Abstract
Adverse prenatal environment, such as intrauterine growth retardation (IUGR), increases the risk for negative neurobehavioral outcomes. IUGR, affecting approximately 10% of all US infants, is a known risk factor for ADHD, schizophrenia spectrum disorders and addiction. Mouse dams were fed a protein deficient (8.5% protein) or isocaloric control (18% protein) diet through pregnancy and lactation (a well validated rodent model of IUGR). Dopamine-related gene expression, dopamine content and behavior were examined in adult offspring. IUGR offspring have 6–8 fold over-expression of dopamine (DA)-related genes (tyrosine hydroxylase (TH) and dopamine transporter) in brain regions related to reward processing (ventral tegmental area (VTA), nucleus accumbens, prefrontal cortex (PFC)) and homeostatic control (hypothalamus), as well as increased number of TH-ir neurons in the VTA and increased dopamine in the PFC. Cyclin-dependent kinase inhibitor 1C (Cdkn1c) is critical for dopaminergic neuron development. Methylation of the promoter region of Cdkn1c was decreased by half and there was a resultant 2–7 fold increase in Cdkn1c mRNA expression across brain regions. IUGR animals demonstrated alterations in dopamine-dependent behaviors, including altered reward-processing, hyperactivity and exaggerated locomotor response to cocaine.
These data describe significant dopamine-related molecular and behavioral abnormalities in a mouse model of IUGR. This animal model, with both face validity (behavior) and construct validity (link to IUGR and dopamine dysfunction) may prove useful in identifying underlying mechanisms linking IUGR and adverse neurobehavioral outcomes such as ADHD.
Keywords: dopamine, neurodevelopmental programming, epigenetics, addiction, perinatal nutrition
A suboptimal prenatal environment, typically indicated by low birth weight or being small for gestational age (SGA), can increase the risk for adverse neurobehavioral outcomes, including ADHD (Hultman et al., 2007; Lahti et al., 2006), schizophrenia spectrum disorders (Susser et al., 2008; St Clair et al., 2005), major affective disorder/depression (Brown et al., 2000; Brown et al., 1995), antisocial personality disorder (Neugebauer et al., 1999) and addiction (Franzek et al., 2008). Intrauterine growth retardation (IUGR), which results in SGA infants, affects roughly 10% of all US births and is associated with adverse pregnancy conditions, such as maternal malnutrition, hypertension, uterine or placental dysfunction, smoking or drug use, and multiple births. While adverse metabolic and cardiovascular outcomes (hypertension, cardiovascular disease, insulin resistance (Gluckman and Hanson, 2008; de Rooij et al., 2007)) have been well characterized in IUGR animal models, the coincident neurobehavioral disabilities and specific CNS abnormalities have received significantly less attention. Early life protein restriction, an established rodent model of IUGR used extensively in rats, has only recently been shown to result in similar metabolic phenotypes in the mouse (Goyal et al., 2009; van Straten et al., 2010; Bol et al., 2009; Chen et al., 2009). Neurobehavioral outcomes in a mouse model of IUGR have not been reported.
Dopamine (DA) participates in locomotor activity, reward, motivation and feeding behavior and its dysfunction is implicated in several neurobehavioral disorders including ADHD and addiction. The central DA system consists of neurons that originate in substantia nigra pars compacta (SNpc), ventral tegmental area (VTA), and project through nigrostriatal (locomotor activity), mesolimbic (reward, feeding), and mesocortical (motivation, emotional) pathways. Dopamine-related gene expression is also prominent within the hypothalamus. A broad range of prenatal insults, including malnutrition, maternal stress or infection and environmental toxins, have been found to alter dopamine function (Wang et al., 2009; Zhou et al., 2009; Palmer et al., 2008; McArthur et al., 2007; Son et al., 2007; Stanwood and Levitt, 2007; Valdomero et al., 2005).
The molecular link between adverse neurobehavioral outcomes and a suboptimal prenatal environment may involve epigenetic changes (DNA methylation, histone methylation or acetylation (Jirtle and Skinner, 2007; Weaver et al., 2004; Szyf, 2009)). Imprinted genes, due to their monoallelic and epigenetically regulated expression, are particularly susceptible to dysregulation as a result of suboptimal prenatal conditions (Jirtle and Skinner, 2007; Kwong et al., 2006). Similarly, disruption of the imprinting status of several imprinted genes in the CNS leads to neurodevelopmental disorders (e.g, Prader-Willi Syndrome, Angelman syndrome) (Davies et al., 2005). Cyclin-dependent kinase inhibitor 1C (Cdkn1c/p57) is an imprinted gene located in an imprinted region on mouse chromosome 7 encoding a cyclin-dependant kinase inhibitor that acts to negatively regulate cell proliferation and, in some tissues, to actively direct differentiation (Smith et al., 2007; Ye et al., 2009). In the brain, Cdkn1c is expressed in dopaminergic neurons where it exerts a crucial role in dopamine neuron differentiation (Joseph et al., 2003; Freed et al., 2008). Furthermore, overexpression of Cdkn1c in a transgenic mouse model leads to embryonic growth retardation and low birth weight, a characteristic IUGR phenotype (Andrews et al., 2007). Igf2, another imprinted gene in this locus, has also been linked to fetal growth (Zeisel, 2009). Therefore, gene expression and differential methylation was determined for genes within the Cdkn1c locus.
IUGR infants are at significant risk for long term neurobehavioral disabilities, including ADHD, depression, and addiction. In utero malnutrition does not lead to major structural or anatomical deficits within the CNS, but rather permanent suboptimal development (Ranade et al., 2008). Experiments in the present manuscript were designed to examine dopaminergic dysregulation at the molecular and behavioral level in a mouse model of IUGR. Vulnerability to epigenetic modifications was determined for dopamine-related genes as well as potentially more vulnerable imprinted genes within the Cdkn1c locus.
Experimental Procedures
Animals and experimental model
C57BL/6J females were mated with DBA/2J males and fed either a control (18% protein) or isocaloric 8.5% low protein (LP) diet during breeding, pregnancy and lactation (diet details below). At birth, litters were culled to 6–8 pups, and at weaning, all offspring were maintained on the control diet. A subset of both control and IUGR animals was placed on a high-fat diet (60% calories from fat) from weaning and tested to determine whether locomotor activity was affected by postnatal diet, as consumption of a Western diet has been shown to affect locomotor activity (Bjursell et al., 2008). One animal per litter was randomly chosen for use in individual experiments, to control for any litter effect. There was no difference in litter size between LP and control pregnancies. Body weights were recorded weekly, and male mice aged 18–20 weeks of age (adulthood) were used in all experiments.
Diet Composition
Total energy content of control diet (Test Diet 5755, Richmond, IN) was 4.09 kcal/g with 18% of total energy calories from protein, 22% from fat, and 60% from carbohydrate. Low protein diet (Test Diet 5769) was 8.5% protein purified diet with total energy content of 4.13 kcal/g with 8.5% of total energy calories from protein, 22% from fat, and 69.5% from carbohydrate. High fat diet (Test Diet 58G9) has a total energy content of 5.21 kcal/g with 18% of total energy calories from protein, 60% from fat, and 22% from carbohydrate.
Sucrose preference and locomotor activity
Sucrose preference was determined in standard cages using a 2 bottle choice test with 4% sucrose (details in Supplemental methods.) Locomotor activity was measured using the Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH), which measures animal movement in the x- and z-axes. While in the CLAMS cages, animals had ad libitum access to powdered diet and water. Food intake was normalized to body weight.
Locomotor response to cocaine
Animals were tested for their locomotor response to cocaine using the CLAMS cages. Animals previously acclimated to the cages were housed in the cages for 3 days. On day 1 animals were left undisturbed, on day 2, they were injected with saline (11am) and on day 3, they were injected with cocaine (11am, 20 mg/kg, IP). Food and water was available ad libitum.
Genomic DNA and Total RNA isolation from brain
Animals were euthanized with an overdose of carbon dioxide, followed by cervical dislocation. This method is consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association and was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. After the animals were killed, the brains were rapidly removed and placed in RNAlater (Ambion, Austin, TX) for four hours before dissections. Specific regions were identified and macrodissected using their approximate mouse stereotaxic coordinates according to Mouse Brain Atlas (NAc, bregma +1.10mm; PFC ~ +2 to Bregma at posterior surface; VTA, bregma −3.64mm). Briefly, brains were positioned in a 1mm mouse brain matrix (ASI Instruments, Warren, MI, USA) abutting a razor blade placed in the first slot. The slot nearest the boundary of medulla was the first landmark. Next, double-edged razor blades were placed in slots one and two mm anterior to that boundary. Next, razor blades were placed in slots one, two and three mm posterior to blade at anterior surface. The anterior set of three blades was removed from the block. Proceeding anterior to posterior, the first section was discarded. From second section (~ +2 to Bregma at posterior surface), cortex was cut from dorsal half of section. From third section (~ +1 to Bregma at posterior surface), the nucleus accumbens was removed. Hypothalamus was dissected from the section in between blade 3 and 4. The slab was placed flat and the cuts were placed on either side of the optic chiasm and dorsal to the third ventricle. The posterior set of blades was removed from the block and VTA/SN region was dissected. Genomic DNA and total RNA were isolated simultaneously using AllPrep DNA/RNA Mini Kit (Qiagen).
