Abstract
Nutritional supplementation has become the standard of care for management of critically ill patients. Traditionally, nutritional support in this patient population was intended to replete substrate deficiencies secondary to stress-induced catabolism. Recognition of the influence of certain nutrients on the immune and inflammatory response of the critically ill has led to the evolution of more sophisticated nutritional strategies and concepts. Administration of immune-enhancing formulas supplemented with a combination of glutamine, arginine, omega-3 fatty acids and nucleotides have been shown in most studies to reduce infectious outcomes. More recently, the separation of nutritional support from the provision of key nutrients, has led to a further appreciation of the immunomodulatory and anti-inflammatory benefits of isolated nutrients, such as glutamine and antioxidants. The purpose of this article is to review the molecular mechanisms that are unique to each class of frequently utilized nutrients. A better understanding of the specific molecular targets of immunonutrients will facilitate application of more refined nutritional therapies in critically ill patients.
Keywords: immune-enhancing formulas, glutamine, argininine, omega-3-fatty acids, antioxidants, pharmaconutrients
INTRODUCTION
Multi-organ failure that occurs as a consequence of sepsis and shock is secondary to an imbalance of inflammatory mediators. Both sepsis and shock lead to dysfunctional inflammation and transient ischemic events that impair the integrity and function of the intestinal mucosa. Subsequent reperfusion events incite the release of pro-inflammatory mediators which worsen the systemic and local inflammatory response. Impaired mucosal blood flow, increased permeability, and altered epithelial integrity lead to over colonization of enteric bacteria, impaired intestinal transit and subsequent bacterial translocation. The provision of intraluminal nutrients to the stressed gut has been shown to reverse shock-induced mucosal hypoperfusion and restore intestinal transit [1]. Clinical studies evaluating the use of immune-enhancing formulas in hospitalized patients have shown a clear benefit with respect to decreased infectious complications, reduced antibiotic requirements and decreased lengths of stay. [2].
The realization that the greatest benefit in clinical outcomes were from studies utilizing specific nutrients, both enteral and parenteral, led to the development of pharmaconutrition. This allows the detachment of nutritional support from the provision of key nutrients that may modulate the inflammatory and immune response associated with critical illness [3]. Trauma, in particular, is characterized by the localized and systemic production and release of multiple pro-inflammatory mediators as well as a parallel release of anti-inflammatory mediators which may be responsible for post-traumatic immunosuppression and increased susceptibility to infections, sepsis, and multiple organ failure seen in these patients [4].
The purpose of this article is to review the molecular mechanisms that are unique to each class of frequently utilized nutrients. A better understanding of the specific molecular targets of immunonutrients will facilitate application of more refined nutritional therapies in critically ill patients.
GLUTAMINE
Clinical Benefits
Glutamine is a conditionally essential nutrient in states of serious illness or injury [5]. Under catabolic conditions, such as sepsis and shock, release of glutamine from muscle tissue serves as a `stress signal' to the organism that leads to gene activation to promote cellular protection and immune regulation [6]. Glutamine is the preferred fuel source for the enterocyte and has been shown to preferentially be absorbed over glucose in settings of gut ischemia [7]. The small intestine is the principal site for glutamine absorption and occurs across the brush border via the epithelial sodium-dependent, neutral amino acid transport system B (ATB○) and to a lesser extent via the sodium-independent, neutral amino acid transport system L [8]. In addition to glutamine's gut protective effects, glutamine is also important in nucleotide synthesis; it is anti-catabolic, has anti-oxidant properties via metabolism to glutathione, and may enhance immune responsiveness. An additional pathway by which glutamine may provide protection is by its contribution to arginine production. In a randomized clinical trial by Houdijk et al, glutamine supplemented enteral nutrition administered to severely injured trauma patients demonstrated increased plasma concentrations of arginine [9]. They later found that the enteral, rather than parenteral, route of administration provided the highest systemic levels of arginine. The described metabolic pathway is that glutamine is a precursor of ornithine, which is converted to citrulline by the intestine; citrulline is then transformed into arginine in the kidney [10, 11]. A recent review on intestinal permeability and systemic infections in critically ill patients concluded that glutamine (both intravenous and enteral) reduced the frequency of systemic infections. Benefit was attributed, in part, to maintenance of intestinal barrier function [12]. A meta-analysis that included 14 randomized trials demonstrated that high dose (> 0.5 gm/kg) glutamine supplementation was beneficial [13]. The majority of these studies, however, used parenteral rather than enteral supplementation.
Four prospective randomized trials in trauma and burn patients tested enteral glutamine as an isolated immune enhancing agent added to standard enteral diets and all demonstrated decreased infectious complications [14-17]. Likewise, a recent prospective randomized clinical trial showed that enteral glutamine administered during shock resuscitation is not only safe, but enhances gastrointestinal tolerance [18]. The potential for improved tolerance without adverse affects suggests that initiation of enteral feeding should be incorporated into early management of critically ill surgical patients.
In the past, parenteral administration of nutrients has come under scrutiny; however, recent randomized studies have shown that parenteral glutamine administration is beneficial in reducing septic morbidity and overall mortality. Investigators have shown a significant reduction in postoperative nosocomial infections after cardiac, vascular and colonic surgery in patients receiving glutamine-supplemented parenteral nutrition [24]. Prospective studies have also shown improved 6 month survival in patients with sepsis and ICU acquired infections who received parenteral glutamine as compared to those on standard isonitrogenous parenteral formulas [25]. Other studies suggest a decreased length of stay in patients receiving parenteral glutamine formulas.
