1 protein levels; however, the dose used appears to be very cytotoxic (98% sub-G1) and may also be a consequence of the large amount of cell death and not a direct inhibition of Plk1 (63).
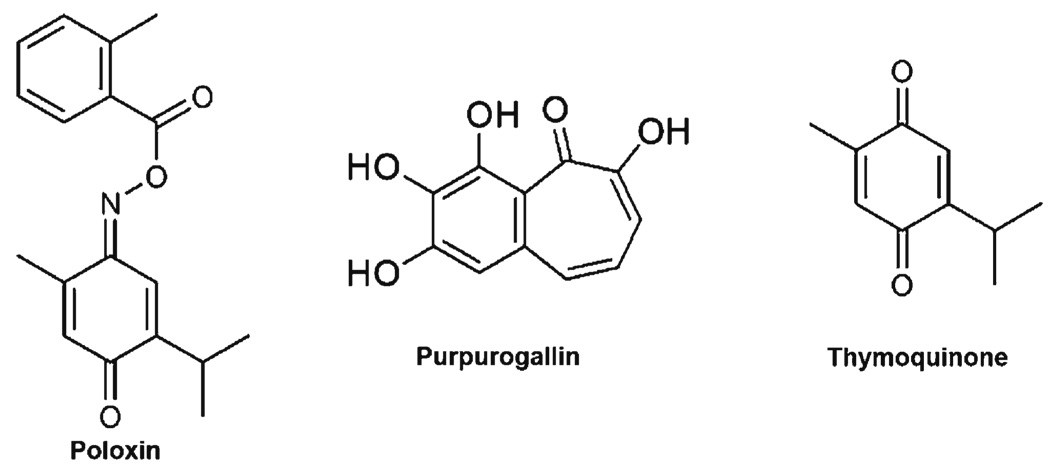
Polo Box Inhibitors
Thymoquinone
Conversely to the widely conserved kinase domain, the PBDs of Plk1 provide a much more specific targeting site to inhibit Plk1 localization and target binding. A few natural compounds have displayed an ability to block PBD binding, leading to a reduction in cell growth. Thymoquinone, a phytochemical found in Nigella sativa seed oil (64), has been shown to possess anti-inflammatory, anti-oxidant, and anti-neoplastic activities; however, the targets of thymoquinone are not well-known (Fig. 3) (65). Using a fluorescence polarization assay based on the binding of the Plk1 PBD to a fluorophore-labeled peptide comprising Plk1’s optimal recognition motif, Reindl et al. screened a chemical library of ~22,000 small molecules for compounds that could interfere with the Plk1 PBD (64,66). From this screen, it was found that the compound Poloxin possessed a PBD interfering IC50 of 4.8 µM and that the closest off-target molecules (Plk2 and Plk3) had IC50 values 4- and 11-fold higher than that of Plk1, indicating a rather specific interference of Plk1. Poloxin itself is not a natural compound, but the backbone of Poloxin is thymoquinone, which possesses a more potent inhibition (IC50 = 1.1 µM) of the Plk1 PBD. However, thymoquinone also inhibits multiple off-target phospho- serine, threonine, and tyrosine binding domains beyond the PBDs of Plk1, including Plk2 and Plk3, the FHA domain of Chk2, the WW domain of Pin1, and the SH2 domain of STAT3. Though the potency of thymoquinone on Plk1 inhibition was made first with its derivative Poloxin, this is a classic example of a natural compound showing strong potency towards a target of interest but with multiple off-target effects. However, thymoquinone provided a backbone for designing a more specific drug, Poloxin. Unfortunately, the more specific drug in this case lost some of its potency.
Fig. 3.
Polo box domain inhibitors. These compounds have been shown to bind to the Polo box domains of Plk1. This binding alters Plk1 localization and binding to Plk1 targets.
Purpurogallin
Another natural compound shown to inhibit the PBD of Plk1 is purpurogallin (PPG), a benzotropolone derived from nutgalls (Fig. 3) (67). Inserting fluorescent monomeric Venus between GST and Plk1 PBD, Watanabe and colleagues created a screening assay by fusing the Wee1A PBD binding site to 96-well plates. After incubating the GST-m Venus-PBD construct with various test compounds, the PBD construct was exposed to the Wee1A wells and unbound PBD washed away. A fluorescent read-out was used to determine the amount of PBD inhibition and binding. From the 2,500 compounds tested, PPG was the most potent, with an IC50 of 0.3 µM (67). Like other Plk1 inhibitors, PPG demonstrates off-target inhibition, including that of Plk2, various tyrosine-specific kinases, HIV integrase, and the Bcl-XL/BH3 peptide interactions. Interestingly, PPG shows little inhibition on Plk3. However, the biggest drawback of PPG is its poor bioavailability (67). In tissue culture, a concentration of 50 µM PPG was needed to observe typical Plk1 inhibitory phenotypes. The authors speculate that this is due to the acidic hydroxyl groups decreasing cell penetration or through oxidation in the media (67). Regardless, they show that the 2-hydroxyl group of PPG is essential using the in vitro model, so modifications to increase stability or cell membrane transport are needed, but without the loss of key structural elements, mainly the 2-hydroxyl group (67).