Gene expression analysis by quantitative Real-Time PCR
For each individual sample, 500 ng of total RNA was used in reverse transcription using High Capacity Reverse Transcription Kit (ABI). Expression of target genes was determined by quantitative RT-PCR using gene-specific TaqMan probes (ABI, Foster City, CA) with TaqMan Gene Expression Master Mix (ABI, Foster City, CA) on the ABI7900HT Real-Time PCR Cycler. Probes used for RT-PCR are listed in supplemental material. The relative amount of each transcript was determined using delta Ct values as previously described (Pfaffl, 2001). Changes in gene expression were calculated using relative quantitation of a target gene against endogenous, unchanged GAPDH and B-ACTN standard.
Methylated DNA Immunoprecipitation (MeDIP) Assay
MeDIP assay was performed as described (Weber et al., 2005). Methylated DNA was immunoprecipitated using 10 µg of mouse monoclonal 5-methylcytidine antibody (Eurogentec) or mouse pre-immune serum. Enrichment in the MeDIP fraction was determined by quantitative RT-PCR using ChIP-qPCR Assay Master Mix (SuperArray) on the ABI7900HT Real-Time PCR Cycler. For all genes examined, primers were obtained from Superarray (ChIP-qPCR Assays (−01) kb tile, SuperArray) for the amplification of genomic regions spanning the CpG sites located approximately 300–500 bp upstream of the transcription start sites (see Supplemental Material for primer sequences). MeDIP results were expressed as fold enrichment of immunoprecipitated DNA for each specific site. To calculate differential occupancy fold change (% enrichment), MeDIP DNA fractions' Ct value were normalized to the Input DNA fraction Ct value (see Supplemental Material). Finally, the normalized level of DNA methylation at a particular site is expressed as relative to control group set to 1.
Immunohistochemistry
Details for tissue processing and immunohistochemistry have been described (Reyes et al., 2003) and are included in the supplemental methods. The primary antibodies were (1) a TH rabbit polyclonal directed against rat TH (Pel Freeze Biologicals, Rogers AR, used at 1:2K) and (2) a c-Fos rabbit polyclonal antiserum directed against a synthetic peptide corresponding to the n-terminal portion (amino acids 5–16) of human Fos protein (Santa Cruz Biotechnologies, Santa Cruz, CA, used at 1:10K). Localization was performed using a conventional avidin-biotin immunoperoxidase method. Differences in the relative abundance of positive cells were estimated by simple cell counting. Counts of stained neurons were made in single sections from multiple animals (n=3–4/group) in either the VTA (TH-ir) or NAc (Fos-ir) defined on the basis of adjoining Nissl stained sections.
Neurochemical Procedure
Dopamine (DA) and its metabolites homovanillic acid (HVA), and 3,4-dihydroxyphenylacetic acid (DOPAC) were measured by high-performance liquid chromatography analysis. Brains were removed from the skull and rinsed in ice cold water to remove any surface blood. First, the brain was partially cut in half into right and left hemisphere. Next, a stiff brush was placed into a hippocampal grove and rotated to displace and remove the hippocampus. After the cortex was peeled apart and collected, the exposed striatum was dissected and harvested. The right and left striata and the cortex were quickly frozen on dry ice, and then stored at −80°C until analysis. Tissue samples were homogenized in 0.1 N perchloric acid with 100 µM EDTA (15 µl/mg of tissue) using a Tissuemizer (Tekmar, Cleveland, OH). Samples were centrifuged at 15,000 rpm for 15 min at 2–8°C. The supernatant was extracted and filtered through 0.45- µm nylon acrodisk syringe filters and analyzed immediately. The Bioanalytical Systems HPLC (West Lafayette, IN) consisted of a PM-80 pump, a Sample Sentinel autosampler, and a LC-4C electrochemical detector. The samples (12 µl) were injected and pumped through a reverse phase microbore column (ODS 3 µm, 1 × 100 mm), at a flow rate of 0.6 ml/min with electrodetection at +0.6 V. Separation for DA and the metabolites was accomplished by using a mobile phase consisting of 90-mM sodium acetate, 35-mM citric acid, 0.34-mM ethylenediamine tetraacetic acid, 1.2-mM sodium octyl sulfate, and 15% methanol v/v at a pH of 4.2 (Mayorga et al., 2001).
Statistical analyses
Data, presented as means ± s.e.m., were analyzed using SPSS statistical package and Excel tools for statistical analysis. Student’s t-test or Mann Whitney U was used to analyze differences between IUGR and control animals, with Bonferroni correction for multiple t-tests (multiple genes within a brain region) applied as warranted. Repeated-measures ANOVA followed by Newman-Keuls posthoc analysis was used to calculate statistically significant difference between groups in Figs 5 and 6. A p-value of 0.05 or lower was considered significant.
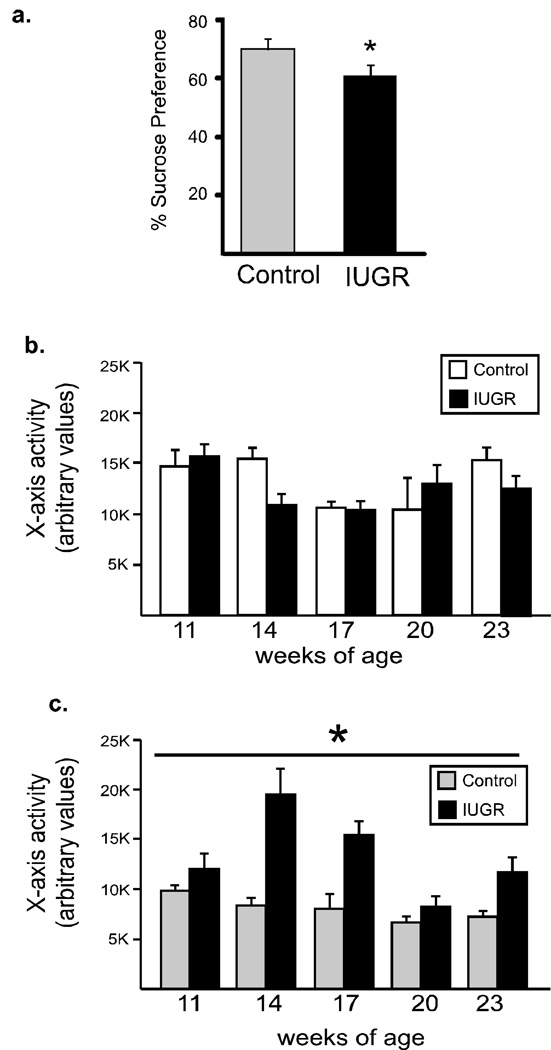
Figure 5. Sucrose intake and locomotor activity.
Sucrose preference (a) was evaluated in IUGR (gray bars) and control (black bars) mice (n=4–5/group). IUGR mice show reduced preference for sucrose. *p <0.05 Locomotor activity was evaluated every 3 weeks in the CLAMS cages for 12 hr, beginning at the onset of lights off. Animals fed the control diet (b) did not differ in activity levels, however, when animals were fed a high-fat diet (c) IUGR animals demonstrated significant hyperactivity (main effect for group, *p<0.003). n=4/group.