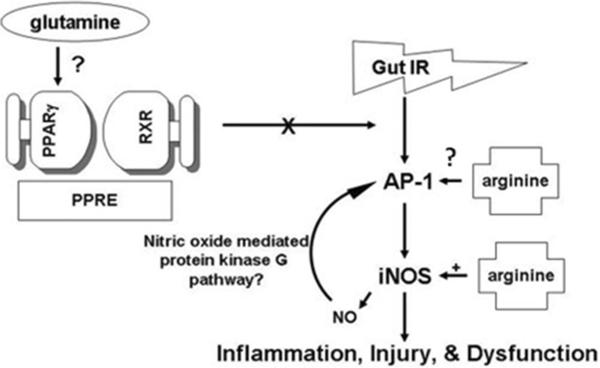
Laboratory studies have shown preservation of gut mucosal integrity, intestinal absorption, and energy (ATP) supplies when glutamine was delivered locally to the post ischemic gut [19]. Gut protection was correlated with transcriptional activation of PPAR-γ and abrogated by the specific PPARγ inhibitor, GW9662 [20]. PPARγ is a ligand activated nuclear hormone initially identified as important in adipocyte development and glucose homeostasis [21]. Once activated by a ligand, PPARγ heterodimerizes with the retinoid X receptor (RXR). This complex then binds to a specific DNA element, PPARγ receptor element (PPRE), in the promoter of target genes. [20, 21]. (Figure 1) The most potent endogenous ligand of PPARγ is 15-deoxy-Δ12,14-prostaglandin J2 (15d PG J2), a product of the cyclooxygenase pathway. Additional, though less well studied endogenous ligands, include members of the lipoxygenase (LOX) pathway, such as hydroxyeicosatetraenoic acid (HETE) and hydroxyoctaolecadienoic acid (HODE). Thus far, 13-HODE, 13-OXO (dehydrogenated 13-HODE) and 15-HETE have been identified in intestinal epithelial cells and may be important in regulation of intestinal inflammation [21, 22]. Lastly, numerous synthetic ligands, such as the glitazones used in the treatment of Type II diabetes mellitus, have also been identified. PPARγ ligands activate transcription via both ligand-dependent and ligand-independent mechanisms. Activation leads to inhibition of pro-inflammatory mediators, such as nuclear factor-κB (NFκB), activated protein-1 (AP-1), signal transducers and activators of transcription 1 (STAT1), and nuclear factor of activated T-cells (NFAT) through ligand-dependent transrepression [23].
Fig. 1. Overview of the proposed mechanisms for differential modulation of gut function by enteral glutamine and arginine in the hypoperfused gut.
Following gut ischemia/reperfusion (IR), the proinflammatory transcription factor, AP-1, is increased, as is the downstream, proinflammatory enzyme iNOS. Both are associated with gut inflammation, mucosal injury, and dysfunction. When the enteral nutrient, arginine, is added to the hypoperfused gut, iNOS expression and AP-1 DNA-binding activity are further increased. Arginine is a known precursor to iNOS, but the mechanism by which arginine increases AP-1 is unknown. There is a recently described, NO-mediated protein kinase G pathway that may explain the increase in AP-1 by arginine. PPARγ is believed to inhibit the proinflammatory pathway at the level of AP-1, protecting the hypoperfused gut. Glutamine increases PPARγ DNA-binding activity and may be a novel PPARγ agonist. When PPAR γ is activated, it heterodimerizes with the retinoic acid receptor (RXR) and then binds to the peroxisome proliferator response element (PPRE), resulting in target gene expression.
In a series of experiments, Wischmeyer et al, have demonstrated that parenteral glutamine is protective against pro-inflammatory induced injury via induction of heat shock proteins (HSPs) [26]. Heat shock proteins are a family of constitutive and inducible stress proteins that confer cellular protection by acting as molecular chaperones to ensure the correct fate of denatured proteins [26, 27]. The inducible isoforms of heat shock proteins, HSP 25 and HSP 72, are thought to confer the greatest cytoprotection. In vitro studies showed that glutamine-induced expression of inducible HSPs resulted in significant cellular protection in response to lethal heat, oxidant, and ischemia-reperfusion injuries [28, 29]. Further studies involving LPS-induced septic shock in rats, confirmed that glutamine induced HSP25 and HSP72 in various organs and was accompanied by decreased host mortality and reduction of end-organ damage. This series of investigations demonstrates the crucial role of glutamine-induced HSPs in cytoprotection and improved survival following various forms of insult.
In addition to heat shock-related protection, intravenous glutamine has also been shown to confer protection by attenuation of systemic pro-inflammatory cytokines by interfering with NFKB and p38/ERK MAPK signal transduction [30]. Interestingly, intravenous glutamine also enhances gut mucosal immunity by promoting proliferation of gut-associated lymphocytes and enhancing IgA secretion [31].