DECREASED PLK1 EXPRESSION
There are a variety of natural compounds demonstrating cell growth inhibitory effects accompanied with the modulation of Plk1. However, these compounds have not been shown to directly inhibit Plk1 activity or PBD function, but rather demonstrate decreases in Plk1 transcription or protein levels after exposure to the compound. Therefore, these compounds are listed as ones that have shown a decrease in Plk1, primarily protein expression, but without complete evaluation on the mechanism leading to decreased Plk1. Thus, these agents (discussed below) show promise; however, attributing their efficacy to Plk1 inhibition, either in whole or in part, needs to be thoroughly examined to conclusively define their mechanism(s) of action.
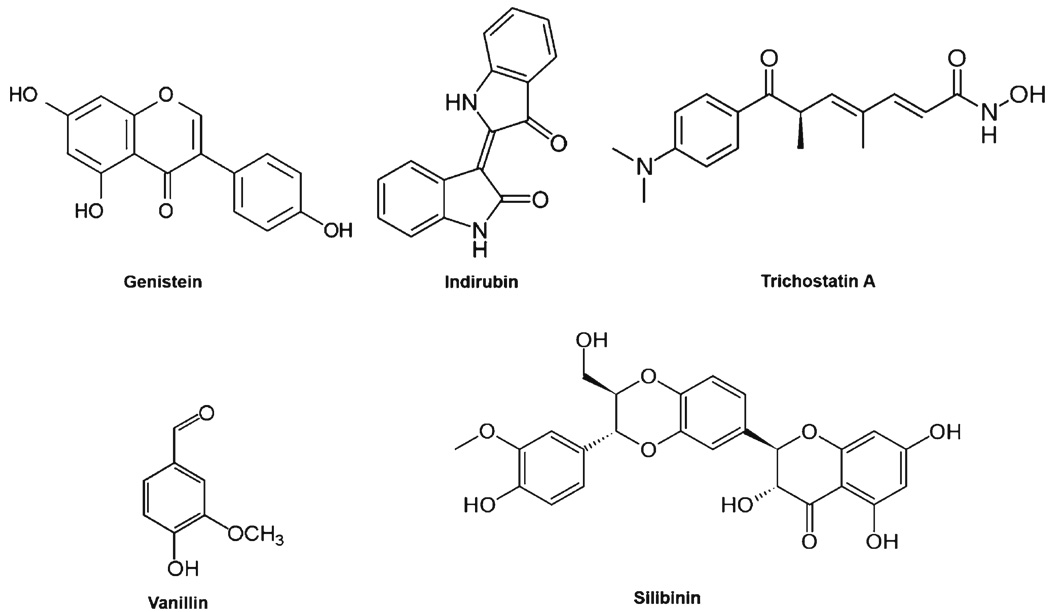
Genistein
Genistein, an isoflavone derived from soybeans, possesses antioxidant and chemopreventive properties leading to reduced cell growth, cell cycle alterations and apoptosis (Fig. 4) (68,69). In a recent study, Ismail and colleagues demonstrated that genistein-induced neuronal apoptosis and G2/M cell cycle arrest is associated with Plk1 down-regulation (70). In this study, treatment with genistein showed some of the typical anti-proliferative effects seen with Plk1 inhibition in cancer cells (70). This primarily includes the reduced cell growth and an increase in S and G2/M phases of the cell cycle leading to increased apoptosis. However, in the case of direct Plk1 inhibition, a strict G2/M cell cycle arrest is seen, rather than an additional arrest in S phase seen with genistein (36,53,54). An S phase arrest with a concomitant G2/M arrest would indicate an effect on the DNA damage response. Indeed, the authors reached the same conclusion (70). The authors also found changes in various DNA damage response and G2/M transition genes and proteins, leading to the conclusion that the primary mechanism for genistein-induced cell death is through an effect on the DNA damage response, possibly through direct DNA damage, and not a direct effect on Plk1 itself (70). Further, following genistein treatment, the authors found an increase in MDC1 (Mediator of DNA damage Checkpoint protein 1), p53, and p21waf1/cip1 mRNA and proteins and an increase in phosphorylated Chk2 at threonine-68 (involved in Chk2 activation during DNA damage repair) and Cdc25C at serine-216 (prevents nuclear translocation, thus inhibiting Cdc2 (Cdk1) activation at the G2/M transition) (70). Further, the authors found a decrease in Cdc2 (Cdk1), Plk1 and Cyclin B1 protein levels and decreased Cyclin B1 phosphorylation, all key regulators of the G2/M transition.
