Abstract
Objective
In order to evaluate the potential use of bupropion as smoking cessation therapy during pregnancy, the aim of this investigation was to determine transplacental transfer and metabolism of bupropion and its distribution among placental tissue and maternal and fetal circuits of the dually perfused placental lobule.
Methods
Placentas obtained from healthy term pregnancies were perfused with bupropion at two concentrations 150 ng/mL and 450 ng/mL, along with the marker compound antipyrine 20 μg/mL. Radioactive isotopes of the two drugs were co-transfused to enhance their detection limits. Concentrations of bupropion and its metabolite were determined by liquid chromatography and liquid scintillation spectrometry.
Results
The fetal/maternal concentration ratio of bupropion was 1.07 ± 0.22. Following 4 hours of its perfusion, 48 ± 6% of bupropion was retained by placental tissue, 32 ± 5% remained in the maternal circuit, and 20 ± 6% was transferred to the fetal circuit. A metabolite of bupropion, threohydrobupropion, was identified.
Conclusions
Bupropion was transferred from the maternal to fetal circuit and was biotransformed by placental tissue enzymes to its metabolite threohydrobupropion. Bupropion and its metabolite did not affect placental tissue viability or functional parameters. These data suggest that bupropion has the potential of being used for smoking cessation during pregnancy and should be further investigated for its safety and efficacy.
Keywords: Bupropion, Transplacental Transfer, Metabolism, Smoking Cessation, Pregnant Smokers
Introduction
The prevalence of smoking among pregnant women in the United States varies markedly, 10 – 30%, depending on data source and maternal characteristics of the study population [1-4]. Smoking during pregnancy is associated with spontaneous abortion, abruption, intrauterine growth restriction, preterm delivery, neonatal mortality, stillbirth and sudden infant death syndrome [5-6]. However, it is the largest modifiable risk factor for pregnancy-related morbidity and mortality. Although the rate of smoking cessation is high due to greater motivation in pregnancy and non-pharmacologic interventions, a significant number of pregnant women, 20 – 30%, fail to achieve this goal and could benefit from the use of pharmacotherapy [1,7-9].
Pharmacotherapeutic agents successfully used for smoking cessation in non-pregnant women include Nicotine Replacement Therapies (NRTs), bupropion, and more recently, varenicline. However, none of the above medications are routinely recommended for pregnant women. The use of NRTs during pregnancy is limited due to concerns about the inherent risk of nicotine to the developing fetus. In addition, the maternal physiological changes that occur with the onset of pregnancy may change the pharmacokinetic parameters of the drug and consequently, its effectiveness reported for non-pregnant women. Previous reports showed that the use of the 15 mg nicotine patch did not achieve the effect observed in non-pregnant patients because of increased in hepatic blood flow and activity of the nicotine biotransforming enzyme CYP2A6 during pregnancy [7,10]. In a recent randomized control trial using 2 mg nicotine gum for the pregnant smoker, there was no increase in smoking cessation rates [11].
Bupropion is a non-nicotinic drug used for smoking cessation. The effectiveness of bupropion for smoking cessation during pregnancy was demonstrated in a prospective, matched, controlled observational study [12]. However, due to the limited available data on the adverse effects of bupropion to the fetus as well as its pharmacokinetics during pregnancy, it is a Class B drug and is not currently approved by the FDA for smoking cessation in pregnant women. Therefore, the potential use of bupropion for treatment of the pregnant woman seeking smoking cessation requires additional preclinical and clinical information on its effectiveness and safety during pregnancy. The first step to obtain the above mentioned information is to determine the role of human placenta in the disposition of bupropion and, consequently, its concentration in the fetal circulation.
In general, placental disposition of a drug determines its concentration in the fetal circulation and is influenced by transfer of the drug across the placenta; its distribution in placental tissue; elimination by placental and fetal metabolic enzymes; and drug excretion and efflux from the feto-placental unit back to the maternal circulation.
The low molecular weight of bupropion and its physicochemical properties indicate that it should be transferred across human placenta from the maternal to fetal circulation by simple diffusion if other factors are not involved. Therefore, intrauterine exposure of the fetus to bupropion should not be ruled out. A therapeutic agent administered during pregnancy could affect normal fetal growth and development either directly, as a result of its concentration in the fetal circulation, or indirectly by affecting the physiologic functions of the placenta. Thus, it is imperative to determine the extent of transplacental transfer of the drug, its retention by the tissue and effects on placental functions. These parameters are best determined in vitro by using the technique of dual perfusion of placental lobule (DPPL). This technique retains the anatomic and functional integrity of placental tissue and has been validated in determining the bidirectional transfer, distribution, and metabolism of numerous drugs and the information obtained can be extrapolated to in vivo conditions at term.
Therefore, the aim of this investigation was to determine placental transfer and metabolism of bupropion; its distribution between the tissue, maternal and fetal circuits of the term DPPL; and the effects of the drug on placental viability and functional parameters.
Methods
Chemicals
[3H]-bupropion (specific activity, 4 Ci/mmol) was custom-synthesized by PerkinElmer Life and Analytical Sciences Custom Synthesis Group. Hydroxybupropion, erythrohydrobupropion, and threohydrobupropion were purchased from Toronto Research Chemicals, Inc (Toronto, Canada). HPLC grade methanol, phosphoric acid and hydrochloric acid were purchased from Fisher Scientific (New Jersey). All other chemicals including radioactive [14C]-antipyrine (specific activity, 6.5 mCi/mmol), bupropion hydrochloride, heptane and isoamyl alcohol were purchased from Sigma-Aldrich (St. Louis, Missouri).
Clinical Material
Placentas from uncomplicated term (37 – 42 weeks) pregnancies (n =17) were obtained immediately after vaginal or abdominal deliveries from the Labor and Delivery Ward of the University of Texas Medical Branch, Galveston, Texas, according to a protocol approved by the Institutional Review Board. Any evidence of maternal infection, systemic disease, and drug or alcohol abuse during pregnancy excluded the placenta from this investigation.