ARGININE
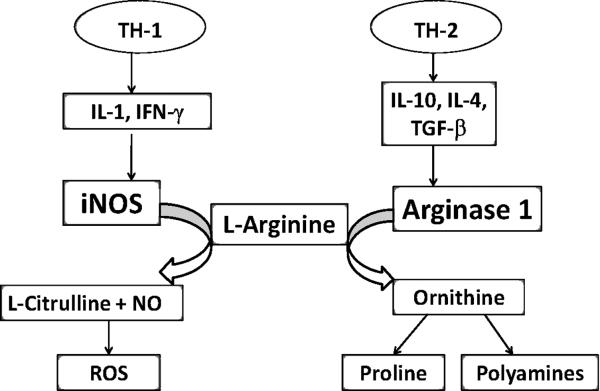
Arginine is a semi essential amino acid obtained both from dietary sources and endogenous synthesis via the urea cycle. The metabolic products of arginine, rather than arginine itself, are the effector molecules that participate in cellular restitution processes and modulate the immune response of the host. Under non stressed conditions, arginine contributes to adequate wound healing, an enhanced immune response, and stimulation of various anabolic hormones. L-arginine is also a unique substrate for the production of nitric oxide (NO). Nitric oxide is generated by oxidation of L-arginine mediated by various isoforms of NO synthases (NOSs) [32]. Sustained production of nitric oxide is thought to be a major contributor to the deleterious effects of postinjury inflammation. Therefore, identification of the metabolic pathways that utilize L-arginine has generated significant interest, as they offer a potential mechanism to control nitric oxide production by regulating the bioavailability of the substrate. The metabolic fate of arginine is determined by two groups of enzymes: the NO synthases and arginases. Two isoforms of arginase exist, Arginase I (AI) and Arginase II (AII), these enzymes are differentially expressed in various organs and cell types [33]. Arginase I is more widely distributed and has been shown to be prevalent in activated macrophages [34, 35]. In addition, arginase I has been shown to be co-localized with ornithine decarboxylase (ODC), which promotes production of polyamines and a subsequent restitution processes in those tissues [34]. Competitive consumption of the substrate arginine by arginase I has been shown to impact the iNOS function by limiting substrate availability [35]. The immune state of the host and associated cytokine expression determines which enzyme, iNOS or Arginase I, is preferentially induced [35](Figure 2). TH-1 immune states, such as sepsis, are associated with the release of the T-helper 1 (TH-1) cytokines, interleukin (IL)-1, tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ), which preferentially induces iNOS expression [36]. The enzymatic activity of iNOS requires dimerization with arginine for proper activation. Once activated, iNOS leads to sustained production of NO, which is an important effector molecule of the cytotoxic actions of the activated macrophages in the hosts' bacteriocidal response. In trauma, a T-helper 2 (TH-2) immune state predominates and the release of IL-4, IL-10 and transforming growth factor beta (TGF-β) increases arginase I expression. Ochoa et al, demonstrated that peripheral mononuclear cells of trauma patients have increased arginase-1 expression, corresponding to increased immune cell arginase activity and decreased plasma arginine and citrulline levels [36]. Increased arginase activity utilizes available arginine for polyamine and proline synthesis, and prevents arginine from being used as a substrate for iNOS dimerization [37] and subsequent nitric oxide production. In addition, it is suggested that polyamines produced by arginine exert negative feedback on the iNOS enzyme. The competition for substrates and intrinsic feedback mechanisms, preferentially employ one of the two pathways for arginine utilization based on the immune state and metabolic needs of the host. Therefore supplemental arginine may be beneficial in trauma patients by altering metabolic pathways in immune cells that leads to reduced nitric oxide production in the post injury period.
Fig. 2. Differential pathways for arginine metabolism.
L-Arginine is differentially metabolized based on the predominant cytokine profile. Th-1 cytokines that accompany the pro-inflammatory response lead to nitric oxide and reactive oxygen species as by-products of arginine. When a TH-2 cytokine profile exists, arginine is metabolized by arginase-1 into proline and polyamines, which promotes cell division and collagen synthesis. IL-1 (interleukin 1), IFN-γ (interferon gamma), iNOS (inducible nitric oxide synthase), NO (nitric oxide), ROS (reactive oxygen species). IL-10 (interkeukin 10), IL-4 (interleukin 4), TGF-β (tumor growth factor beta).
Conversely, Heyland et al have suggested that arginine supplementation increases nitric oxide production, amplifying the systemic inflammatory response syndrome (SIRS), and increasing mortality in critically ill septic patients [38]. Rodent models of ischemia-reperfusion mediated gut injury also demonstrate a deleterious effect of arginine administration by a similar mechanism [39]. However, Ward et al in a gut ischemia/reperfusion model and Mailman in a hemorrhagic shock model both demonstrated protection by enteral arginine [40, 41]. Data with intravenous arginine is similarly controversial [42-45]. The reasons for these disparate results are unclear; the timing, route of administration, and severity of shock, are all likely to be important contributing factors.