Fig. 4.
Compounds shown to decrease Plk1 mRNA or protein levels. Treatment of cells with these compounds has been shown to decrease Plk1 mRNA and/or protein levels. The cause of this decrease in some instances is due to a G1 or S arrest, which would naturally decrease Plk1 levels due to Plk1 transcription and translation occurring mainly leading up to and during mitosis.
Vanillin
Vanillin is the principal ingredient of vanilla bean extract and is widely used as a flavoring agent (Fig. 4). Studies have shown that vanillin inhibits mutagenesis induced by chemical and physical mutagens, and suppresses the invasion and migration of cancer cells (71). Cheng and colleagues used the oligonucleotide microarray approach to study gene expression profile of vanillin-treated human hepatocarcinoma cells (71). In this study, the microarray data followed by gene ontology investigation found that vanillin affected clusters of genes involved in cell cycle and apoptosis (71). Plk1 was one gene found to be repressed by vanillin, and this observed effect was found to be dose-dependent and most prominent at concentrations of 5 mM. Additional studies demonstrated that vanillin treatment resulted in the typical G2/M phase cell cycle arrest and apoptosis profile seen with Plk1 inhibition in a dose-dependent manner in cultured human colorectal cancer cells (72). Similar to genistein, however, it is not clear whether or not the effects on Plk1 down-regulation by vanillin is an upstream effect on the DNA damage repair mechanism, rather than on Plk1 itself.
Silibinin
Silibinin, a polyphenolic flavonoid, is an important bio-active constituent of silymarin, the extract of Silybum maranium (milk thistle) seed (Fig. 4). Several studies have shown cancer chemopreventive effects of silibinin in a variety of model systems (reviewed in (73–75)). Cui and colleagues have shown that silibinin, at 80 mg/kg and 160 mg/kg, reduced the growth of HuH7 human hepatocellular carcinoma xeno-grafts in nude mice, and this growth inhibitory effect was found to be accompanied with an inhibition of Plk1 (76). In earlier studies, silibinin has shown typical effects of Plk1 inhibition. For example, silibinin was found to cause caspase-independent apoptosis as well as decreased levels of Cyclin B1 in human colon carcinoma HT-29 cells (77) and G2/M cell cycle arrest in human epidermoid carcinoma A431 cells.
Trichostatin A
A recent study has demonstrated that the histone deacetylase (HDAC) inhibitor, Trichostatin A (TSA), resulted in a marked down-regulation of multiple mitosis-associated genes in HeLa cells (78). TSA, originally derived from Streptomyces hygroscopicus in 1976, has been shown to possess potent anti-fungal as well as potent class I and II HDAC inhibitor properties (Fig. 4) (79,80). Noh and colleagues have shown that TSA treatment resulted in a delay at the G2/M transition and spindle checkpoint slippage leading to cell death. In addition, the authors also showed that these phenotypes are due to an increase in p21waf1/cip1 transcription with a corresponding decrease in Plk1, Cyclin B1 and Survivin (78). These results are similar to those seen with genistein above, and may be attributed to the effects of TSA on the DNA damage checkpoint as well. In a paper by Jong-Soo Lee, it was shown that TSA can induce an ATM-dependent DNA damage response, indicating that the G2/M slippage and mitotic defects seen by Noh and colleagues may be due to an upstream defect during S phase or the G2/M transition (81).
Indirubin
5′-nitro-indirubinoxime (5′-NIO), a derivative of the natural compound indirubin that is found in the plant Danggui Longhui Wan, has been reported to possess therapeutic activities against neurodegenerative disorders, inflammation, and chronic myelogenous leukemia (Fig. 4) (82). Several studies on various indirubin derivatives have attributed their antitumor activity to the inhibition of a wide range of kinases, including the Cdks, GSK3β, and Jnk (83–86). Kim and colleagues synthesized the indirubin derivative 5′-NIO and found that it possesses greater antitumor effects than the most common derivative, indirubin-3′-monoxime, both in vitro and in vivo (87,88). The data from this study showed a shift from 64% of control cells in G1 or S phase to 30% of 5′-NIO treated cells and a corresponding shift from 24% of control cells in G2/M to 50% of 5′-NIO treated cells, suggesting a strong G2/M phase arrest (82). However, other than reduced Plk1 protein after 5′-NIO treatment, this paper did not evaluate any direct effects of 5′-NIO on Plk1 activity, binding, transcription or protein stability; therefore, no conclusions on Plk1 inhibition can be made, and considering the promiscuous kinase inhibitory effects of other indirubin derivatives, the antitumor effects of 5′-NIO may be due to inhibition of multiple kinases.