Dual Perfusion of Term Human Placental Lobule
The technique of DPPL was used as previously described by Miller et al. and an earlier report from our laboratory [13-14]. Briefly, each placenta was examined for tears, and 2 chorionic vessels (one artery and vein) supplying a single intact peripheral cotyledon were cannulated with 3F and 5F umbilical catheters, respectively. The cotyledon was trimmed and placed in the perfusion chamber with the maternal surface upward. The intervillous space on the maternal side was perfused by 2 catheters piercing the basal plate. The flow rate of the perfusate medium in the fetal and maternal circuits was 2.8 and 12 mL/min, respectively. The perfusion medium was made of tissue culture medium M199 (Sigma, St. Louis, MO) supplemented with: Dextran 40 (7.5 g/L in the maternal and 30 g/L in the fetal reservoir), 25 IU/mL heparin, 40 mg/L gentamicin sulfate, 80 mg/L sulfamethoxazole, and 16 mg/L trimethoprim. The maternal perfusate was equilibrated with a gas mixture made of 95% O2, 5% CO2, and the fetal perfusate with a mixture of 95% N2, 5% CO2. Sodium bicarbonate was added to the maternal and fetal circuits to maintain the pH at 7.4 and 7.35, respectively. All experiments were carried out at a temperature of 37 °C.
Each placenta was perfused for an initial control period of one hour. The control period allowed the tissue to stabilize to its new environment and to determine the baseline levels for the viability and functional parameters. Perfusion was terminated if one of the following occurred: fetal arterial pressure exceeding 50 mm Hg, a volume loss in fetal circuit in excess of 2 mL/h, or a pO2 difference between fetal vein and artery less than 60 mmHg, indicating inadequate perfusion overlap between the two circuits.
Binding of bupropion to components of the perfusion medium, glassware and tubing
The binding of [3H]-bupropion to human serum albumin (HSA), added to the perfusion medium, was determined using gel filtration (desalting columns, Sephadex G25M, PD-10). The void and total volumes of the column were approximately 4.0 and 9.0 mL, respectively. Each placenta was perfused with the drug in the presence of HSA (30 mg/ml) for a period of 4 hours. Aliquots (2 mL) from the perfusion medium of the maternal and fetal reservoirs were loaded on the gel filtration column, and eluted with potassium phosphate buffer. The eluent was collected in fractions of 1 ml, and the content of [3H]-bupropion in each fraction was determined using a liquid scintillation spectrometer. The bupropion bound to HSA was eluted in the void volume of the column, while the free drug was eluted in the total volume.
Binding of [3H]-bupropion to glassware, teflon and tygon type of tubing utilized in the perfusion system was determined by re-circulating drug in the model system in the absence of a placenta. At the end of 4 hours of perfusion, the initial amount of [3H]-bupropion in the medium declined by 40%. Binding to glassware and teflon tubing was negligible. Therefore, the observed decline in the amount of [3H]-bupropion was due to its binding to the tygon type of tubing used in the peristaltic pumps. However, after addition of 30 mg/mL of HSA to the perfusion medium, the binding of [3H]-bupropion to tygon tubing was reduced by 25%.
Transplacental transfer and distribution of bupropion
The control period, which was the initial period of 1 hour, was followed by the experimental period of 4 hours. The latter was initiated by replacing the medium of the maternal and fetal reservoirs and the addition of HSA in its in vivo concentration at near term of 30 mg/ml, [15]. Bupropion 150 ng/ml or 450 ng/ml together with 1.5 μCi of its [3H]-isotope was added to the maternal reservoir. The concentrations of bupropion selected for this investigation were based on the range of the concentrations determined in the plasma of patients under treatment with 150 mg of bupropion [16]. The non-ionizable, lipophilic marker compound antipyrine (AP) 20 μg/mL and its [14C]-isotope (1.5μCi) were co-transfused with bupropion to account for interplacental variations and to normalize the transfer of bupropion. The perfusion system was used in its closed-closed configuration (re-circulation of the medium).
Samples from the maternal artery and fetal vein, in 0.5 mL aliquots, were taken at 0, 5, 10, 15, 20, 30, 40, 50, 60, 90, 120, 150, 180, 210 and 240 minutes and the amount of radioactivity in both maternal and fetal perfusate were determined in each experiment by liquid scintillation spectrometry that used both the [3H] and [14C]-channels simultaneously (1900TR; Packard Instruments, Inc, Shelton, CT). The concentration of bupropion in all samples was calculated after correcting for the specific activity as previously reported [14].
At the end of experiment, the perfused area was dissected from the adjoining placental tissue, weighed, and homogenized in a volume of saline equal to four times its weight. 1 mL of 1M NaOH was added to 1 mL of the homogenate and the samples were incubated for 12 hours at 60 °C in the dark to allow for luminescence decay. Scintillation cocktail was added to each sample and the concentration of each drug was determined.
The effect of bupropion on placental tissue viability and functional parameters
The adverse effects of 150 ng/ml and 450 ng/ml of bupropion on the perfused placental tissue were evaluated by determining its functional (human chorionic gonadotropin [hCG] release) and viability parameters (oxygen delivery, transfer and consumption) [14]. Samples (250 μl) were collected from the maternal perfusate every 30 minutes during each control and experimental periods, centrifuged at 1000g for 10 minutes at 4 °C, and the supernatant stored at -70 °C until the concentrations of hCG were determined by an IRMA kit (Diagnostics Production Corp., Los Angeles, CA).
In vitro hCG release from explant cultures of different placentas covers a wide concentration range and reflects the variability in maternal serum levels of the hormone between individuals during pregnancy [17]. Therefore, the amounts of hCG released during the control period were set at 100% and those during the experimental period as a percentage of the control.
Oxygen delivery, transfer and consumption were used as indicators of placental viability. Values determined for the initial control period represented baseline levels and were compared with those obtained during the following experimental period of 4 hours during which bupropion was transfused. Samples (100 μL) were collected every 15 minutes from both circuits and immediately analyzed for pH, pO2, and pCO2 by using a blood gas analyzer (Instrumentation Laboratory, Lexington, MA).