NUCLEOTIDES
Nucleotides are low molecular weight intracellular compounds that play an active role in cellular proliferation and immune modulation. Nucleotides are composed of a purine and pyridimine backbone with a ribose and one or more phosphate groups [46]. They serve as building blocks for DNA, RNA and ATP and are coenzyme components of flavin adenine dinucleotide (FAD), Nicotinamide adenine dinucleotide (NAD) and coenzyme A. Nucleotides are available through two major pathways: de novo synthesis or a salvage pathway. The former requires synthesis from amino acid precursors while the later is much less time and energy-dependent and involves linkage of a ribose phosphate moiety to available free bases [46]. The availability of free bases is the rate-limiting factor for host utilization of the salvage pathway. This is thought to be critically important in rapidly dividing cells, such as lymphocytes and enterocytes during times of stress when cells rely heavily on the salvage pathway. The modulating effects of nucleotides on intestinal and immune cells are considered to be overlapping entities. Intestinal epithelial cells that comprise the gut associated lymphoid tissue (GALT) are a well recognized as a source of cytokines and antigen presentation [47]. A number of studies have demonstrated that parenteral supplementation of nucleotides leads to increased immune responsiveness such as enhanced lymphocyte blastogenesis, decreased bacterial translocation [48] and fewer episodes of graft rejection [49]. The most well-defined models that demonstrate the influence of nucleotides on immune function are those evaluating the host response against allografts. Preformed nucleotides are postulated to be required for optimal T cell proliferation and responsiveness to antigens. Functional in vivo studies evaluating lymphocytes have showed decreased lymphocyte proliferation in response to mitogens and decreased IL-2 production that is restored with RNA and uracil supplementation [49]. These findings translated into improved cardiac allograft survival in animals fed a nucleotide-free diet [47]. Overall, these studies suggest that dietary nucleotides are substrates needed for optimal T cell maturation, which influences T cell effector functions.
Nucleotide supplementation is also thought to augment the nonspecific host response to infection by altering the intestinal microflora environment, which is best demonstrated by reduced infections in infants receiving nucleotide supplemented formulas[47, 51]. In these instances, nucleotides are thought to act as prebiotics, facilitating the proliferation of beneficial flora. Finally, nucleotides are also thought to maintain the integrity of gut mucosal barrier function. Nucleotide supplementation mitigates the effects of endotoxin-induced mucosal damage. This mechanism leads to reduced bacterial translocation in LPS-induced sepsis models [52]. Similarly, parenteral supplementation of nucleotides reduced total parenteral nutrition (TPN)-induced mucosal atrophy and permeability [48]. In conclusion, the benefits of nucleotide supplementation on the immune system and maintenance of enterocyte integrity have led to the inclusion of nucleotides in immune enhancing formulas.
OMEGA-3 FATTY ACIDS
Fish oils leads to suppression of excessive endothelial activity and decreased production of pro-inflammatory mediators [53]. The active components of fish oils are eixosapentaenoic (EPA) and docosahexaenoic acid (DHA). These compounds are further classified as n-3 polyunsaturated fatty acids. Omega-3 fatty acids (ω-3 FA) have demonstrated significant anti-inflammatory properties which are thought to occur through three principal mechanisms: (1) displacement of arachidonic acid from cellular membranes, (2) differential prostaglandin E2 and LTB4 production, and (3) diminished nuclear factor kappa B (NF-κB) and AP-1 activation [54]. To further elucidate the molecular mechanisms of the anti-inflammatory properties of fatty acid supplementation, a lipopolysaccharide (LPS)-stimulated macrophage model has been utilized to evaluate the effect of omega-3 fatty acids on induction of pro-inflammatory mediators. In this experimental model, ω-3 FA were shown to attenuate pro-inflammation by down regulation of TNF-α through inactivation of the NF-κB signal transduction pathway [55] and inhibition of AP-1 [56]. However, down regulation of TNF-α after treatment with ω-3 FA only approached 45-50%. To account for the full anti-inflammatory potential of ω-3 FA, other factors such as nitric oxide were examined. Pre-treatment with an ω-3 FA emulsion demonstrated decreased production of nitric oxide via down regulation of iNOS protein expression [57]. These findings offer a plausible mechanism for reduction of inflammation-associated tissue damage that occurs with ω-3 FA supplementation. There have been three prospective randomized trials of enteral supplementation with omega-3 fatty acids in critically ill (primarily medical) patients and all have shown beneficial effects on respiratory mechanics [58-60]. Unfortunately, these studies all included additional supplements, making assignment of benefit to the omega-3 fatty acids difficult, and all utilized a high fat diet as the control. Nonetheless, results are promising and omega-3 fatty acid supplementation should be considered for use in patients with acute respiratory distress syndrome.
ANTIOXIDANTS
Immune enhancing nutrients are directed at ameliorating both metabolic and immune dysfunction that occurs in critical illness. The therapeutic value of most supplemental nutrients is attributed to their immunomodulating potential. However, oxidative stress also causes significant damage to mucosal barriers in states of hyper inflammation. Intrinsic defense mechanisms against reactive oxygen species (ROS)-induced cell injury include special enzymes known as anti-oxidants that catalyze the breakdown of ROS [61] (Figure 3). Well known antioxidants include superoxide dismutase, catalase and glutathione peroxidase, which are associated with cofactors selenium, zinc, manganese, and iron. In critically ill patients, endogenous supplies of anti-oxidants and their co-factors are significantly depleted and the ability to detoxify damaging radical oxygen species is greatly compromised. Investigators have shown that low levels of plasma selenium are inversely related to the degree of systemic inflammation in intensive care unit (ICU) patients with the systemic inflammatory response syndrome (SIRS) [62]. Ongoing oxidative stress is associated with cell injury, potentiation of the SIRS response, and increased morbidity and mortality in critically ill patients [62, 63]. Antioxidant replacement strategies have demonstrated overall reduction in mortality and specific end organ protection. In a cohort of trauma patients, those supplemented with antioxidants had a lower incidence of multiple organ failure, reduced ICU length of stay and a shorter duration of mechanical ventilation [64]. Likewise, Collier et al demonstrated a significant risk reduction in mortality in severely injured patients who received high-dose antioxidants compared to historical controls [65]. A systemic review of aggregated clinical trials in critically ill patients demonstrated an overall reduction in mortality with antioxidant supplementation [66]. After subgroup analysis, selenium appeared to be the premier antioxidant, having a greater association with reduced mortality as compared to nonselenium antioxidant strategies. The REDOX trial is a large prospective, randomized, double blinded multicenter trial currently in progress that is designed to evaluate mortality in critically ill patients with evidence of hypoperfusion receiving supplementation with antioxidants alone, glutamine alone, or a combination of glutamine and antioxidants [61]. Results of the Phase I dose-escalating study failed to show any adverse effect on organ function from the nutrients but did show a reduction in markers of oxidative stress, greater preservation of glutathione levels, and an improvement in mitochondrial function [67]. This important study should provide definitive data on the efficacy of glutamine and antioxidant supplementation in critically ill patients.