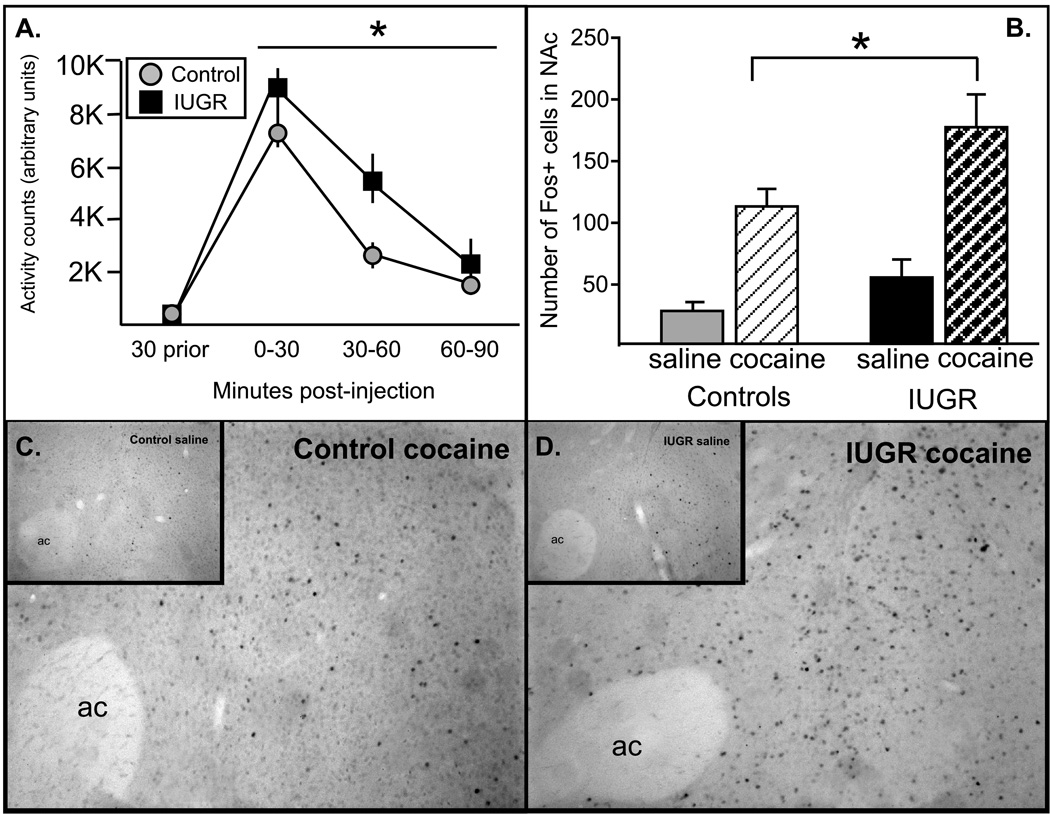
Figure 6. Locomotor and neuronal response to cocaine.
(A). Locomotor response to cocaine (20 mg/kg, IP) in IUGR (black square) and control (grey circles) was measured. IUGR animals had a significantly greater response to cocaine, particularly at 30–60 min post-injection (n=10–12/group). (B–D) Immunohistochemistry was used to localize the expression of the immediate early gene protein product, c-Fos, a marker of general neuronal activation, within the NAc. As expected, there were few activated (Fos-ir) cells in saline-injected animals, which did not differ between groups (insets, C and D). In response to cocaine (20 mg/kg, IP), a significant increase in Fos-ir cells was observed in the NAc, and this response was significantly greater in IUGR animals (*p<.05, n=3 mice/group).
RESULTS
Experimental animal model of intrauterine growth retardation (IUGR)
In order to eliminate any potential adverse effect of handling on the development of offspring behavior and CNS gene expression (Murgatroyd et al., 2009), a separate group of dams and offspring were used to determine the maternal response to the LP diet (Supplemental Table 1). Consumption of the low protein diet did not affect maternal weight gain through pregnancy, litter size, or pregnancy duration. There was a nonsignificant trend for a lower birthweight in the IUGR animals, however, by weaning IUGR animals weighed significantly less. Comparable to IUGR infants, animals from maternal LP pregnancies, termed IUGR mice, weighed 37% less at weaning than animals from control pregnancies, (n=5, t-test, p <0.002) and maintained 10–15% lower weight than the controls until postnatal week 9 (Supplemental Fig 1). Body weight of IUGR mice normalized by 10 weeks of age. Therefore, when tested at 18–20 week of age, animals in the IUGR group weighed on average the same as controls.
The mesocorticolimbic and hypothalamic dopamine (DA) system is altered in IUGR adult animals
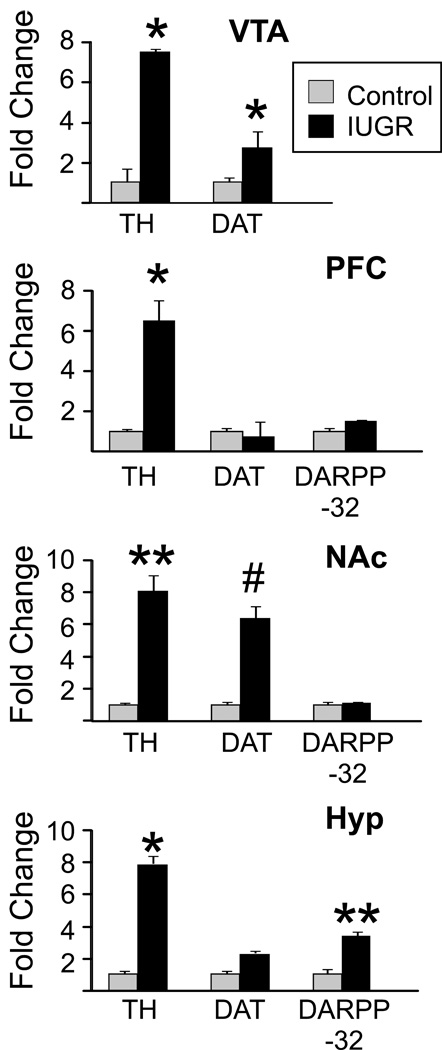
Experiments were initiated to determine the extent to which dopamine-related genes were altered within specific CNS regions in IUGR animals. Quantitative real-time PCR (qRT-PCR) was used to measure the relative expression of genes involved in the synthesis (TH, tyrosine hydroxylase), reuptake (DAT, dopamine reuptake transporter), and degradation of dopamine (COMT, catechol-O-methyltransferase) and modulation and transmission of dopamine signal (dopamine receptor D1, dopamine receptor D2, and dopamine- and cyclic AMP-regulated phosphoprotein DARPP-32) in brain regions associated with reward processing (VTA, PFC, NAc) and homeostasis (hypothalamus) of adult IUGR and control animals (Fig 1). The expression of TH was upregulated 6–8-fold in VTA, PFC, NAc and hypothalamus of IUGR animals (n=5, t-test, p=0.019, 0.012, 0.008, 0.012, respectively). Additionally, IUGR animals showed overexpression of DAT, with a 3–4- fold increase in the VTA (n = 5, t-test, p=0.017), a nearly 8- fold (n = 5, t-test, p<0.004) increase in the NAc, and a nonsignificant (p=.042; Bonferroni corrected α-level=.02) increase in the hypothalamus. DARPP-32 expression was significantly increased only in the hypothalamus of IUGR animals (n=5, t-test, p<0.01). On the other hand, expression levels of dopamine receptors, D1 and D2, or COMT did not differ (data not shown) regardless of the brain region. Taken together, these data demonstrate that IUGR due to exposure to prenatal and early postnatal LP diet results in altered expression of key dopaminergic signaling molecules within mesocorticolimbic circuitry and in the hypothalamus.
Figure 1. Dopamine-related gene expression within mesocorticolimbic circuitry and hypothalamus of IUGR and control mice.
mRNA expression levels of dopamine pathway related genes TH, DAT, and DARPP-32 in the ventral tegmental area (VTA), nucleus accumbens (NAc), prefrontal cortex (PFC) and hypothalamus of control (grey bars) and IUGR (black bars) mice was determined by qRT-PCR (n=5/group). Expression level of each gene examined was normalized to the GAPDH and β-Actin levels and expressed relative to the control group set to 1. n=5/group. Values are mean ± s.e.m. *p < 0.05, **p <.01, #p <.005.