CONCLUSION
For centuries, many natural compounds, particularly those found in edible fruits, vegetables and beverages, have shown important preventive as well as therapeutic properties against several disease conditions, including cancer. The mechanisms of action of these agents are being extensively studied, and novel agents with beneficial health effects are being continuously discovered. However, several thousands of known and hitherto unknown agents have not yet been tested for their specificity against any particular pathway or targets, such as Plk1. Thus, given the known functions and effects of Plk1 inhibition, coupled with its wide overexpression pattern in cancer, a naturally occurring Plk1 inhibitor with low or no toxicity will be immensely useful in prevention as well as treatment of cancer. This is particularly important since a wide range of studies have shown that Plk1 inhibition is lethal to cancer cells but spares the normal cells. Thus, a naturally occurring non-toxic Plk1 inhibitor, aimed at regulating the enzymatic activity, target binding, localization, mRNA transcription, or inhibition of Plk1 protein stability, could be used for cancer chemoprevention. This does not exclude the non-specific natural Plk1 inhibitors with multiple target-affecting abilities (including Plk1), as even promiscuous inhibitors may be useful as chemopreventative agents. Further, some compounds do demonstrate strong enough affinity or specificity to be used as scaffolds for drug design, which has been the case for many drugs used regularly today. One example listed above is Poloxin, a derivative of thymoquinone (64). Poloxin was actually developed without knowledge of its Plk1 PBD inhibitory mechanism, but once revealed, it was also found that the original backbone, thymoquinone, also possessed a more potent, albeit less specific, inhibition of Plk1 as well (64).
ACKNOWLEDGEMENTS
This work was partially supported (Pre-doctoral Train-eeship to Travis Schmit) by the Molecular and Environmental Toxicology Center Training Grant (T32ES007015) from the NIEHS, NIH.
REFERENCES
- 1.Vuorelaa P, Leinonenb M, Saikkuc P, Tammelaa P, Rauhad JP, Wennberge T, et al. Natural products in the process of finding new drug candidates. Curr Med Chem. 2004;11:1375–1389. doi: 10.2174/0929867043365116. [DOI] [PubMed] [Google Scholar]
- 2.Cheung CH, Coumar MS, Hsieh HP, Chang JY. Aurora kinase inhibitors in preclinical and clinical testing. Expert Opin Investig Drugs. 2009;18:379–398. doi: 10.1517/13543780902806392. [DOI] [PubMed] [Google Scholar]
- 3.Coumar MS, Cheung CH, Chang JY, Hsieh HP. Advances in Aurora kinase inhibitor patents. Expert Opin Ther Pat. 2009;19:321–356. doi: 10.1517/13543770802646949. [DOI] [PubMed] [Google Scholar]
- 4.Johansson M, Persson JL. Cancer therapy: targeting cell cycle regulators. Anticancer Agents Med Chem. 2008;8:723–731. doi: 10.2174/187152008785914833. [DOI] [PubMed] [Google Scholar]
- 5.Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009;8:547–566. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- 6.Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009;10:265–275. doi: 10.1038/nrm2653. [DOI] [PubMed] [Google Scholar]
- 7.Berg T. Small-molecule inhibitors of protein–protein interactions. Curr Opin Drug Discov Devel. 2008;11:666–674. [PubMed] [Google Scholar]
- 8.McInnes C, Mezna M, Fischer PM. Progress in the discovery of polo-like kinase inhibitors. Curr Top Med Chem. 2005;5:181–197. doi: 10.2174/1568026053507660. [DOI] [PubMed] [Google Scholar]
- 9.Schmit TL, Ahmad N. Regulation of mitosis via mitotic kinases: new opportunities for cancer management. Mol Cancer Ther. 2007;6:1920–1931. doi: 10.1158/1535-7163.MCT-06-0781. [DOI] [PubMed] [Google Scholar]
- 10.Schoffski P. Polo-like kinase (PLK) inhibitors in preclinical and early clinical development in oncology. Oncologist. 2009;14:559–570. doi: 10.1634/theoncologist.2009-0010. [DOI] [PubMed] [Google Scholar]
- 11.Sunkel CE, Glover DM. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci. 1988;89(Pt 1):25–38. doi: 10.1242/jcs.89.1.25. [DOI] [PubMed] [Google Scholar]