Fig 3. Antioxidant enzymes catalyze the breakdown of reactive oxygen species (ROS).
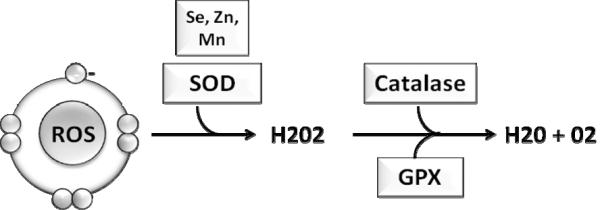
Antioxidant enzymes are the first line of defense against free radical damage. Superoxide dismutase (SOD) is associated with various cofactors, selenium (Se), zinc (Zn) and manganese (Mn); it initiates the breakdown of free radicals into hydrogen peroxide (H202). Hydrogen peroxide, which is also harmful to the host, is further catabolized into water and oxygen by catalase and glutathione peroxidase (GPX).
CONCLUSION
In summary, the rationale and benefit of immune enhancing nutrients and anti-oxidants in surgical and trauma patients has been carefully reviewed. The replacement of macronutrients under catabolic conditions has obvious benefits. Additionally, the use of combination therapy is emerging as a strategy not only to replace deficits, but more importantly to modulate metabolic and immune pathways, a concept known as pharmaconutrition. A better understanding of each supplements' specific molecular targets will facilitate application of more refined nutritional therapies in critically ill patients.
Acknowledgments
Funded by NIGMS RO1GM077282
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grossie VB, Jr., Weisbrodt NW, Moore FA, Moody F. Ischemia/reperfusion-induced disruption of rat small intestine transit is reversed by total enteral nutrition. Nutrition. 2001 Nov-Dec;17(11-12):939–43. doi: 10.1016/s0899-9007(01)00668-2. [DOI] [PubMed] [Google Scholar]
- 2.Moore F. Effects of immune enhancing diets on infectious morbidity and multiple organ failure. J Parenter Enteral Nutr. 2001;25(2 suppl):S36–S43. doi: 10.1177/014860710102500209. [DOI] [PubMed] [Google Scholar]
- 3. http://www.criticalcarenutrition.com.
- 4.Moore FA, Sauaia A, Moore EE, Haenel JB, et al. Post injury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Wischmeyer PE. Glutamine: Mode of action in critical illness. Crit Care Med. 2007;35(No. 9 (suppl.)):S541–S544. doi: 10.1097/01.CCM.0000278064.32780.D3. [DOI] [PubMed] [Google Scholar]
- 6.Wischmeyer PE. The glutamine story: where are we now? Current Opin Crit Car. 2006;12:142–148. doi: 10.1097/01.ccx.0000216582.87674.a4. [DOI] [PubMed] [Google Scholar]
- 7.Kles KA, Tappenden KA. Hypoxia differentially regulates nutrient transport in rat jejunum regardless of luminal nutrient present. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1336–G1342. doi: 10.1152/ajpgi.00055.2002. [DOI] [PubMed] [Google Scholar]
- 8.Choudry HA, Souba WW, Lin CM, et al. Stimulation of expression of the intestinal glutamine transporter ATB○ in tumor-bearing rats. Ann Surg Onc. 2006;13:1747–1753. doi: 10.1245/s10434-006-9115-8. [DOI] [PubMed] [Google Scholar]
- 9.Houdijk AP, Rijnsburger ER, Jansen J, et al. Randomised trial of glutamine-enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet. 1998;352:772–776. doi: 10.1016/S0140-6736(98)02007-8. [DOI] [PubMed] [Google Scholar]
- 10.Boelens PG, Melis GC, van Leeuwen PA, et al. Route of administration (enteral or parenteral) affects the contribution of L-glutamine to de novo L-arginine synthesis in mice: a stable-isotope study. Am J Physiol Endocrin Metab. 2006;291:E683–E690. doi: 10.1152/ajpendo.00252.2005. [DOI] [PubMed] [Google Scholar]
- 11.Van de Poll, Siroen MP, van Leeuwen PA, et al. Interorgan amino acid exchange in humans: consequences for arginine and citrulline metabolism. Am J Clin Nutr. 2007;85:167–172. doi: 10.1093/ajcn/85.1.167. [DOI] [PubMed] [Google Scholar]
- 12.De-Souza DA, Greene LJ. Intestinal permeability and systematic infections in critically ill patients: Effect of glutamine. Crit Care Med. 2005;33(No. 5):1125–1135. doi: 10.1097/01.ccm.0000162680.52397.97. [DOI] [PubMed] [Google Scholar]
- 13.Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: A systematic review of the evidence. Crit Care Med. 2002;30:2022–9. doi: 10.1097/00003246-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Houdijk APJ, Rijnsburger ER, Jansen J, Wesdorp RIC, Weiss JK, McCamish MA, Teerlink, Meuwissen SGM, Haarman HJ, Thijs LG, Van Leeuwen PAM. Randomized trial of glutamine-enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet. 1998;352:772–776. doi: 10.1016/S0140-6736(98)02007-8. [DOI] [PubMed] [Google Scholar]