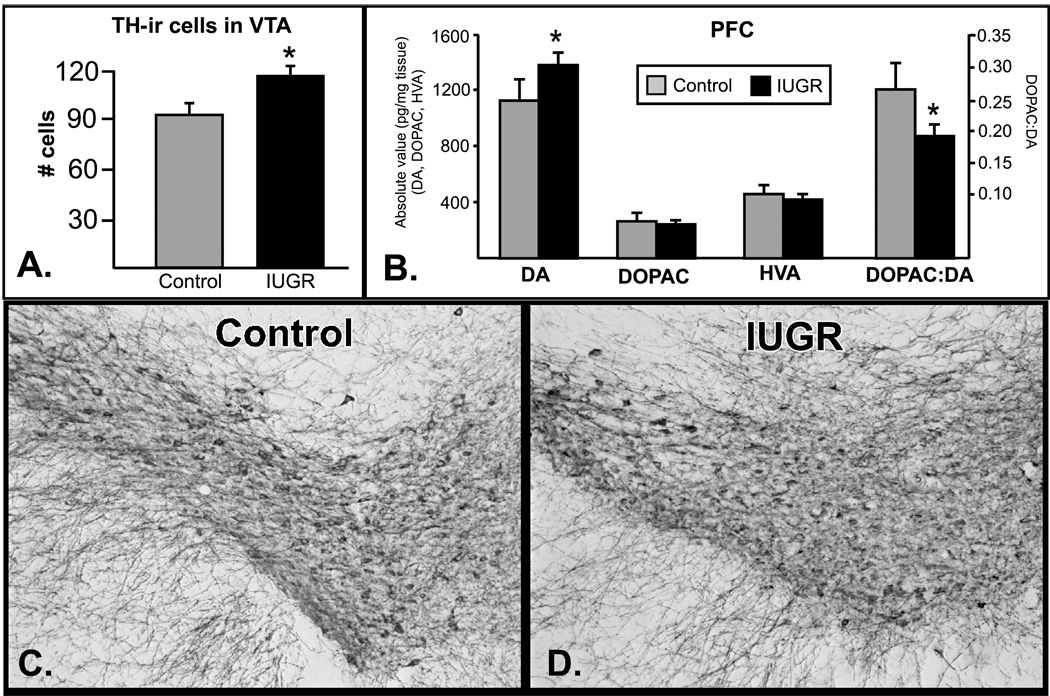
Experiments were then conducted to examine the number of TH-ir neurons within the VTA, the primary source of dopaminergic innervation to the PFC and VTA. As predicted based on the increased TH mRNA in the VTA, there were nearly 30% more TH+ neurons in the VTA of IUGR animals. (Fig 2A, p<.05, t-test, representative images are shown in 2C (control) and 2D (IUGR)). Dopamine and dopamine metabolites (3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA)) were measured in PFC and striatum, projection fields of the VTA. A significant increase in DA was detected in the PFC, as well as a significant decrease in the ratio of DOPAC:DA, an indication of dopamine turnover (p<.05, Mann-Whitney U). No changes were evident in DOPAC or HVA concentrations in PFC, and there were no significant differences in DA or DA metabolites within the striatum.
Figure 2. Increased PFC dopamine and TH-ir cells in the VTA.
Top left: Dopamine and dopamine metabolites were measured in the PFC of control (grey) and IUGR animals (black, n=6/group). There was a significant increase in dopamine, and a significant decrease in dopamine turnover (DOPAC:DA ratio). Top right: A significant increase in TH-ir neurons was seen in the VTA (n=4/group). Representative images are shown in the lower panels (control-left, IUGR-right). *p<.05.
Perinatal protein restriction affects promoter methylation of Cdkn1c
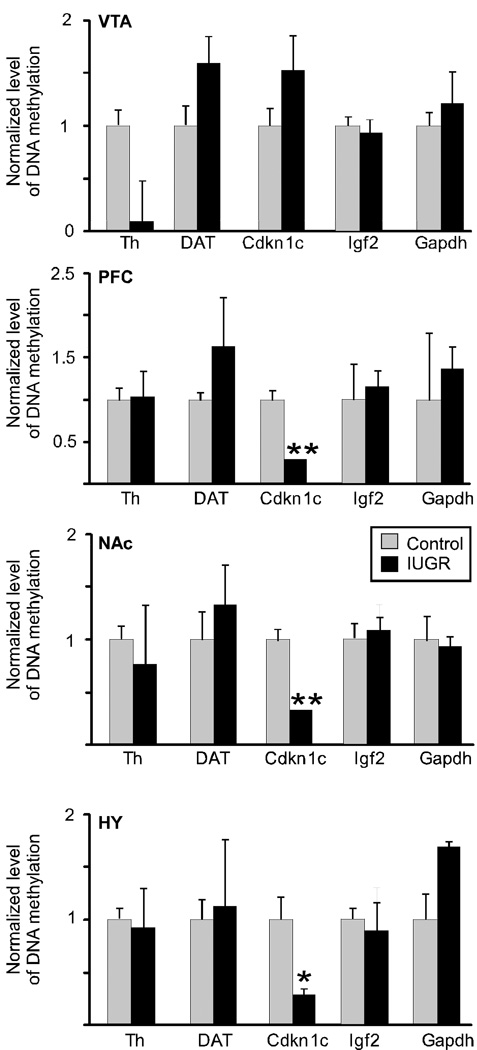
We then examined gene-specific promoter methylation within the CpG islands of TH and DAT, genes that were significantly overexpressed in IUGR animals. We used affinity purification of methylated DNA (methyl DNA immunoprecipitation (MeDIP) assay) followed by qRT-PCR to determine methylation levels of proximal promoter regions of TH and DAT (Fig 3). The amount of 5-meC-DNA enriched fraction in the promoters of TH and DAT of IUGR animals did not differ significantly from the controls in any of the brain regions examined.
Figure 3. DNA methylation changes at regulatory regions of dopamine related genes in IUGR mice.
The enrichment of DNA methylation relative to input genomic DNA at proximal promoter regions of TH, DAT, Cdkn1c, Igf2 and Gapdh was quantified in ventral tegmental area (VTA), prefrontal cortex (PFC), nucleus accumbens (NAc), and hypothalamus (Hyp) by qRT-PCR after MeDIP. No differences were observed in TH, DAT or IGF2, however, the Cdkn1c promoter region was significantly hypomethylated in PFC, NAc, and hypothalamus. n=3/group. Values are mean ± s.e.m. *p <0.05, **p<.01.
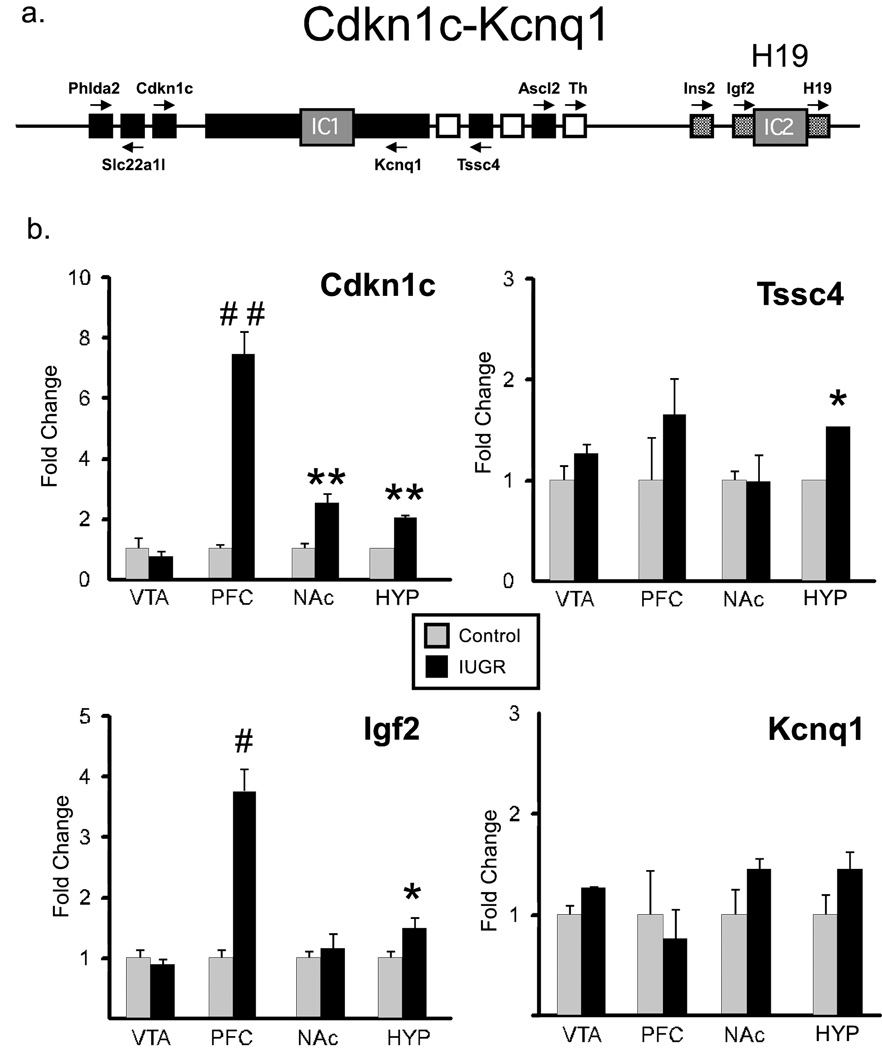
We next examined methylation status of several imprinted genes in the Cdkn1c-Igf2 locus on mouse Ch7 (Fig 4a). Expression of genes located in Cdkn1c-Igf2 locus can be regulated by environmental inputs during development (Kwong et al., 2006; Waterland et al., 2006), are important for differentiation of neuronal precursor cells into mature DA neurons (Joseph et al., 2003; Freed et al., 2008), and neuronal-specific overexpression of Cdkn1c leads to embryonic growth retardation (Andrews et al., 2007). We performed MeDIP analysis of proximal promoter regions (~350 bp upstream of transcription start site) of the Cdkn1c and Igf2 genes. Adult IUGR animals showed a 50–75% reduction in methylation of Cdkn1c promoter in PFC, NAc, and hypothalamus compared to controls (Fig 3; n=3, t-test, p=.01, p=.008, p=.013, respectively), while Igf2 promoter methylation did not differ in any brain region tested. The primarily unmethylated, control Gapdh promoter was not affected.