The effect of 4 hours of perfusion on placental tissue viability and functional parameters was determined in a separate set of control experiments. Placentas (n=6) were perfused with the medium devoid of bupropion. Samples from maternal and fetal circuits were taken and analyzed as described above.
Metabolism of bupropion
The three major metabolites of bupropion: hydroxyburpropion, erythrohydrobupropion and threohydrobupropion, if formed, retain the [3H]-label present in the parent compound (Figure 1). The biotransformation of bupropion during its perfusion was investigated. At the end of the 4 hour experimental period, 20 ml of medium were collected from the maternal and fetal reservoirs. Isoamyl alcohol in heptane (1.5%) was added to the medium and shaken vigorously for 10 minutes. The extraction was repeated and organic layers of both were combined and evaporated to 1 ml under a stream of nitrogen. The organic phase was then transferred to a centrifuge tube containing 250 μl 0.1 M HCl and vortexed for 1 min. The organic layer was discarded and the aqueous layer was dried at 35°C under a stream of nitrogen. The dry residue was reconstituted in 100 μl of mobile phase and filtrated through a 0.45 μm membrane filter. A volume of 50 μl of each sample was analyzed by HPLC.
Figure 1.
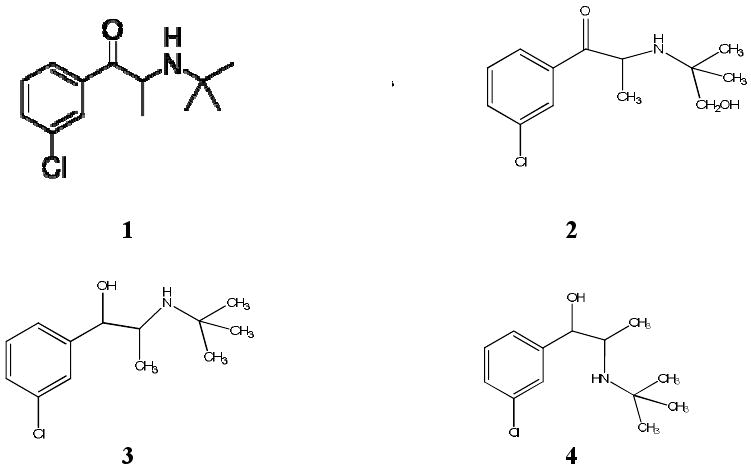
Structure of bupropion and its metabolites. (1) Bupropion; (2) hydroxybupropion; (3) erythrohydrobupropion; (4) threohydrobupropion. Bupropion is biotransformed in human liver by: 1) hydroxylation of the tertiary-butyl chain to OH-bupropion and 2) by reduction of the carbonyl group giving rise to the two isomers threohydrobupropion and erythrohydrobupropion [30].
At the end of the experimental period, 10 grams of the perfused tissue was excised, homogenized and used for extraction of bupropion and its metabolites as described above. The recovery of bupropion and its metabolites, hydroxybupropion, erythrohydrobupropion, and threohydrobupropion, from the extraction procedure was determined by comparing the peak areas of aliquots containing known concentrations of bupropion and its metabolites to the peak areas obtained from the same amounts of bupropion, hydroxybupropion, erythrohydrobupropion and threohydrobupropion dissolved in the mobile phase. The recovery of all compounds under these experimental conditions was 70 – 75%.
The purity of [3H]-bupropion used in this investigation was determined by the same elution time as non-radioactive compound and no impurities were detected. The stability of [3H]- bupropion to radioactive damage or chemical degradation during 4 hours recirculation in the perfusion system was determined in the absence of placenta.
Instrumentation and chromatographic conditions
The HPLC system consisted of a Waters 600E Multisolvent Delivery System (Waters Corporation, Milford, MA), a 717 autosample, and 2487 dual λ absorbance detector coupled with the β-RAM flow-through on-line detector (model 4, IN/US Systems, Tampa, FLA). The HPLC system was controlled by Millennium chromatography manager (Waters, Milford, MA) and ScintFlow SA (Version 1.3.3, IN/US Systems, Inc, FLA). The mobile phase consisted of methanol and 25 mM phosphate buffer adjusted to pH 5.3 with phosphoric acid (85%) before addition of methanol (40:60 v/v). The separation was achieved on Waters Symmetry C18 column (150 × 4.6 mm, 5 μm) connected with a Phenomenex C18 guard column (4×3.0 mm) at ambient temperature. The flow rate of mobile phase was 1 ml/min and wavelength of UV detector was set at 254 nm. The split ratio of eluent to radioactive detector was 100% and the ratio of scintillation cocktail to eluent was 3.
Data and statistical analysis
Oxygen transfer, and consumption were calculated according to the methods of Wier and Miller [18].
All reported values are expressed as mean ± S.D. Statistical significance of the differences observed between bupropion-treated and control placentas and between the control and experimental periods for each placenta were calculated by two-tailed t test and considered significant when P <0.05.
Results
Bupropion biotransformation by placental tissue during its perfusion
The retention times for the radiolabeled standards under our experimental conditions were as follows: hydroxybupropion, 6.7 minutes; erythrohydrobupropion, 7.7 minutes; threohydrobupropion, 8.5 minutes; and bupropion, 10.9 minutes. A HPLC chromatogram of the eluted peaks of the standard compounds of bupropion and its metabolites are presented in Figure 2A.
Figure 2.