- 12.Llamazares S, Moreira A, Tavares A, Girdham C, Spruce BA, Gonzalez C, et al. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 1991;5:2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- 13.Lee KS, Yuan YL, Kuriyama R, Erikson RL. Plk is an M-phase-specific protein kinase and interacts with a kinesin-like protein, CHO1/MKLP-1. Mol Cell Biol. 1995;15:7143–7151. doi: 10.1128/mcb.15.12.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–1658. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macurek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455:119–123. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 16.Toyoshima-Morimoto F, Taniguchi E, Shinya N, Iwamatsu A, Nishida E. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature. 2001;410:215–220. doi: 10.1038/35065617. [DOI] [PubMed] [Google Scholar]
- 17.Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 1995;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- 18.Parker LL, Therton-Fessler S, Piwnica-Worms H. p107wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc Natl Acad Sci U S A. 1992;89:2917–2921. doi: 10.1073/pnas.89.7.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roshak AK, Capper EA, Imburgia C, Fornwald J, Scott G, Marshall LA. The human polo-like kinase, PLK, regulates cdc2/ cyclin B through phosphorylation and activation of the cdc25C phosphatase. Cell Signal. 2000;12:405–411. doi: 10.1016/s0898-6568(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 20.van Leuken R, Clijsters L, van Zon W, Lim D, Yao X, Wolthuis RM, et al. Polo-like kinase-1 controls Aurora A destruction by activating APC/C-Cdh1. PLoS One. 2009;4:e5282. doi: 10.1371/journal.pone.0005282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan EH, Santamaria A, Sillje HH, Nigg EA. Plk1 regulates mitotic Aurora A function through betaTrCP-dependent degradation of hBora. Chromosoma. 2008;117:457–469. doi: 10.1007/s00412-008-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De LM, Lavia P, Guarguaglini G. A functional interplay between Aurora-A, Plk1 and TPX2 at spindle poles: Plk1 controls centrosomal localization of Aurora-A and TPX2 spindle association. Cell Cycle. 2006;5:296–303. doi: 10.4161/cc.5.3.2392. [DOI] [PubMed] [Google Scholar]
- 23.Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 24.Peters JM. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- 25.Reimann JD, Freed E, Hsu JY, Kramer ER, Peters JM, Jackson PK. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell. 2001;105:645–655. doi: 10.1016/s0092-8674(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 26.Moshe Y, Boulaire J, Pagano M, Hershko A. Role of polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome. Proc Natl Acad Sci U S A. 2004;101:7937–7942. doi: 10.1073/pnas.0402442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen DV, Loktev AV, Ban KH, Jackson PK. Plk1 regulates activation of the anaphase promoting complex by phosphorylating and triggering SCFbetaTrCP-dependent destruction of the APC Inhibitor Emi1. Mol Biol Cell. 2004;15:5623–5634. doi: 10.1091/mbc.E04-07-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckerdt F, Strebhardt K. Polo-like kinase 1: target and regulator of anaphase-promoting complex/cyclosome-dependent proteolysis. Cancer Res. 2006;66:6895–6898. doi: 10.1158/0008-5472.CAN-06-0358. [DOI] [PubMed] [Google Scholar]
- 29.Burkard ME, Maciejowski J, Rodriguez-Bravo V, Repka M, Lowery DM, Clauser KR, et al. Plk1 self-organization and priming phosphorylation of HsCYK-4 at the spindle midzone regulate the onset of division in human cells. PLoS Biol. 2009;7:e1000111. doi: 10.1371/journal.pbio.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Randall CL, Burkard ME, Jallepalli PV. Polo kinase and cytokinesis initiation in mammalian cells: harnessing the awesome power of chemical genetics. Cell Cycle. 2007;6:1713–1717. doi: 10.4161/cc.6.14.4501. [DOI] [PubMed] [Google Scholar]
- 31.Burkard ME, Randall CL, Larochelle S, Zhang C, Shokat KM, Fisher RP, et al. Chemical genetics reveals the requirement for polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc Natl Acad Sci U S A. 2007;104:4383–4388. doi: 10.1073/pnas.0701140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simizu S, Osada H. Mutations in the Plk gene lead to instability of Plk protein in human tumour cell lines. Nat Cell Biol. 2000;2:852–854. doi: 10.1038/35041102. [DOI] [PubMed] [Google Scholar]
- 33.Smith MR, Wilson ML, Hamanaka R, Chase D, Kung H, Longo DL, et al. Malignant transformation of mammalian cells initiated by constitutive expression of the polo-like kinase. Biochem Biophys Res Commun. 1997;234:397–405. doi: 10.1006/bbrc.1997.6633. [DOI] [PubMed] [Google Scholar]