Figure 4. a. Cdkn1c-Igf2 locus.
Schematic representation of Cdkn1c-Igf2 genes with two imprinted centers (IC1 and IC2) regulating the expression of genes in this locus. Black and gray boxes indicate maternally and paternally imprinted genes, respectively. White boxes represent biallelically expressed genes. Arrows are the direction of transcription. b. CNS expression of Cdkn1c-Igf2 genes. Expression of Cdkn1c-Igf2 locus genes Kcnq1, Cdkn1c, Tssc4, and Igf2 in VTA, PFC, NAc and hypothalamus of control (gray bars), IUGR (black bars) mice was assayed by RT-PCR. n=5/group. Data are means ± s.e.m. *p <0.05, **p<.01, # p<.005, ##p<.001.
Brain-region specific overexpression of genes in imprinted Cdkn1c - Igf2 locus that regulate dopamine neuron differentiation
We next asked whether hypomethylation of the Cdkn1c promoter in IUGR animals was accompanied by altered expression of genes in the Cdkn1c-Igf2 gene cluster (Fig 4b). We observed a statistically significant increase in the expression of Cdkn1c in PFC, NAc and hypothalamus of IUGR animals (n=5, t-test, p=0.0001, p=0.012, p=0.013, respectively), all regions in which promoter hypomethylation was observed. Igf2 was overexpressed in PFC and hypothalamus (n=5, t-test, p=0.004, and p=0.03), while Tssc4 (tumor-suppressing subchromosomal transferable fragment 4) showed a significant increase in hypothalamus only (n=5, t-test, p=0.037). On the other hand, the relative expression of potassium voltage-gated channel Q1(Kcnq1), as well as pleckstrin homology-like domain A2 (Phlda2) and solute carrier family 22 (Slc22a1) gene were not affected by IUGR in any of the brain regions tested (data not shown). Interestingly, out of 7 genes that were tested in the Cdkn1c-Igf2 locus, only the genes shown to be important in differentiation and specification of DA neurons (TH, Cdkn1c, Igf2 (Freed et al., 2008)) were overexpressed in IUGR offspring.
Perinatal exposure to low protein diet alters dopamine-associated behaviors
Altered sucrose preference and locomotor behavior in IUGR offspring
We evaluated the response to a natural reward, sucrose, using a two-bottle, 4% sucrose solution preference test. All mice preferred sucrose over water, but that preference was significantly decreased in IUGR mice (Fig 5a, p=.02). Locomotor activity during the dark period (active phase for mice, n=4 mice/condition) was also assessed every 3 weeks beginning at 14 weeks of age in a cohort of mice. When animals were fed the control diet, there was no difference in locomotor activity (Fig 5b). However, when animals (both controls and IUGR animals) were placed on a HF diet at weaning, the IUGR animals demonstrated a significant hyperactivity (Fig 5c). Oneway repeated measures ANOVA revealed a significant interaction between group and time (F(4,20) = 24.6, p=.0008), with significant main effects for both group and time (F(1,20)=38.0, p <.003, F(4,20)=19.4, p< .003, respectively).
Augmentation of locomotor response and c-fos activation after acute cocaine administration in IUGR offspring
IUGR and control animals (n=10–12/ group) were tested for their locomotor response to acute cocaine administration (20 mg/kg) (Fig 6). There were no differences in locomotor activity in response to the saline injection on d2 or in the 30 min prior to cocaine injection on d3 (Fig 6A). In response to cocaine, both groups of animals had an increase in locomotor activity (significant main effect for time (F(3, 60)=65.6, p<.0001), however, IUGR offspring had a significantly greater response to the cocaine (significant main effect for group; F(1, 60)=4.36, p<.05).
Additionally, expression of the immediate early gene product, Fos (a generic marker of neuronal activation), was quantified in the NAc 2 hrs following saline or cocaine injection. As expected, there were few Fos-ir cells in saline-injected animals (6C and 6D, insets) and both control and IUGR animals showed an increase in Fos-ir cells in the NAc after cocaine injection (20 mg/kg), a response shown previously to be associated with the locomotor response to cocaine (Hooks et al., 1991). Consistent with the exaggerated locomotor response, there was a significant increase in the number of activated cells present in the NAc of IUGR versus control animals (Fig 6B; 176 ± 27 cells 112±14 cells, respectively, p=.03).
DISCUSSION
Adverse prenatal environment has been linked to increased risk of adult neurobehavioral disorders and chronic metabolic diseases. These data highlight significant vulnerability of the dopamine system to early life protein restriction. We utilized maternal protein restriction during pregnancy and lactation, a well characterized rat model for IUGR, which has been used extensively to examine both metabolic and CNS outcomes. Recent studies (Goyal et al., 2009; van Straten et al., 2010; Bol et al., 2009; Chen et al., 2009) combined with our data confirm this as a valid mouse model of IUGR. As the first report of adverse neurobehavioral outcomes in a mouse, we report large increases in TH and DAT mRNA expression, increased number of TH-ir cells in the VTA and increased dopamine in the PFC. These changes in dopamine expression are paralleled by disruptions in DA-dependent behaviors, including hyperactivity and increased locomotor response to cocaine. Additionally, we identified hypomethylation and overexpression of Cdkn1c, which is critical for driving dopamine neuron development and differentiation and which has been linked to IUGR.
Early life exposure to LP diet increased expression of TH in all regions examined, while upregulation of DAT and DARPP-32 was evident in only select brain regions. Importantly, we also observed an increase in TH-ir neurons within the VTA, and an increase in DA within the PFC. Dopamine levels within the striatum were not altered, suggesting that VTA to PFC dopaminergic projections may be uniquely sensitive to this perinatal challenge or that the increased expression of DAT in the NAc (which was not elevated in the PFC) participated in normalizing dopamine levels. These data complement reports of increased whole brain DA levels (Chen et al., 1997; Marichich et al., 1979), TH activity (Marichich et al., 1979), and altered dopamine receptor binding in the striatum in rats from protein-restricted pregnancies (Palmer et al., 2008; Marichich et al., 1979). TH-producing neurons within the PFC are likely non-classical TH-producing interneurons (Asmus et al., 2008) although little is known about these cells, including their final transmitter phenotype. These neurons lack additional downstream enzymes necessary for catecholamine synthesis and these neurons have been termed “dopaergic” (Ugrumov et al., 2004). Interestingly, both classical and non-classical catecholaminergic cell groups responded similarly to the early life protein restriction.
Altered DA tone has significant implications for human health, given the broad range of normal and pathological behaviors that rely on dopamine. Studies in infants have shown that reduced fetal growth represents a consistent risk for ADHD (Hultman et al., 2007; Lahti et al., 2006; Mick et al., 2002). Although the relative importance of dopamine in ADHD pathology has been debated (Gonon, 2009), there is a wealth of data that support a role for dopamine dysfunction in ADHD pathology (van der Kooij and Glennon, 2007; Madras et al., 2005; DiMaio et al., 2003). Overexpression of DAT in the NAc (similar to the increased accumbens DAT expression observed in the IUGR mice) was shown to increase impulsivity and risk taking behavior (Adriani et al., 2009), prominent behavioral components of ADHD, and methylphenidate administration was shown to decrease DAT expression in the striatum (Moll et al., 2001), which may contribute to its therapeutic effectiveness. However, decreases in DAT (and D2/D3) in the left midbrain and accumbens have been linked to measures of inattention in ADHD patients (Volkow et al., 2009), highlighting the difficulty of replicating the totality and heterogeneity of ADHD symptoms in any one animal model. Hyperactivity (Wilson, 2000), a component of ADHD, was also present in the IUGR mice, but was only evident in animals fed a high fat diet. While the explanation for this is currently unknown, consumption of a high fat diet is known to alter dopaminergic activity (Lee et al., 2009). It is possible that the dopaminergic alterations due to IUGR establish an underlying vulnerability to hyperactivity and when coupled with altered dopamine signaling in response to consumption of high fat diet, hyperactivity emerged. The present data suggest that altered DA in response to a suboptimal perinatal environment may contribute to some of the behavioral components of ADHD observed in IUGR infants.