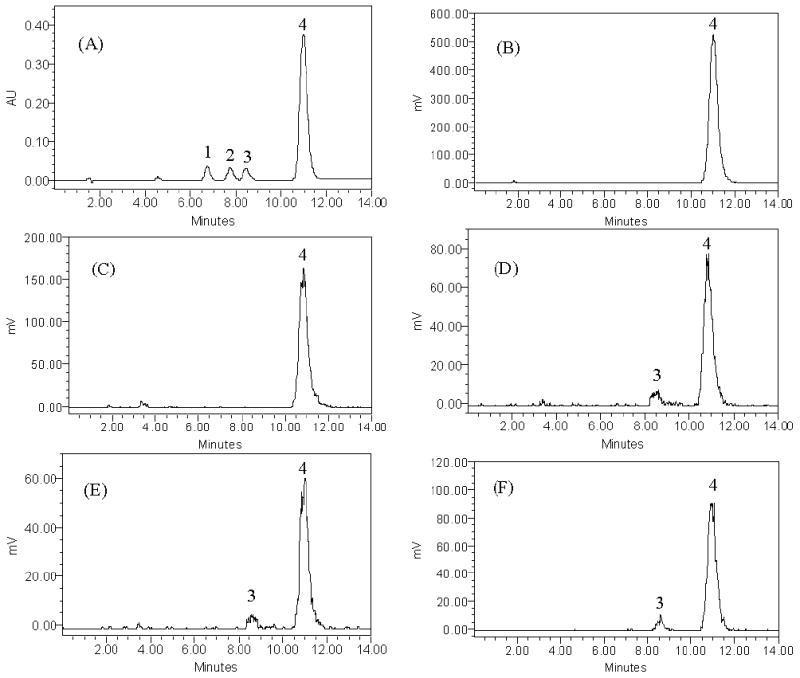
HPLC elution profiles of the standard compounds of bupropion and its metabolites.
Figure 2A: HPLC elution profile of the standard compounds of bupropion and its metabolites as detected by UV at 254 nm. (1): hydroxybupropion (6.7 minutes), (2): erythrohydrobupropion (7.7 minutes), (3): threohydropbupropion (8.5 minutes), (4): bupropion (10.9 minutes).
Figure 2B: HPLC elution profile of standard custom-synthesized [3H]-bupropion as detected by β-RAM-flow-through on-line detector.
Figure 2C-F: Chromatograms of perfusion medium and tissue homogenate after 240 minutes of perfusion as detected by β-RAM flow-through on-line detector.
(C): Maternal perfusate in absence of placenta.
(D): Maternal perfusate in presence of placenta.
(E): Fetal perfusate in presence of placenta.
(F): Placental tissue homogenate.
Two major peaks at 8.5 minutes and 10.9 minutes are detected in the maternal and fetal perfusate and placental tissue, corresponding to the metabolite (3) and parent drug (4).
Preliminary analyses were performed to determine the purity of the custom-synthesized [3H]-bupropion as well as its chemical stability and presence of radioactive damage. Chromatograms revealed that the retention time of eluted [3H]-isotope used in this investigation corresponded to that of bupropion standard (Figure 2B). Since we did not find elution of additional peaks to bupropion in the perfusion medium that had re-circulated in the perfusion system for 4 hours, in the absence of placenta at 37°C, it was concluded that [3H]-bupropion was chemically stable and radioactive damage did not occur during experimentation (Figure 2C).
The elution profile (chromatograms) of aliquots from the maternal (Figure 2D) and fetal (Figure 2E) perfusates, and placental tissue homogenate (Figure 2F) revealed the elution of two major peaks at 8.5 min and 10.9 minutes and each retained the [3H] nuclide. The retention time of eluted peaks corresponded to that of threohydrobupropion and bupropion standards, respectively (Figure 2A). The formation of the threohydrobupropion was determined in samples obtained at the end of 4 hours of perfusion. The ratio of metabolite to parent drug in the maternal and fetal circuits and placental tissue were 0.08, 0.07 and 0.06, respectively. Therefore, the metabolic activity of placental enzymes in the biotransformation of bupropion during 4 hours of perfusion was low.
Binding of bupropion to HSA
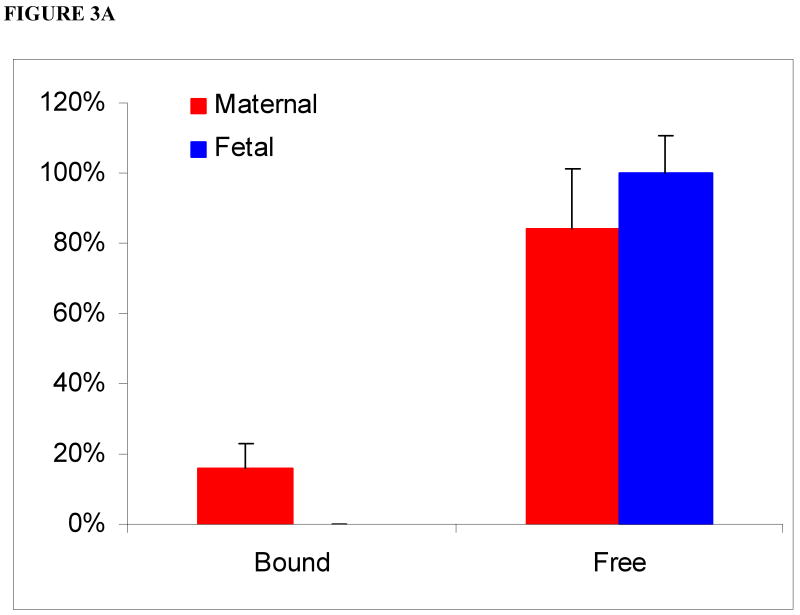
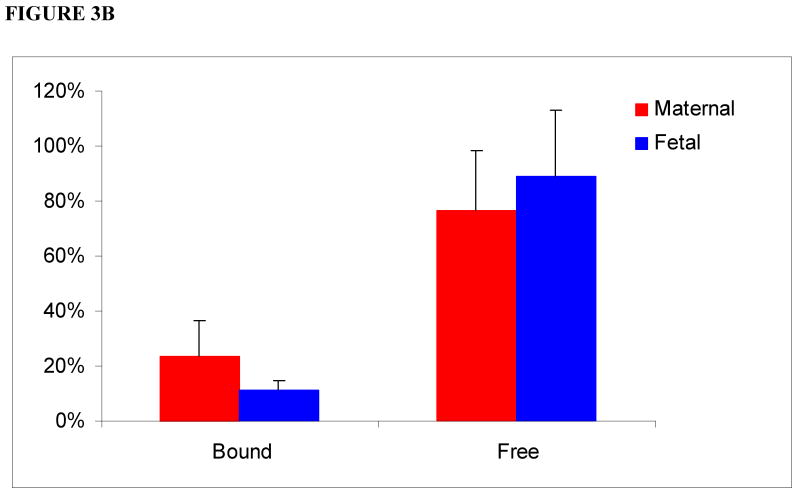
In the media of the maternal circuit devoid of HSA, 17 ± 7% of bupropion was bound to protein and eluted in the void volume. The presence of a bound fraction of bupropion in aliquots of maternal perfusate can be explained by its binding to placental tissue proteins released from the perfused lobule to the maternal circuit only [19]. In aliquots from the fetal circuit, bupropion was eluted in the total volume of the column only, i.e., as a free drug indicating that tissue proteins are not released into the fetal circuit (Figure 3A).