- 34.Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I. Polo-like kinases (Plks) and cancer. Oncogene. 2005;24:287–291. doi: 10.1038/sj.onc.1208272. [DOI] [PubMed] [Google Scholar]
- 35.Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- 36.Schmit TL, Zhong W, Setaluri V, Spiegelman VS, Ahmad N. Targeted depletion of polo-like kinase (Plk) 1 through lentiviral shRNA or a small-molecule inhibitor causes mitotic catastrophe and induction of apoptosis in human melanoma cells. J Invest Dermatol. 2009;129:2843–2853. doi: 10.1038/jid.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmit TL, Zhong W, Nihal M, Ahmad N. Polo-like kinase 1 (Plk1) in non-melanoma skin cancers. Cell Cycle. 2009;8:2697–2702. doi: 10.4161/cc.8.17.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weichert W, Denkert C, Schmidt M, Gekeler V, Wolf G, Kobel M, et al. Polo-like kinase isoform expression is a prognostic factor in ovarian carcinoma. Br J Cancer. 2004;90:815–821. doi: 10.1038/sj.bjc.6601610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weichert W, Schmidt M, Gekeler V, Denkert C, Stephan C, Jung K, et al. Polo-like kinase 1 is overexpressed in prostate cancer and linked to higher tumor grades. Prostate. 2004;60:240–245. doi: 10.1002/pros.20050. [DOI] [PubMed] [Google Scholar]
- 40.Weichert W, Kristiansen G, Winzer KJ, Schmidt M, Gekeler V, Noske A, et al. Polo-like kinase isoforms in breast cancer: expression patterns and prognostic implications. Virchows Arch. 2005;446:442–450. doi: 10.1007/s00428-005-1212-8. [DOI] [PubMed] [Google Scholar]
- 41.Weichert W, Schmidt M, Jacob J, Gekeler V, Langrehr J, Neuhaus P, et al. Overexpression of polo-like kinase 1 is a common and early event in pancreatic cancer. Pancreatology. 2005;5:259–265. doi: 10.1159/000085280. [DOI] [PubMed] [Google Scholar]
- 42.Weichert W, Kristiansen G, Schmidt M, Gekeler V, Noske A, Niesporek S, et al. Polo-like kinase 1 expression is a prognostic factor in human colon cancer. World J Gastroenterol. 2005;11:5644–5650. doi: 10.3748/wjg.v11.i36.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weichert W, Ullrich A, Schmidt M, Gekeler V, Noske A, Niesporek S, et al. Expression patterns of polo-like kinase 1 in human gastric cancer. Cancer Sci. 2006;97:271–276. doi: 10.1111/j.1349-7006.2006.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kneisel L, Strebhardt K, Bernd A, Wolter M, Binder A, Kaufmann R. Expression of polo-like kinase (PLK1) in thin melanomas: a novel marker of metastatic disease. J Cutan Pathol. 2002;29:354–358. doi: 10.1034/j.1600-0560.2002.290605.x. [DOI] [PubMed] [Google Scholar]
- 45.Strebhardt K, Kneisel L, Linhart C, Bernd A, Kaufmann R. Prognostic value of pololike kinase expression in melanomas. JAMA. 2000;283:479–480. doi: 10.1001/jama.283.4.479. [DOI] [PubMed] [Google Scholar]
- 46.Yamada S, Ohira M, Horie H, Ando K, Takayasu H, Suzuki Y, et al. Expression profiling and differential screening between hepatoblastomas and the corresponding normal livers: identification of high expression of the PLK1 oncogene as a poor-prognostic indicator of hepatoblastomas. Oncogene. 2004;23:5901–5911. doi: 10.1038/sj.onc.1207782. [DOI] [PubMed] [Google Scholar]
- 47.Nogawa M, Yuasa T, Kimura S, Tanaka M, Kuroda J, Sato K, et al. Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J Clin Invest. 2005;115:978–985. doi: 10.1172/JCI23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilmartin AG, Bleam MR, Richter MC, Erskine SG, Kruger RG, Madden L, et al. Distinct concentration-dependent effects of the polo-like kinase 1-specific inhibitor GSK461364A, including differential effect on apoptosis. Cancer Res. 2009;69:6969–6977. doi: 10.1158/0008-5472.CAN-09-0945. [DOI] [PubMed] [Google Scholar]
- 49.Ikezoe T, Yang J, Nishioka C, Takezaki Y, Tasaka T, Togitani K, et al. A novel treatment strategy targeting polo-like kinase 1 in hematological malignancies. Leukemia. 2009;23:1564–1576. doi: 10.1038/leu.2009.94. [DOI] [PubMed] [Google Scholar]
- 50.Rudolph D, Steegmaier M, Hoffmann M, Grauert M, Baum A, Quant J, et al. BI 6727, a polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin Cancer Res. 2009;15:3094–3102. doi: 10.1158/1078-0432.CCR-08-2445. [DOI] [PubMed] [Google Scholar]