Altered reward processing (Haenlein and Caul, 1987) is also a component of ADHD. IUGR mice demonstrated a decrease in sucrose preference, reflecting a decreased responsiveness to rewarding stimuli. Individual differences in sucrose consumption (high preference versus low preference) positively predict amphetamine self-administration (DeSousa et al., 2000) and correlate with D2 binding in the striatum (Tonissaar et al., 2006). Decreased sucrose preference in response to stress has also been associated with increased dopamine in the hypothalamus, striatum and PFC (Bekris et al., 2005), which is similar to our observation of increased DA in the PFC and increased TH in the hypothalamus. It is important to note, however that dopamine deficient mice have been shown to have an intact sucrose preference (Cannon and Palmiter, 2003), and additional neurotransmitter systems are clearly involved. An extensive literature has documented decreased sucrose preference in response to chronic mild stress, commonly interpreted as an animal model of “anhedonia”. Accordingly, this decrease can be ameliorated by antidepressant treatment (i.e., broad acting tricyclics such as imiprimine (Bekris et al., 2005), as well as selective serotonin reuptake inhibitors like citalopram (Rygula et al., 2006)). In addition to dopamine, decreased activity of serotonin in the PFC has also been linked to reductions in sucrose preference (Bekris et al., 2005) and citalopram can reverse both the behavior and the neurochemical changes (Rygula et al., 2006). Of note, however, citalopram also decreases striatal dopamine and increases D2 binding (Dewey et al., 1995), indicating that an interaction between serotonin and dopamine systems is likely to be important in mediating sucrose preference. Activation of the reward system by drugs of abuse (Martinez et al., 2007; Ron and Jurd, 2005), coupled with underactivation of reward circuitry in response to natural rewards (i.e., sucrose) may act synergistically in the development of addiction (Koob and Le Moal, 2008). These two behaviors were noted in IUGR animals (exaggerated response to cocaine and decreased sucrose preference), suggesting that the IUGR animals may represent an animal model of increased addiction risk as a result of suboptimal prenatal environment. These findings are consistent with other observations in the literature linking malnutrition and addictive behavior in rodents (Palmer et al., 2008; Valdomero et al., 2007; Valdomero et al., 2006; Shultz et al., 1999) and humans (Franzek et al., 2008).
In an effort to define potential mechanisms driving the observed gene expression changes, we investigated the methylation status of specific target genes. A recent report examined genome-scale methylation status of CpG islands in the liver in response to maternal malnutrition (van Straten et al., 2010), and found that less than 0.5% of CpG islands were differentially methylated. Further, they observed equal amounts of hyper- and hypomethylation, suggesting that limited substrate (e.g., methyl donors) due to the low protein diet was not likely to be responsible for the differential methylation patterns. Further, CpG islands within promoter regions are known to demonstrate varying degrees of sensitivity to aberrant methylation (Feltus et al., 2003), and here we report that TH, DAT and Igf2 are not susceptible to differential methylation, but Cdkn1c is vulnerable to differential methylation in response to early life protein restriction. Hypomethylation and subsequent overexpression of Cdkn1c in IUGR animals is notable for three primary reasons: (1) Cdkn1c is involved in DA neuron differentiation, (2) neural overexpression of Cdkn1c results in growth retardation (Andrews et al., 2007) and (3) as an imprinted locus, this region may be more susceptible to epigenetic dysregulation as a result of suboptimal prenatal conditions. Cdkn1c is important for neuronal migration and coordination of the timing of progenitor cell cycle exit in developing mouse neocortex (Ye et al., 2009; Itoh et al., 2007) and most likely partners with Nurr1 to promote terminal differentiation of DA neurons (Joseph et al., 2003; Freed et al., 2008). Interestingly, Igf2, overexpressed in the PFC and hypothalamus of IUGR mice, has also been shown to play a role in DA neuron development (Vazin et al., 2009). Counts of TH-ir neurons were made within the VTA, as this is the primary source of dopaminergic innervation of the PFC where an increase in DA was detected, even though we did not detect an increase in Cdkn1c in this region. It is possible that elevations in Cdkn1c were not detected using qRT-PCR in a mixed (VTA/SN) dissection or it is possible that Cdkn1c levels were elevated at an earlier timepoint in development. At this time, it is premature to define Cdkn1c overexpression as causative in the dopaminergic changes observed in the IUGR animals. However, given its dysregulation in this model of IUGR, the importance of Cdkn1c to dopamine neuron development, and the link between neuronal overexpression of Cdkn1c and embryonic growth retardation, our data highlight Cdkn1c as an important molecule for future research within this context. Only a subset of genes in Cdkn1c-Kcnq1 locus was differentially expressed in IUGR animals, which implies that the methylation status of IC1 (see Fig 4a), normally inherited from gametes, is not responsible for observed gene expression changes. Instead the Cdkn1c promoter is hypomethylated in IUGR offspring, which is established during the post-implantation period (Bhogal et al., 2004), identifying this as a vulnerable timeframe for long-lasting epigenetic dysregulation of Cdkn1c gene.
In these studies, maternal exposure to the low protein diet extended through the entirety of breeding, pregnancy and lactation. At birth, rodents are considered more “immature” than humans, with significant brain development occurring postnatally (e.g., postnatal days 3–10 are thought to mirror the third trimester of a human pregnancy (Livy et al., 2003) with precise pairing of developmental time periods dependent upon brain region (Clancy et al., 2007). Therefore, to model the entirety of human gestation, we chose to maintain the low protein diet through pregnancy and lactation. A number of factors may participate in driving the observed neurodevelopmental phenotype, including the prenatal protein restriction of the pup or the maternal endocrine response (Langley-Evans et al., 1996). Postnatal maternal behavior has been shown to modify the methylation status of the glucocorticoid receptor (Weaver et al., 2004) and estrogen receptor (Champagne et al., 2006), and differences in maternal care between the groups are possible (i.e., while the direct effect of protein malnutrition on the maternal behavior has not been reported, protein-deprived rat pups nursed by control dams experienced an increase in active nursing and grooming (Massaro et al., 1974)). Defining the causative factor(s) for the observed phenotypes remains an area of active research. As these experiments represented an initial evaluation of maternal low protein diet on neurobehavioral outcomes in the offspring, experiments were necessarily limited with regard to age of assessment (adults) and sex (males). Future work can now be directed at defining other key variables. For example, an examination of the critical period for exposure to low protein diet (e.g., early versus late gestation, inclusion or exclusion of lactation) will provide important information on specific windows of vulnerability. Further, future studies may extend the analyses to female offspring, as certain developmental programming effects have been shown to be sex-specific (Skinner et al., 2008) or other assessment timepoints that represent particularly vulnerable ages (e.g., adolescence).
In conclusion, early life protein deficiency results in a mouse model of IUGR with persistent alterations in dopamine circuitry and dopamine-dependent behaviors. Additionally, we have identified hypomethylation and overexpression of Cdkn1c, a molecule known to have a causative role in growth retardation and to play a critical role in dopamine neuron development. This mouse model of IUGR may represent a novel animal model for ADHD, as well as other disorders linked to both IUGR and dopamine dysfunction (schizophrenia and addiction), with both face validity (behaviors) and construct validity (IUGR-induced dopaminergic dysregulation), which would provide an additional tool to explore questions of mechanism and potential therapeutics.
Supplementary Material
Acknowledgements
The current work was supported by NIH DK064086 (Reyes) and MH087978 (Reyes).
Abbreviations
- CNS
central nervous system
- CDKN1C
cyclin-dependent kinase inhibitor 1C
- DA
dopamine
- DAT
dopamine reuptake transporter
- Hyp
hypothalamus
- IUGR
intrauterine growth retardation
- LP
low protein
- NAc
nucleus accumbens
- PFC
prefrontal cortex
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE/CONFLICTS OF INTEREST
Z Vucetic, K Totoki, H. Schoch, KW Whitaker, T. E. Hill-Smith, and TM Reyes declare no conflicts of interest, either financial or otherwise. Irwin Lucki has been on the Scientific Advisory Board for Wyeth and has received research support from AstraZeneca, Wyeth, Forest and Epix pharmaceutical companies during the past 3 years.
Contributor Information
Zivjena Vucetic, Department of Pharmacology, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA.
Kathy Totoki, Department of Pharmacology, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA.
Hannah Schoch, Department of Pharmacology, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA.
Keith W. Whitaker, Department of Biochemistry, Scripps Florida, Jupiter, FL 33458, USA
Tiffany Hill-Smith, Department of Psychiatry, Institute for Translational Medicine and Therapeutics, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA.
Irwin Lucki, Department of Psychiatry, Institute for Translational Medicine and Therapeutics, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA.
Teresa M. Reyes, Department of Pharmacology, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA.