Figure 3.
Relative amounts of bound and free bupropion in maternal and fetal media in absence (3A) and presence (3B) of human serum albumin (HSA) following its perfusion for 4 hours.
(3A) In media of the maternal circuit devoid of added HSA: 17 ± 17% of bupropion was bound and the remainder was free. In the fetal circuit, bupropion was in its free form.
(3B) In presence of 30 mg/mL HSA (physiologic concentration) added to media of both maternal and fetal circuits: elution of bupropion in the void volume of the column increased to 24 ± 12% in the maternal circuit. In the fetal circuit, bupropion was eluted 11 ± 4% as bound fraction.
In the presence of 30 mg/mL HSA, added to both maternal and fetal circuits, the elution of bupropion in the void volume from the maternal circuit increased to 24 ± 12%. Moreover, 11 ± 4% of bupropion was eluted as a bound fraction in the fetal circuit (Figure 3B). Taken together, this data indicate that binding of bupropion to HSA of the perfusion medium was low and most likely did not affect its transfer across human placenta.
Placental transfer and distribution of bupropion
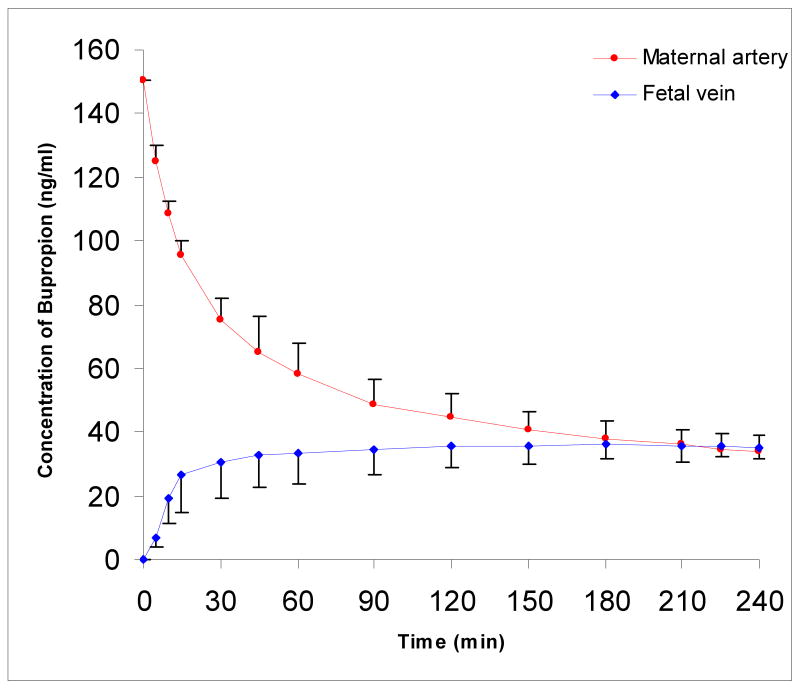
In this investigation, the transplacental transfer of AP was approximately 50%, and consistent with previous reports from our laboratory [14, 20]. The decline in bupropion concentration in the maternal circuit (150 ng/ml) was biphasic (Figure 4). In the initial phase, the decline was rapid and lasted 30 minutes. In the second phase, i.e., the remaining 210 minutes of the experimental period, the decline was slow. Bupropion appeared in the fetal circuit within the first five minutes of perfusion. The concentration of bupropion in the fetal circuit at the end of the experiment reached 35 ± 4 ng/mL, which represents 24 ± 3% of its initial concentration in the maternal circuit. Equilibrium was achieved in the fetal circuit after 180 minutes from time “0”. At the end of the 4-hour period, the fetal/maternal concentration ratio of bupropion was 1.07 ± 0.22.
Figure 4.
Transplacental transfer of bupropion during 4 hours of perfusion. The decline in bupropion concentration in the maternal circuit was biphasic: a rapid decline in the initial 30 minutes, followed by a slower decline in the remaining 210 minutes.
Transplacental transfer of bupropion at its higher concentration (450 ng/ml) was similar to that observed for its lower concentration. The transfer of bupropion was biphasic and the decline in its concentration in the maternal circuit and increase in the fetal circuit was three-folds of that observed for the lower concentration. The concentration of bupropion in the fetal circuit at the end of the experiment was 104 ng/ml ± 8 ng/ml, 23 ± 2% of its initial concentration and its fetal/maternal concentration ratio was 1.00 ± 0.14, similar to the lower bupropion concentration, indicating that the transfer of bupropion is dose-independent.
At the end of the 4-hour experimental period, the distribution of bupropion was independent of its initial concentration as follows: 48 ± 6% of the drug was retained by placental tissue, 32 ± 5% remained in the maternal circuit, and 20± 6% was transferred to the fetal circuit (Figure 5).
Figure 5.