- 15.Garrel D, Patenaude J, Nedelec B, Samson L, Dorais J, Champoux J, D'Elia M, Bernier J. Decreased mortality and infectious morbidity in adult burn patients given enteral glutamine supplements: A prospective, controlled, randomized clinical trial. Crit Care Med. 2003;31:2444–2449. doi: 10.1097/01.CCM.0000084848.63691.1E. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Jiang Z, Sun Y, Wang X, Ma E, Wilmore D. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: a randomized, double-blind, controlled clinical trial. JPEN. 2003;27:241–245. doi: 10.1177/0148607103027004241. [DOI] [PubMed] [Google Scholar]
- 17.Conejero R, Bonet A, Grau T, Esteban A, Mesejo A, Montejo JC, Lopez J, Acosta JA. Effect of a glutamine-enriched enteral diet on intestinal permeability and infectious morbidity at 28 days in critically ill patients with systemic inflammatory response syndrome: a randomized, single-blind, prospective, multicenter study. Nutrition. 2002;18:716–721. doi: 10.1016/s0899-9007(02)00847-x. [DOI] [PubMed] [Google Scholar]
- 18.McQuiggan M, Kozar R, Sailors RM, et al. Enteral glutamine during active shock resuscitation is safe and enhances tolerance of enteral feeding. J Parent Enteral Nut. 2008;32:28–35. doi: 10.1177/014860710803200128. [DOI] [PubMed] [Google Scholar]
- 19.Kozar RA, Schultz SG, Hassoun HT, DeSoignie R, Weisbrodt NW, Haber MH, Moore FA. The type of sodium-coupled solute modulates small bowel mucosal injury, transport function and ATP after Ischemia/reperfusion injury in rats. Gastroenterology. 2002;123(3):810–6. doi: 10.1053/gast.2002.35389. [DOI] [PubMed] [Google Scholar]
- 20.Sato N, Moore FA, Smith MA, Zou L, Olufemi-Moore S, Schultz SG, Kozar RA. Differential induction of PPAR{gamma} by luminal glutamine and iNOS by luminal arginine in the rodent post ischemic small bowel. Am J Physiol Gastrointest Liver Physiol. 2006;290:616–623. doi: 10.1152/ajpgi.00248.2005. [DOI] [PubMed] [Google Scholar]
- 21.Altmann R, Hausmann M, Spottl T, Gruber M, Bull AW, Menzel K, Vogl D, Herfarth H, Scholmerich J, Falk W, Rogler G. 13-Oxo-ODE is an endogenous ligand for PPARγ in human colonic epithelial cells. Biochem Pharmacol. 2007;74:612–622. doi: 10.1016/j.bcp.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 22.Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, et al. Interleukin-4 dependent production of PPAR gamma ligands in macrophages by 12/15-liopenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 23.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferators-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 24.Estivariz CF, Griffith DP, Luo M, et al. Efficacy of parenteral nutrition supplemented with glutamine dipeptide to decrease hospital infections in critically ill surgical patients. J Parenter Enteral Nutr. 2008;32(4):389–402. doi: 10.1177/0148607108317880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths RD, Allen KD, Andrews FJ, Jones C. Infection, multiple organ failure, and survival in the intensive care unit: influence of glutamine-supplemented parenteral nutrition on acquired infection. Nutrition. 2002;17(7-8):546–52. doi: 10.1016/s0899-9007(02)00817-1. [DOI] [PubMed] [Google Scholar]
- 26.Wischmeyer PE, Kahana MD, Wolfson R, Hongyu R, et al. Glutamine induces heat shock protein and protects against endotoxin shock in the rat. J Appl Physiol. 2001;90:2403–2410. doi: 10.1152/jappl.2001.90.6.2403. [DOI] [PubMed] [Google Scholar]
- 27.Hartl F. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 28.Musch MW, Hayden D, Sugi K, Strasu D, Chang EB. Cell-specific induction for hsp72-mediated protection by glutamine against oxidant injury in IEC 18 cells. Proc Assoc Am Phys. 1998;110(2):136–139. [PubMed] [Google Scholar]
- 29.Wischmeyer PE, Kahana MD, Vandehoek TL, et al. Glutamine reduces cell death and induces recovery of contractile function in cardiac myocytes after ischemia/reperfusion injury. Crit Care Med. 2001;28:A33. [Google Scholar]
- 30.Singleton KD, Beckey VE, Wischmeyer PE. Glutamine prevents activation of NF-κB and stress kinas pathways, attenuates inflammatory cytokine release, and prevents acute respiratory distress syndrome (ARDS) following sepsis. Shock. 2005;24:583–589. doi: 10.1097/01.shk.0000185795.96964.71. [DOI] [PubMed] [Google Scholar]