- 51.Emmitte KA, Andrews CW, Badiang JG, vis-Ward RG, Dickson HD, Drewry DH, et al. Discovery of thiophene inhibitors of polo-like kinase. Bioorg Med Chem Lett. 2009;19:1018–1021. doi: 10.1016/j.bmcl.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 52.Mross K, Frost A, Steinbild S, Hedbom S, Rentschler J, Kaiser R, et al. Phase I dose escalation and pharmacokinetic study of BI 2536, a novel polo-like kinase 1 inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2008;26:5511–5517. doi: 10.1200/JCO.2008.16.1547. [DOI] [PubMed] [Google Scholar]
- 53.Santamaria A, Neef R, Eberspacher U, Eis K, Husemann M, Mumberg D, et al. Use of the novel Plk1 inhibitor ZK-thiazolidinone to elucidate functions of Plk1 in early and late stages of mitosis. Mol Biol Cell. 2007;18:4024–4036. doi: 10.1091/mbc.E07-05-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steegmaier M, Hoffmann M, Baum A, Lenart P, Petronczki M, Krssak M, et al. BI 2536, a potent and selective inhibitor of pololike kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17:316–322. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 55.Stevenson CS, Capper EA, Roshak AK, Marquez B, Eichman C, Jackson JR, et al. The identification and characterization of the marine natural product scytonemin as a novel antiproliferative pharmacophore. J Pharmacol Exp Ther. 2002;303:858–866. doi: 10.1124/jpet.102.036350. [DOI] [PubMed] [Google Scholar]
- 56.Garcia-Pichel F, Sherry ND, Castenholz RW. Evidence for an ultraviolet sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium Chlorogloeopsis sp. Photochem Photobiol. 1992;56:17–23. doi: 10.1111/j.1751-1097.1992.tb09596.x. [DOI] [PubMed] [Google Scholar]
- 57.Sorrels CM, Proteau PJ, Gerwick WH. Organization, evolution, and expression analysis of the biosynthetic gene cluster for scytonemin, a cyanobacterial UV-absorbing pigment. Appl Environ Microbiol. 2009;75:4861–4869. doi: 10.1128/AEM.02508-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, et al. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC, Jr, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y, Shreder KR, Gai W, Corral S, Ferris DK, Rosenblum JS. Wortmannin, a widely used phosphoinositide 3-kinase inhibitor, also potently inhibits mammalian polo-like kinase. Chem Biol. 2005;12:99–107. doi: 10.1016/j.chembiol.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y, Jiang N, Wu J, Dai W, Rosenblum JS. Polo-like kinases inhibited by wortmannin. Labeling site and downstream effects. J Biol Chem. 2007;282:2505–2511. doi: 10.1074/jbc.M609603200. [DOI] [PubMed] [Google Scholar]
- 62.Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, et al. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000;6:909–919. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- 63.Kang GY, Lee ER, Kim JH, Jung JW, Lim J, Kim SK, et al. Downregulation of PLK-1 expression in kaempferol-induced apoptosis of MCF-7 cells. Eur J Pharmacol. 2009;611:17–21. doi: 10.1016/j.ejphar.2009.03.068. [DOI] [PubMed] [Google Scholar]
- 64.Reindl W, Yuan J, Kramer A, Strebhardt K, Berg T. Inhibition of polo-like kinase 1 by blocking polo-box domain-dependent protein–protein interactions. Chem Biol. 2008;15:459–466. doi: 10.1016/j.chembiol.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 65.Gali-Muhtasib H, Roessner A, Schneider-Stock R. Thymoquinone: a promising anti-cancer drug from natural sources. Int J Biochem Cell Biol. 2006;38:1249–1253. doi: 10.1016/j.biocel.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 66.Elia AE, Cantley LC, Yaffe MB. Proteomic screen finds pSer/ pThr-binding domain localizing Plk1 to mitotic substrates. Science. 2003;299:1228–1231. doi: 10.1126/science.1079079. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe N, Sekine T, Takagi M, Iwasaki J, Imamoto N, Kawasaki H, et al. Deficiency in chromosome congression by the inhibition of Plk1 polo box domain-dependent recognition. J Biol Chem. 2009;284:2344–2353. doi: 10.1074/jbc.M805308200. [DOI] [PubMed] [Google Scholar]
- 68.Kulling SE, Lehmann L, Metzler M. Oxidative metabolism and genotoxic potential of major isoflavone phytoestrogens. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:211–218. doi: 10.1016/s1570-0232(02)00215-5. [DOI] [PubMed] [Google Scholar]