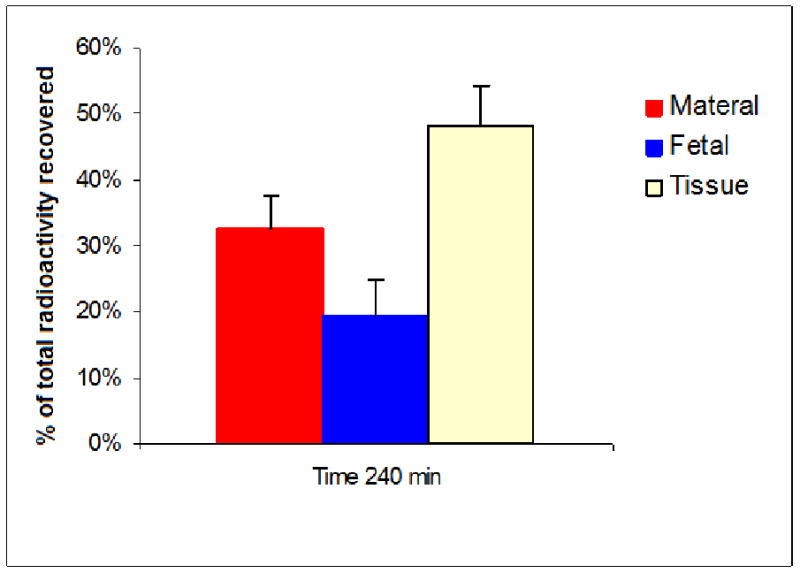
Distribution of bupropion between the placental tissue, maternal and fetal circuits.
Effect of bupropion and its metabolite on trophoblast tissue viability and functional parameters
Oxygen delivery from the maternal to fetal circuit ranged between 11.0 ± 2.9 and 16.4 ± 4.5 ml/min.kg at bupropion concentrations of 150 and 450 ng/ml. These values were independent of its concentration and were not significantly different from those observed for control placentas. All values are shown in Table 1. All values are within the normal range established in our laboratory for control placentas (perfused for the same duration of time but in the absence of drug) and indicate that bupropion at the transfused concentrations does not adversely affect oxygen transfer and consumption.
Table 1.
Effect of bupropion on oxygen transfer and consumption during 4 hours of perfusion. Each value represents the mean of at least 5 placentas ± standard deviation. Release of hCG varies widely between placentas in vivo and in vitro. Therefore, the amount of hormone released from each placenta during the control period was set at 100 and that released during the experimental period as a percent.
| Concentration of Bupropion | O2 transfer | O2 consumption | ||
|---|---|---|---|---|
| Experimental period (mL/min kg) | % Control perioda | Experimental period (mL/min kg) | % of Control perioda | |
| Control placentasb | 0.15 ± 0.2 | 42 ± 28 | 5.0 ± 1.9 | 97 ± 20 |
| 150 ng/mL | 0.22 ± 0.1 | 56 ± 21 | 6.6 ± 1.6 | 114 ± 37 |
| 450 ng/mL | 0.09 + 0.03 | 43 + 16 | 3.7 + 1.2 | 98 + 38 |
Tissue was perfused with medium only during the control period. The values obtained during the experimental period were expressed as a percent of that obtained during the control period.
No drug was added in the experimental period of control placentas.
The release of the trophoblast tissue-specific hormone hCG during transfusion of bupropion is used as an indicator of placental function and a decline in its rate suggests an adverse effect. The rate of hCG release during the control period was set at 100% and that during the experiment as a percentage of it. In control placentas, in absence of HSA, the release of hCG at the end of the experimental period was 72 ±14%. In experiments where bupropion, irrespective of its dose, was perfused in absence of HSA, the release of hCG at the end of the experimental period was not statistically different from that of control placentas, i.e. in absence of bupropion. On the other hand, when bupropion was transfused in presence of HSA, the release of hCG at the end of the experimental period reached 94 ± 12% (P=0.02). Since hCG release from tissue perfused with bupropion and in absence of HSA was not different from control placentas, it could be attributed to the effect of the added protein. Moreover, the increase in hCG release in presence of HSA observed in this report is in agreement with that reported for albumin stimulation of release of the hormone from term trophoblast explants in culture [21].
Taken together, these data indicate that at the concentrations tested, bupropion does not adversely affect placental viability and functional parameters.
Discussion
Bupropion is an antidepressant used for treatment of women in their childbearing age, who are at increased risk of developing depressive episodes during pregnancy and postpartum. In addition, women suffering from depression are more likely to be involved in other high-risk behaviors including cigarette smoking [22]. The advantage of bupropion, over NRT, during pregnancy is the absence fetal exposure to nicotine. The efficacy of bupropion for smoking cessation in adult males and non-pregnant females has been well established [23, 24]. Nevertheless, the use of bupropion during pregnancy requires additional information on its disposition as well as its potential risk to fetal development; which could be either due to direct toxicity of bupropion, or indirect. Since bupropion acts as a nicotinic cholinergic receptor blocker, it could potentially interfere with cholinergic signaling of the placenta or fetal tissues. Therefore, the aim of this investigation is to provide information on the disposition (transplacental transfer, distribution and metabolism) of bupropion by the human placenta.
The technique of the DPPL lobule has been used in our laboratory for the last decade to obtain data on transplacental transfer, distribution and metabolism of several drugs used for treatment of the pregnant patient. Data from our laboratory and others have demonstrated that this ex-vivo technique can serve as a powerful tool in providing fundamental information for subsequent in vivo investigations of therapeutics for the pregnant patient.
The transfer and distribution of bupropion across the dually perfused human placental lobule is compared and normalized to the transfer of AP as a marker compound [14, 25]. AP was selected because of its physicochemical properties namely, low molecular weight, 188, non-ionizable at pH 7.4 (pKa 1.4), and consequently its rapid transfer across the perfused lobule [26]. Moreover, AP is preferentially distributed into the aqueous maternal and fetal circuits with minimal retention in the tissue due its low octanol/water partition coefficient, 0.33, as well as its minimal binding to plasma proteins and human serum albumin [14, 19, 26].