- 31.Lai YN, Yeh SL, Lin MT, Shang HF, Yeh CL, Chen WJ. Glutamine supplementation enhances mucosal immunity in rats with gut-derived sepsis. Nutrition. 2004;20:286–291. doi: 10.1016/j.nut.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Ckless K, van der Vliet A, Janssen-Heininger Y. Oxidative-nitrosative stress and post-translational protein modifications: implications to lung structure-function relations. Arginase modulates NF-kappaB activity via a nitric oxide-dependent mechanism. Am J Respir Cell Mol Biol. 2007 Jun;36(6):645–53. doi: 10.1165/rcmb.2006-0329SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo PK, Aguirre CC, Tsoa RW, Kern RM, Grody WW, Cederbaum SD, Iyer RK. Widespread expression of arginase I in mouse tissues. Biochemical and physiological implications. J Histochem Cytochem. 2003 Sep;51(9):1151–1160. doi: 10.1177/002215540305100905. [DOI] [PubMed] [Google Scholar]
- 34.Chang CI, Liao JC, Kuo L. Arginase modulates nitric oxide production in activated macrophages. Am J Physiol. 1998 Jan;274(1 Pt 2):H342–8. doi: 10.1152/ajpheart.1998.274.1.H342. [DOI] [PubMed] [Google Scholar]
- 35.Ochoa JB, Bernard AC, O'Brien WE, Griffen MM, et al. Arginase 1 expression and activity in human mononuclear cells after injury. Ann Surg. 2001;233:393–399. doi: 10.1097/00000658-200103000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popovic P, Zeh HJ, Ochoa JB. Arginine and Immunity. J. Nutr. 2007;137:1681S–1686S. doi: 10.1093/jn/137.6.1681S. [DOI] [PubMed] [Google Scholar]
- 37.Bansal V, Ochoa JB. Argnine availability, arginase and the immune response. Curr Opin Clin Nutr Metab Care. 2003 Mar;6(2):223–8. doi: 10.1097/00075197-200303000-00012. Review. [DOI] [PubMed] [Google Scholar]
- 38.Heyland DK, Samis A. Does immunonutrition in patients with sepsis do more harm than good? Intensive Care Med. 2003;29:667–671. doi: 10.1007/s00134-003-1710-6. [DOI] [PubMed] [Google Scholar]
- 39.Sato N, Moore FA, Kone BC, Zou L. Differential induction of PPAR-γ by luminal glutamine and iNOS by luminal arginine in the rodent post ischemic small bowel. Am J Physiol Gastrointest Liver Physiol. 2006;290:G616–G623. doi: 10.1152/ajpgi.00248.2005. [DOI] [PubMed] [Google Scholar]
- 40.Ward DT, Lawson SA, Gallagher CM, Conner WC, Shea-Donohue T. Sustained nitric oxide production via L-arginine administration ameliorates effect of intestinal ischemia-reperfusion. J Surg Res. 2000;89:13–19. doi: 10.1006/jsre.1999.5795. [DOI] [PubMed] [Google Scholar]
- 41.Mailman D. Modulation of hemorrhagic shock by intestinal mucosal N-nitro-L arginine and L-arginine in the anesthetized rat. Shock. 1999;12:155–160. doi: 10.1097/00024382-199908000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Hua TC, Moochhala SM. Influence of L-arginine, aminoguanidine, and NG-nitro-L-arginine methyl ester (L-name) on the survival rate in a rat model of hemorrhagic shock. Shock. 1999;11:51–57. doi: 10.1097/00024382-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Daughters K, Waxman K, Nguyen H. Increasing nitric oxide production improves survival in experimental hemorrhagic shock. Resuscitation. 1996;31:141–144. doi: 10.1016/0300-9572(95)00922-1. [DOI] [PubMed] [Google Scholar]
- 44.Fukatsu K, Ueno C, Maeshima Y, Hara E, Nagayoshi H, Omata J, Mochizuki H, Hiraide H. Effects of L-arginine infusion during ischemia on gut perfusion, oxygen tension, and circulating myeloid cell activation in murine gut ischemia/reperfusion model. JPEN J Parenter Enteral Nutr. 2004;228:224–231. doi: 10.1177/0148607104028004224. [DOI] [PubMed] [Google Scholar]
- 45.Jacob TD, Ochoa JB, Udekwu AO, Wilkinson J, Murray T, Billiar TR, Simmons RL, Marion DW, Peitzman AB. Nitric oxide production is inhibited in trauma patients. J Trauma. 1993;35:590–597. doi: 10.1097/00005373-199310000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Cosgrove M. Perinatal and infant nutrition. Nucleotides. Nutrition. 1998;14(10):748–751. doi: 10.1016/s0899-9007(98)00075-6. [DOI] [PubMed] [Google Scholar]
- 47.Gil A. Modulation of the immune response mediated by dietary nucleotides. Eur J Clin Nutr. 2002 Aug;56(Suppl 3):S1–4. doi: 10.1038/sj.ejcn.1601475. [DOI] [PubMed] [Google Scholar]
- 48.Iwasa Y, Iwasa M, Ohmori Y, Fkutomi T, Ogoshi S. The effect of the administration of nucleosides and nucleotides for parenteral use. Nutrition. 2000;16:598–602. doi: 10.1016/s0899-9007(00)00325-7. [DOI] [PubMed] [Google Scholar]
- 49.Kulkarni AD, Rudolph FB, Van Buren CT. The role of dietary sources of nucleotides in immune function: review. J Nutr. 1994;124(8 Suppl):1442S–1446S. doi: 10.1093/jn/124.suppl_8.1442S. [DOI] [PubMed] [Google Scholar]