REFERENCES
- Adriani W, Boyer F, Gioiosa L, Macri S, Dreyer JL, Laviola G. Increased impulsive behavior and risk proneness following lentivirus-mediated dopamine transporter over-expression in rats' nucleus accumbens. Neuroscience. 2009;159:47–58. doi: 10.1016/j.neuroscience.2008.11.042. [DOI] [PubMed] [Google Scholar]
- Andrews SC, Wood MD, Tunster SJ, Barton SC, Surani MA, John RM. Cdkn1c (p57Kip2) is the major regulator of embryonic growth within its imprinted domain on mouse distal chromosome 7. BMC Dev Biol. 2007;7:53. doi: 10.1186/1471-213X-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmus SE, Anderson EK, Ball MW, Barnes BA, Bohnen AM, Brown AM, Hartley LJ, Lally MC, Lundblad TM, Martin JB, Moss BD, Phelps KD, Phillips LR, Quilligan CG, Steed RB, Terrell SL, Warner AE. Neurochemical characterization of tyrosine hydroxylase-immunoreactive interneurons in the developing rat cerebral cortex. Brain Res. 2008;1222:95–105. doi: 10.1016/j.brainres.2008.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res. 2005;161:45–59. doi: 10.1016/j.bbr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Bhogal B, Arnaudo A, Dymkowski A, Best A, Davis TL. Methylation at mouse Cdkn1c is acquired during postimplantation development and functions to maintain imprinted expression. Genomics. 2004;84:961–970. doi: 10.1016/j.ygeno.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Bjursell M, Gerdin AK, Lelliott CJ, Egecioglu E, Elmgren A, Tornell J, Oscarsson J, Bohlooly-Y M. Acutely reduced locomotor activity is a major contributor to Western diet-induced obesity in mice. Am J Physiol Endocrinol Metab. 2008;294:E251–E260. doi: 10.1152/ajpendo.00401.2007. [DOI] [PubMed] [Google Scholar]
- Bol VV, Delattre AI, Reusens B, Raes M, Remacle C. Forced catch-up growth after fetal protein restriction alters the adipose tissue gene expression program leading to obesity in adult mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R291–R299. doi: 10.1152/ajpregu.90497.2008. [DOI] [PubMed] [Google Scholar]
- Brown AS, Susser ES, Lin SP, Neugebauer R, Gorman JM. Increased risk of affective disorders in males after second trimester prenatal exposure to the Dutch hunger winter of 1944–45. Br J Psychiatry. 1995;166:601–606. doi: 10.1192/bjp.166.5.601. [DOI] [PubMed] [Google Scholar]
- Brown AS, van Os J, Driessens C, Hoek HW, Susser ES. Further evidence of relation between prenatal famine and major affective disorder. Am J Psychiatry. 2000;157:190–195. doi: 10.1176/appi.ajp.157.2.190. [DOI] [PubMed] [Google Scholar]
- Cannon CM, Palmiter RD. Reward without dopamine. J Neurosci. 2003;23:10827–10831. doi: 10.1523/JNEUROSCI.23-34-10827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Chen JC, Turiak G, Galler J, Volicer L. Postnatal changes of brain monoamine levels in prenatally malnourished and control rats. Int J Dev Neurosci. 1997;15:257–263. doi: 10.1016/s0736-5748(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Chen JH, Martin-Gronert MS, Tarry-Adkins J, Ozanne SE. Maternal protein restriction affects postnatal growth and the expression of key proteins involved in lifespan regulation in mice. PLoS One. 2009;4:e4950. doi: 10.1371/journal.pone.0004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Davies W, Isles AR, Wilkinson LS. Imprinted gene expression in the brain. Neurosci Biobehav Rev. 2005;29:421–430. doi: 10.1016/j.neubiorev.2004.11.007. [DOI] [PubMed] [Google Scholar]
- de Rooij SR, Painter RC, Holleman F, Bossuyt PM, Roseboom TJ. The metabolic syndrome in adults prenatally exposed to the Dutch famine. Am J Clin Nutr. 2007;86:1219–1224. doi: 10.1093/ajcn/86.4.1219. [DOI] [PubMed] [Google Scholar]
- DeSousa NJ, Bush DE, Vaccarino FJ. Self-administration of intravenous amphetamine is predicted by individual differences in sucrose feeding in rats. Psychopharmacology (Berl) 2000;148:52–58. doi: 10.1007/s002130050024. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Alexoff D, Ding YS, King P, Pappas N, Brodie JD, Ashby CR., Jr Serotonergic modulation of striatal dopamine measured with positron emission tomography (PET) and in vivo microdialysis. J Neurosci. 1995;15:821–829. doi: 10.1523/JNEUROSCI.15-01-00821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio S, Grizenko N, Joober R. Dopamine genes and attention-deficit hyperactivity disorder: a review. J Psychiatry Neurosci. 2003;28:27–38. [PMC free article] [PubMed] [Google Scholar]
- Feltus FA, Lee EK, Costello JF, Plass C, Vertino PM. Predicting aberrant CpG island methylation. Proc Natl Acad Sci U S A. 2003;100:12253–12258. doi: 10.1073/pnas.2037852100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzek EJ, Sprangers N, Janssens AC, Van Duijn CM, Van De Wetering BJ. Prenatal exposure to the 1944–45 Dutch 'hunger winter' and addiction later in life. Addiction. 2008;103:433–438. doi: 10.1111/j.1360-0443.2007.02084.x. [DOI] [PubMed] [Google Scholar]
- Freed WJ, Chen J, Backman CM, Schwartz CM, Vazin T, Cai J, Spivak CE, Lupica CR, Rao MS, Zeng X. Gene expression profile of neuronal progenitor cells derived from hESCs: activation of chromosome 11p15.5 and comparison to human dopaminergic neurons. PLoS ONE. 2008;3:e1422. doi: 10.1371/journal.pone.0001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int J Obes (Lond) 2008;32 Suppl 7:S62–S71. doi: 10.1038/ijo.2008.240. [DOI] [PubMed] [Google Scholar]
- Gonon F. The dopaminergic hypothesis of attention-deficit/hyperactivity disorder needs re-examining. Trends Neurosci. 2009;32:2–8. doi: 10.1016/j.tins.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Goyal R, Goyal D, Leitzke A, Gheorghe CP, Longo LD. Brain Renin-Angiotensin System: Fetal Epigenetic Programming by Maternal Protein. Restriction During Pregnancy Reprod Sci. 2009 doi: 10.1177/1933719109351935. [DOI] [PubMed] [Google Scholar]
- Haenlein M, Caul WF. Attention deficit disorder with hyperactivity: a specific hypothesis of reward dysfunction. J Am Acad Child Adolesc Psychiatry. 1987;26:356–362. doi: 10.1097/00004583-198705000-00014. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991;9:121–128. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Hultman CM, Torrang A, Tuvblad C, Cnattingius S, Larsson JO, Lichtenstein P. Birth weight and attention-deficit/hyperactivity symptoms in childhood and early adolescence: a prospective Swedish twin study. J Am Acad Child Adolesc Psychiatry. 2007;46:370–377. doi: 10.1097/01.chi.0000246059.62706.22. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Masuyama N, Nakayama K, Nakayama KI, Gotoh Y. The cyclin-dependent kinase inhibitors p57 and p27 regulate neuronal migration in the developing mouse neocortex. J Biol Chem. 2007;282:390–396. doi: 10.1074/jbc.M609944200. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B, Wallen-Mackenzie A, Benoit G, Murata T, Joodmardi E, Okret S, Perlmann T. p57(Kip2) cooperates with Nurr1 in developing dopamine cells. Proc Natl Acad Sci U S A. 2003;100:15619–15624. doi: 10.1073/pnas.2635658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Miller DJ, Ursell E, Wild AE, Wilkins AP, Osmond C, Anthony FW, Fleming TP. Imprinted gene expression in the rat embryo-fetal axis is altered in response to periconceptional maternal low protein diet. Reproduction. 2006;132:265–277. doi: 10.1530/rep.1.01038. [DOI] [PubMed] [Google Scholar]
- Lahti J, Raikkonen K, Kajantie E, Heinonen K, Pesonen AK, Jarvenpaa AL, Strandberg T. Small body size at birth and behavioural symptoms of ADHD in children aged five to six years. J Child Psychol Psychiatry. 2006;47:1167–1174. doi: 10.1111/j.1469-7610.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Phillips GJ, Benediktsson R, Gardner DS, Edwards CR, Jackson AA, Seckl JR. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 1996;17:169–172. doi: 10.1016/s0143-4004(96)80010-5. [DOI] [PubMed] [Google Scholar]
- Lee AK, Mojtahed-Jaberi M, Kyriakou T, Aldecoa-Otalora Astarloa E, Arno M, Marshall NJ, Brain SD, O'Dell SD. Effect of high-fat feeding on expression of genes controlling availability of dopamine in mouse hypothalamus. Nutrition. 2009 doi: 10.1016/j.nut.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Madras BK, Miller GM, Fischman AJ. The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1397–1409. doi: 10.1016/j.biopsych.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Marichich ES, Molina VA, Orsingher OA. Persistent changes in central catecholaminergic system after recovery of perinatally undernourished rats. J Nutr. 1979;109:1045–1050. doi: 10.1093/jn/109.6.1045. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Massaro TF, Levitsky DA, Barnes RH. Protein malnutrition in the rat: its effects on maternal behavior and pup development. Dev Psychobiol. 1974;7:551–561. doi: 10.1002/dev.420070607. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Dalvi A, Page ME, Zimov-Levinson S, Hen R, Lucki I. Antidepressant-like behavioral effects in 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) receptor mutant mice. J Pharmacol Exp Ther. 2001;298:1101–1107. [PubMed] [Google Scholar]