Bupropion is a relatively low molecular weight compound, 239.7, and at physiologic pH 7.4, the ratio of its ionized/non-ionized fraction in the perfusion medium is approximately 60/40 (pKa 7.6). These properties suggest its transfer across placenta but to a lesser extent than AP. The octanol/water partition coefficient of bupropion is 3.21, i.e. higher than AP, and thus favors its distribution into hydrophobic compartments such as placental tissue.
Data cited in this report indicate that bupropion is rapidly transferred across the placenta and appearing in the fetal circuit within 5 minutes. The transfer of bupropion to the fetal circuit, at the end of the perfusion, accounted for approximately 20± 6% of that added to the maternal circuit. Under the same experimental conditions, almost 50% of the added AP is transferred to the fetal circuit i.e. the transfer of bupropion is approximately half of AP.
One of the factors that may affect the extent of bupropion transfer and distribution across the placenta is its binding to plasma and tissue proteins. It is well known that the concentration of plasma proteins is reduced in pregnancy, largely due to decreased concentration of albumin (43 mg/ml in non-pregnant women to 29 mg/ml). Therefore, the binding of bupropion to HSA (30 mg/ml) was investigated utilizing gel filtration chromatography. We detected only a 10% change in protein binding of bupropion with the addition of 30 mg/ml of HSA. Since HSA is the major plasma protein, and appears to have a small effect on total protein binding of bupropion, it is unlikely that the decrease in serum albumin associated with the onset of pregnancy would have a significant effect on bupropion pharmacokinetics.
Bupropion is relatively liphophilic (partition coefficient 3.21). Therefore, at the end of the experimental period, approximately half of it (48 ± 6%) was retained by the placental lobule, and the remainder distributed between maternal 32 ± 5% and fetal circuits 20± 6%. The observed biphasic decline in bupropion concentration in the maternal circulation and its distribution are in agreement with the two-step process for transfer of lipophilic compounds [27]. The first step is uptake of the drug from the maternal circuit by syncytiotrophoblast, and the second, its transfer/release from the tissue to the fetal circuit.
Moreover, by the end of 4 hours, bupropion retained by the placental tissue was metabolized to threohydrobupropion which was consequently released to the maternal and fetal circuits (Figure 2D-F). The ratio of metabolite to parent compound in the maternal (0.08) and fetal (0.07) circuits were higher than in the placental tissue (0.06) and is likely due to the more polar nature of the metabolite than the drug. This explains the preferential distribution of the metabolite into the aqueous compartments.
Human liver biotransforms bupropion to hydroxybupropion, threohydrobupropion and erythrohydrobupropion [28]. The major hepatic metabolite formed is hydroxybupropion and its pharmacologic activity is almost 50% that of the parent compound while the other two have very low activity [28]. Accordingly, the biotransformation of bupropion, during its perfusion, to threohydrobupropion underscores the difference between placental and hepatic metabolism of the drug. Similar differences in the biotransformation of the hypoglycemic drug glyburide by placental and hepatic microsomes have been reported [29].
Finally, neither bupropion nor its metabolite, formed during perfusion, had any apparent adverse effects on placental tissue as determined by the viability (oxygen transfer and consumption) and functional (hCG release) parameters. Therefore, these data indicate that at the concentrations tested, 150 ng/ml and 450 ng/ml, bupropion and its metabolite, threohydrobupropion, do not adversely affect the determined functions which are necessary for normal fetal growth and development.
In summary, dual perfusion of placental lobule with a drug has been widely accepted as an approximation of the in vivo conditions at term. Therefore, the data obtained in this investigation suggest that both bupropion and its metabolite threohydrobupropion, formed by the placenta, are most likely transferable to the fetal circulation during pregnancy. The absence of any observed adverse effects for either bupropion or its metabolite on placental tissue warrants further investigation of their toxicity. This obtained information could render this drug available for women seeking smoking cessation during their pregnancy and thus, help to prevent poor neonatal outcomes associated with exposure to nicotine.
Acknowledgments
The authors sincerely appreciate the support of the physicians and nurses of the Labor & Delivery Ward of the John Sealy Hospital, the teaching hospital at UTMB, Galveston, Texas, and the Perinatal Research Division of the Department of Obstetrics & Gynecology. The authors greatly appreciate the Publication, Grant, & Media Support area of the Department of Obstetrics & Gynecology. This work was supported by a NIDA grant RO1DA024094 to TN.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.LeCLere F, Wilson J. Smoking behavior of recent mothers, 18 – 44 years of age, before and after pregnancy: United States, 1990. Advance Data. 1997;288:1–11. [PubMed] [Google Scholar]
- 2.Benowitz N, Dempsey D, Goldenberg R, Hughes J, Dolan-Mullen P, Ogburn P, Oncken C, Orleans C, Slotkin T, Whiteside H, Yaffe S. The use of pharmacotherapies for smoking cessation during pregnancy. Tobacco Control. 2000;9(Suppl III):iii91–iii94. doi: 10.1136/tc.9.suppl_3.iii91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammoud A, Bujold E, Sorokin Y, Schild C, Krapp M, Baumann P. Smoking in Pregnancy revisited: Findings from a large population-based study. AJOG. 2005;192:1856–63. doi: 10.1016/j.ajog.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 4.Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine and Tobacco Research. 2004;6(2):S125–140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists. Smoking Cessation During Pregnancy. Washington D.C.: The College; 2005. Committee Opinion No.316. [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Smoking during pregnancy – United States, 1990 – 2002. MMWR Morb Mortal Wkly Rep. 2004;53(39):911–5. [PubMed] [Google Scholar]