- 50.Van Buren CT, Kulkarni AD, Rudolph FB. The role of nucleotides in adult nutrition. J Nutr. 1994 Jan;124(1 Suppl):160S–164S. doi: 10.1093/jn/124.suppl_1.160S. [DOI] [PubMed] [Google Scholar]
- 51.Uauy R, Quan R, Gil A. Role of nucleotides in intestinal development and repair: implications for infant nutrition. J Nutr. 1994 Aug;124(8 Suppl):1436S–1441S. doi: 10.1093/jn/124.suppl_8.1436S. [DOI] [PubMed] [Google Scholar]
- 52.Kulkarni A, Fanslow W, Higley H, Pizzini RP, Rudolph FB, VanBuren CT. Expression of the immune cell surface markers in vivo and immune competence in mice by dietary nucleotides. Transplan Proc. 1989;21:121–124. [PubMed] [Google Scholar]
- 53.Furst P, Kuhn KS. Fish oil emulsions: what benefits can they bring? Clinical Nutrition. 2000;19(1):7–14. doi: 10.1054/clnu.1999.0072. [DOI] [PubMed] [Google Scholar]
- 54.Razzak A, Aldrich C, Babcock TA, Saied A, et al. Attenuation of iNOS in an LPS-Stimulated Macrophage Model by Omega-3 Fatty Acids is Independent of COX-2 Derived PGE2. Journal of Surgical Research. 2008;145:344–250. doi: 10.1016/j.jss.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Novak T, Babcock TA, JHO DH, Helton WS, Espat J. NF-κB inhibition by ω-3 fatty acids modulates LPS-stimulated macrophage TNF-α transcription. Am J of Physiol Lung Cell Mol Physiol. 2003;284:L84–L89. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- 56.Babcock TA, Kurland A, Helton WS, Rahman A, et al. Inhibition of Activator Protein-1 Transcription Factor Activation by ω-3 Fatty Acid Modulation of Mitogen-Activated Protein Kinase Signaling Kinases. Journal of Parenteral and Enteral Nutrition. 2003;27(3):176–181. doi: 10.1177/0148607103027003176. [DOI] [PubMed] [Google Scholar]
- 57.Aldridge C, Razzak A, Babcock TA, Helton WS, et al. Lipopolysaccharide-Stimulated RAW 264.7 Macrophage Inducible Nitric Oxide Synthase and Nitric Oxide Production Is Decreased by and Omega-3 Fatty Acid Lipid Emulsion. Journal of Surgical Research. 2008;149(2):296–302. doi: 10.1016/j.jss.2007.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singer P, Theilla M, Fisher H, et al. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med. 2006;34(4):1033–1038. doi: 10.1097/01.CCM.0000206111.23629.0A. [DOI] [PubMed] [Google Scholar]
- 59.Pontes-Arruda A, Aragao AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med. 2006;34:2325–2333. doi: 10.1097/01.CCM.0000234033.65657.B6. [DOI] [PubMed] [Google Scholar]
- 60.Gadek JE, DeMichele SJ, Karlstad MD, et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999;27:1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Heyland DK, Dhaliwal R, Day AG, Muscedere J, et al. Reducing Deaths due to Oxidative Stress (The REDOXS Study): rationale and study design for a randomized trial of glutamine and antioxidant supplementation in critically-ill patients. Proceedings of the Nutrition Society. 2006;65:250–263. doi: 10.1079/pns2006505. [DOI] [PubMed] [Google Scholar]
- 62.Forceville X, Vitoux D, Gauzit R, Combes A, et al. Selenium, systemic immune response syndrome, sepsis and outcome in critically ill patients. Crit Car Med. 1998;26:1536–1544. doi: 10.1097/00003246-199809000-00021. [DOI] [PubMed] [Google Scholar]
- 63.Goode HF, Cowley HC, Walker BE, Howdle PD, Webster NR. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Critical Care Medicine. 1995;23:646–651. doi: 10.1097/00003246-199504000-00011. [DOI] [PubMed] [Google Scholar]
- 64.Nathens AB, Neff MJ, Jurkovich GJ, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg. 2002;236:814–822. doi: 10.1097/00000658-200212000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Collier BR, Giladi A, Dossett LA, et al. Impact of High-dose antioxidants on outcomes in acutely injured patients. J Parenter Enteral Nutr. 2008;32:384–388. doi: 10.1177/0148607108319808. [DOI] [PubMed] [Google Scholar]
- 66.Heyalnd DK, Dhaliwal R, Suchner U, Berger M. Antioxidant nutrients: a systemic review of trace elements and vitamins in the critically ill patient. Int Car Med. 2005;31:327–337. doi: 10.1007/s00134-004-2522-z. [DOI] [PubMed] [Google Scholar]
- 67.Heyland DK, Dhaliwalm R, Day A, et al. Optimizing the dose of glutamine dipeptides and antioxidants in critically ill patients: a phase I dose-finding study. J Parenter Enteral Nutr. 2007;31:109–118. doi: 10.1177/0148607107031002109. [DOI] [PubMed] [Google Scholar]