- McArthur S, McHale E, Gillies GE. The size and distribution of midbrain dopaminergic populations are permanently altered by perinatal glucocorticoid exposure in a sex-region-and time-specific manner. Neuropsychopharmacology. 2007;32:1462–1476. doi: 10.1038/sj.npp.1301277. [DOI] [PubMed] [Google Scholar]
- Mick E, Biederman J, Prince J, Fischer MJ, Faraone SV. Impact of low birth weight on attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2002;23:16–22. doi: 10.1097/00004703-200202000-00004. [DOI] [PubMed] [Google Scholar]
- Moll GH, Hause S, Ruther E, Rothenberger A, Huether G. Early methylphenidate administration to young rats causes a persistent reduction in the density of striatal dopamine transporters. J Child Adolesc Psychopharmacol. 2001;11:15–24. doi: 10.1089/104454601750143366. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Neugebauer R, Hoek HW, Susser E. Prenatal exposure to wartime famine and development of antisocial personality disorder in early adulthood. JAMA. 1999;282:455–462. doi: 10.1001/jama.282.5.455. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Brown AS, Keegan D, Siska LD, Susser E, Rotrosen J, Butler PD. Prenatal protein deprivation alters dopamine-mediated behaviors and dopaminergic and glutamatergic receptor binding. Brain Res. 2008;1237:62–74. doi: 10.1016/j.brainres.2008.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SC, Rose A, Rao M, Gallego J, Gressens P, Mani S. Different types of nutritional deficiencies affect different domains of spatial memory function checked in a radial arm maze. Neuroscience. 2008;152:859–866. doi: 10.1016/j.neuroscience.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci. 2003;23:5607–5616. doi: 10.1523/JNEUROSCI.23-13-05607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Jurd R. The "ups and downs" of signaling cascades in addiction. Sci STKE. 2005;2005:re14. doi: 10.1126/stke.3092005re14. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Hiemke C, Fuchs E, Ruther E, Havemann-Reinecke U. Citalopram counteracts depressive-like symptoms evoked by chronic social stress in rats. Behav Pharmacol. 2006;17:19–29. doi: 10.1097/01.fbp.0000186631.53851.71. [DOI] [PubMed] [Google Scholar]
- Shultz PL, Galler JR, Tonkiss J. Prenatal protein restriction increases sensitization to cocaine-induced stereotypy. Behav Pharmacol. 1999;10:379–387. doi: 10.1097/00008877-199907000-00005. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One. 2008;3:e3745. doi: 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Choufani S, Ferreira JC, Weksberg R. Growth regulation, imprinted genes, and chromosome 11p15.5. Pediatr Res. 2007;61:43R–47R. doi: 10.1203/pdr.0b013e3180457660. [DOI] [PubMed] [Google Scholar]
- Son GH, Chung S, Geum D, Kang SS, Choi WS, Kim K, Choi S. Hyperactivity and alteration of the midbrain dopaminergic system in maternally stressed male mice offspring. Biochem Biophys Res Commun. 2007;352:823–829. doi: 10.1016/j.bbrc.2006.11.104. [DOI] [PubMed] [Google Scholar]
- St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, Zheng X, Gu N, Feng G, Sham P, He L. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA. 2005;294:557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Levitt P. Prenatal exposure to cocaine produces unique developmental and long-term adaptive changes in dopamine D1 receptor activity and subcellular distribution. J Neurosci. 2007;27:152–157. doi: 10.1523/JNEUROSCI.4591-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser E, St Clair D, He L. Latent effects of prenatal malnutrition on adult health: the example of schizophrenia. Ann N Y Acad Sci. 2008;1136:185–192. doi: 10.1196/annals.1425.024. [DOI] [PubMed] [Google Scholar]
- Szyf M. The early life environment and the epigenome. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbagen.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Tonissaar M, Herm L, Rinken A, Harro J. Individual differences in sucrose intake and preference in the rat: circadian variation and association with dopamine D2 receptor function in striatum and nucleus accumbens. Neurosci Lett. 2006;403:119–124. doi: 10.1016/j.neulet.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Ugrumov MV, Melnikova VI, Lavrentyeva AV, Kudrin VS, Rayevsky KS. Dopamine synthesis by non-dopaminergic neurons expressing individual complementary enzymes of the dopamine synthetic pathway in the arcuate nucleus of fetal rats. Neuroscience. 2004;124:629–635. doi: 10.1016/j.neuroscience.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Valdomero A, Bussolino DF, Orsingher OA, Cuadra GR. Perinatal protein malnutrition enhances rewarding cocaine properties in adult rats. Neuroscience. 2006;137:221–229. doi: 10.1016/j.neuroscience.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Valdomero A, Isoardi NA, Orsingher OA, Cuadra GR. Pharmacological reactivity to cocaine in adult rats undernourished at perinatal age: behavioral and neurochemical correlates. Neuropharmacology. 2005;48:538–546. doi: 10.1016/j.neuropharm.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Valdomero A, Velazquez EE, de Olmos S, de Olmos JS, Orsingher OA, Cuadra GR. Increased rewarding properties of morphine in perinatally protein-malnourished rats. Neuroscience. 2007;150:449–458. doi: 10.1016/j.neuroscience.2007.09.006. [DOI] [PubMed] [Google Scholar]
- van der Kooij MA, Glennon JC. Animal models concerning the role of dopamine in attention-deficit hyperactivity disorder. Neurosci Biobehav Rev. 2007;31:597–618. doi: 10.1016/j.neubiorev.2006.12.002. [DOI] [PubMed] [Google Scholar]
- van Straten EM, Bloks VW, Huijkman NC, Baller JF, Meer H, Lutjohann D, Kuipers F, Plosch T. The liver X-receptor gene promoter is hypermethylated in a mouse model of prenatal protein restriction. Am J Physiol Regul Integr Comp Physiol. 2010;298:R275–R282. doi: 10.1152/ajpregu.00413.2009. [DOI] [PubMed] [Google Scholar]
- Vazin T, Becker KG, Chen J, Spivak CE, Lupica CR, Zhang Y, Worden L, Freed WJ. A novel combination of factors, termed SPIE, which promotes dopaminergic neuron differentiation from human embryonic stem cells. PLoS One. 2009;4:e6606. doi: 10.1371/journal.pone.0006606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yan JY, Lo YK, Carvey PM, Ling Z. Dopaminergic and serotoninergic deficiencies in young adult rats prenatally exposed to the bacterial lipopolysaccharide. Brain Res. 2009;1265:196–204. doi: 10.1016/j.brainres.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Waterland RA, Lin JR, Smith CA, Jirtle RL. Post-weaning diet affects genomic imprinting at the insulin-like growth factor 2 (Igf2) locus. Hum Mol Genet. 2006;15:705–716. doi: 10.1093/hmg/ddi484. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weber M, Davies JJ, Wittig D, Oakeley EJ, Haase M, Lam WL, Schubeler D. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Genet. 2005;37:853–862. doi: 10.1038/ng1598. [DOI] [PubMed] [Google Scholar]
- Wilson MC. Coloboma mouse mutant as an animal model of hyperkinesis and attention deficit hyperactivity disorder. Neurosci Biobehav Rev. 2000;24:51–57. doi: 10.1016/s0149-7634(99)00064-0. [DOI] [PubMed] [Google Scholar]
- Ye W, Mairet-Coello G, Pasoreck E, Dicicco-Bloom E. Patterns of p57Kip2 expression in embryonic rat brain suggest roles in progenitor cell cycle exit and neuronal differentiation. Dev Neurobiol. 2009;69:1–21. doi: 10.1002/dneu.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. Epigenetic mechanisms for nutrition determinants of later health outcomes. Am J Clin Nutr. 2009;89:1488S–1493S. doi: 10.3945/ajcn.2009.27113B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Zhang Z, Zhu Y, Chen L, Sokabe M, Chen L. Deficits in development of synaptic plasticity in rat dorsal striatum following prenatal and neonatal exposure to low-dose bisphenol A. Neuroscience. 2009;159:161–171. doi: 10.1016/j.neuroscience.2008.12.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.