- 7.Wisborg K, Henriksen T, Jespersen L, Secher N. Nicotine patches for pregnant smokers: a randomized controlled study. Obstet Gynecol. 2000;96:967–71. doi: 10.1016/s0029-7844(00)01071-1. [DOI] [PubMed] [Google Scholar]
- 8.Fingerhut L, Kleinman J, Kendrick J. Smoking before, during and after pregnancy. American Journal of Public Health. 1990;80:541–4. doi: 10.2105/ajph.80.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huster C, Chrismon J, Reddy M. Trends and effects of cigarette smoking among girls and women in the United Sates, 1965 – 1993. J Am Med Women's Assoc. 1996;51:11–18. [PubMed] [Google Scholar]
- 10.Dempsey D, Jacob P, III, Benowitz NL. Accelerated metabolism of nicotine and continine in pregnant smokers. J Pharmacol Exp Ther. 2002;301(2):594–8. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- 11.Oncken C, Dornelas E, Greene J, Sankey H, Glassmann A, Feinn R, Kranzler HR. Nicotine gum for pregnant smokers: a randomized control trial. Obstet Gynecol. 2008;112(4):859–67. doi: 10.1097/AOG.0b013e318187e1ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan B, Einarson A, Koren G. Effectiveness of bupropion for smoking cessation during pregnancy. Journal of Addictive Diseases. 2005a;24(2):19–23. doi: 10.1300/J069v24n02_02. [DOI] [PubMed] [Google Scholar]
- 13.Miller R, Wier P, Maulik D, Di Sant'Agnese P. Human placenta in vitro: characterization during 12 hours of dual perfusion. Contrib Gynecol Obstet. 1985;13:77–84. [PubMed] [Google Scholar]
- 14.Nanovskaya T, Deshmukh S, Brooks M, Ahmed M. Transplacental transfer and metabolism of buprenorphine. J Pharmacol Exp Ther. 2002;300:26–33. doi: 10.1124/jpet.300.1.26. [DOI] [PubMed] [Google Scholar]
- 15.Krauer B, Dayer P, Anner R. Changes in serum albumin and alpha acid glycoprotein concentrations during pregnancy: an analysis of fetal-maternal pairs. BJOG. 1984;91(9):875–881. doi: 10.1111/j.1471-0528.1984.tb03700.x. [DOI] [PubMed] [Google Scholar]
- 16.Kirchheiner J, Klein C, Meineke I, Sasse J, Zanger U, Murdter T, Roots I, Brockmoller J. Bupropion and 4-OH-bupropion pharmacokinetics in relation to genetic polymorphisms in CYP2B6. Pharmacogenetics. 2003;13:619–626. doi: 10.1097/00008571-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Cemerikic B, Cheng J, Agbas A, Ahmed MS. Opioids regulate the release of human chorionic gonadotropin hormone from trophoblast tissue. Life Sci. 1991;49:813–824. doi: 10.1016/0024-3205(91)90246-8. [DOI] [PubMed] [Google Scholar]
- 18.Wier P, Miller R. Oxygen transfer as an indicator of perfusion variability in the isolated human placental lobule. Contr Gynecol Obstet. 1985;13:127–131. [PubMed] [Google Scholar]
- 19.Nanovskaya T, Nekhayeva I, Hankins G, Ahmed M. Effect of human serum albumin on transplacental transfer of glyburide. Biochem Pharmacol. 2006;72(5):632–9. doi: 10.1016/j.bcp.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Nanovskaya T, Patrikeeva S, Hemauer S, Fokina V, Mattison D, Hankins G, Ahmed M. Effect of albumin on transplacental transfer and distribution of rosiglitazone and glyburide. The Journal of Maternal-Fetal and Neonatal Medicine. 2008;21(3):197–207. doi: 10.1080/14767050801929901. [DOI] [PubMed] [Google Scholar]
- 21.Cirelli N, Lebrun P, Gueurning C, Delongne-Desnoeck J, Vanbellinghen A, Graff G, Meuris S. Physiologic concentrations of albumin stimulate chorionic gonadotropin and placental lactogen release from human term placental explants. Hum Reprod. 2001;16(3):441–8. doi: 10.1093/humrep/16.3.441. [DOI] [PubMed] [Google Scholar]
- 22.Zuckerman B, Amaro H, Bauchner H, Cabral H. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160:1107–11. doi: 10.1016/0002-9378(89)90170-1. [DOI] [PubMed] [Google Scholar]
- 23.Tonnesen P, Tonstad S, Hjalmarson A, Lebargy F, VanSpiegel P, Hider A, Sweet R, Townsend J. A multicentre, randomized, double-blind, placebo-controlled, 1-year study of bupropion SR for smoking cessation. J Intern Med. 2003;254(2):184–92. doi: 10.1046/j.1365-2796.2003.01185.x. [DOI] [PubMed] [Google Scholar]
- 24.Dalsgareth O, Hansen N, Soes-Peterson U, Evald T, Hoegholm A, Barber J, Vestbo J. A multicenter, randomized, double-blind, placebo-controlled, 6-month trial of bupropion hydrochloride sustained-release tablets as an aid to smoking cessation in hospital employees. Nicotine Tob Res. 2004;6(1):55–61. doi: 10.1080/14622200310001656867. [DOI] [PubMed] [Google Scholar]
- 25.Schneider H. Techniques: in vitro perfusion of human placenta. In: Sastry B, editor. Placental Toxicology. Florida: CRC press; 1995. pp. 1–26. [Google Scholar]
- 26.Wu Y, Cross S, Roberts M. Influence of physicochemical parameters and perfusion flow rate on the distribution of solutes in the isolated perfused rat hindlimb determined by the impulse-response technique. J Pharm Sci. 1995;84:1020–1027. doi: 10.1002/jps.2600840820. [DOI] [PubMed] [Google Scholar]
- 27.Sastry BVR. Techniques to study human placental transport. Adv Drug Deliv Rev. 1999;38:17–39. doi: 10.1016/s0169-409x(99)00004-6. [DOI] [PubMed] [Google Scholar]
- 28.Schroeder DH. Metabolism and kinetics of bupropion. J Clin Psychiatry. 1983;44(5 Pt 2):79–81. [PubMed] [Google Scholar]
- 29.Zharikova O, Ravindran S, Nanovskaya T, Hill R, Hankins GD, Ahmed MS. Kinetics of glyburide metabolism by hepatic and placental microsomes of human and baboon. Biochem Pharmacol. 2007;73(12):2012–9. doi: 10.1016/j.bcp.2007.03.005. [DOI] [PubMed] [Google Scholar]