- 69.Taylor CK, Levy RM, Elliott JC, Burnett BP. The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutr Rev. 2009;67:398–415. doi: 10.1111/j.1753-4887.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- 70.Ismail IA, Kang KS, Lee HA, Kim JW, Sohn YK. Genistein-induced neuronal apoptosis and G2/M cell cycle arrest is associated with MDC1 up-regulation and PLK1 down-regulation. Eur J Pharmacol. 2007;575:12–20. doi: 10.1016/j.ejphar.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 71.Cheng WY, Hsiang CY, Bau DT, Chen JC, Shen WS, Li CC, et al. Microarray analysis of vanillin-regulated gene expression profile in human hepatocarcinoma cells. Pharmacol Res. 2007;56:474–482. doi: 10.1016/j.phrs.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 72.Ho K, Yazan LS, Ismail N, Ismail M. Apoptosis and cell cycle arrest of human colorectal cancer cell line HT-29 induced by vanillin. Cancer Epidemiol. 2009;33:155–160. doi: 10.1016/j.canep.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 73.Kaur M, Agarwal R. Silymarin and epithelial cancer chemo-prevention: how close we are to bedside? Toxicol Appl Pharmacol. 2007;224:350–359. doi: 10.1016/j.taap.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mann CD, Neal CP, Garcea G, Manson MM, Dennison AR, Berry DP. Phytochemicals as potential chemopreventive and chemotherapeutic agents in hepatocarcinogenesis. Eur J Cancer Prev. 2009;18:13–25. doi: 10.1097/CEJ.0b013e3282f0c090. [DOI] [PubMed] [Google Scholar]
- 75.Ramasamy K, Agarwal R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008;269:352–362. doi: 10.1016/j.canlet.2008.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cui W, Gu F, Hu KQ. Effects and mechanisms of silibinin on human hepatocellular carcinoma xenografts in nude mice. World J Gastroenterol. 2009;15:1943–1950. doi: 10.3748/wjg.15.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agarwal C, Singh RP, Dhanalakshmi S, Tyagi AK, Tecklenburg M, Sclafani RA, et al. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- 78.Noh EJ, Lim DS, Jeong G, Lee JS. An HDAC inhibitor, trichostatin A. induces a delay at G2/M transition, slippage of spindle checkpoint, and cell death in a transcription-dependent manner. Biochem Biophys Res Commun. 2009;378:326–331. doi: 10.1016/j.bbrc.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 79.Tsuji N, Kobayashi M, Nagashima K, Wakisaka Y, Koizumi K. A new antifungal antibiotic, trichostatin. J Antibiot (Tokyo) 1976;29:1–6. doi: 10.7164/antibiotics.29.1. [DOI] [PubMed] [Google Scholar]
- 80.Yoshida M, Horinouchi S, Beppu T. Trichostatin A and trapoxin: novel chemical probes for the role of histone acetylation in chromatin structure and function. Bioessays. 1995;17:423–430. doi: 10.1002/bies.950170510. [DOI] [PubMed] [Google Scholar]
- 81.Lee JS. Activation of ATM-dependent DNA damage signal pathway by a histone deacetylase inhibitor, trichostatin A. Cancer Res Treat. 2007;39:125–130. doi: 10.4143/crt.2007.39.3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim SA, Kim SW, Chang S, Yoon JH, Ahn SG. 5′-nitro-indirubinoxime induces G2/M cell cycle arrest and apoptosis in human KB oral carcinoma cells. Cancer Lett. 2009;274:72–77. doi: 10.1016/j.canlet.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 83.Hoessel R, Leclerc S, Endicott JA, Nobel ME, Lawrie A, Tunnah P, et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999;1:60–67. doi: 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- 84.Nam S, Buettner R, Turkson J, Kim D, Cheng JQ, Muehlbeyer S, et al. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc Natl Acad Sci U S A. 2005;102:5998–6003. doi: 10.1073/pnas.0409467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eisenbrand G, Hippe F, Jakobs S, Muehlbeyer S. Molecular mechanisms of indirubin and its derivatives: novel anticancer molecules with their origin in traditional Chinese phytomedicine. J Cancer Res Clin Oncol. 2004;130:627–635. doi: 10.1007/s00432-004-0579-2. [DOI] [PubMed] [Google Scholar]
- 86.Leclerc S, Garnier M, Hoessel R, Marko D, Bibb JA, Snyder GL, et al. Indirubins inhibit glycogen synthase kinase-3 beta and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors? J Biol Chem. 2001;276:251–260. doi: 10.1074/jbc.M002466200. [DOI] [PubMed] [Google Scholar]
- 87.Kim SA, Kim YC, Kim SW, Lee SH, Min JJ, Ahn SG, et al. Antitumor activity of novel indirubin derivatives in rat tumor model. Clin Cancer Res. 2007;13:253–259. doi: 10.1158/1078-0432.CCR-06-1154. [DOI] [PubMed] [Google Scholar]
- 88.Kim SH, Choi SJ, Kim YC, Kuh HJ. Anti-tumor activity of noble indirubin derivatives in human solid tumor models in vitro. Arch Pharm Res. 2009;32:915–922. doi: 10.1007/s12272-009-1614-2. [DOI] [PubMed] [Google Scholar]