Abstract
Background
Administration of mesenchymal stem cells (MSCs) is an effective therapy to repair cardiac damage after myocardial infarction (MI) in experimental models. However, the mechanisms of action still need to be elucidated. Our group has recently suggested that MSCs mediate their therapeutic effects primarily via paracrine cytoprotective action. Furthermore, we have shown that MSCs overexpressing Akt1 (Akt-MSCs) exert even greater cytoprotection than unmodified MSCs. Thus far, little has been reported on the metabolic characteristics of infarcted hearts treated with stem cells. Here we hypothesize that Akt-MSC administration may influence the metabolic processes involved in cardiac adaptation and repair after MI.
Methods and Results
MI was performed in rats randomised in four groups: sham group and animals treated with control MSCs, Akt-MSCs or phosphate buffer solution (PBS). High energy metabolism and basal 2-deoxy-glucose (2-DG) uptake were evaluated on isolated hearts using phosphorous-31 nuclear magnetic resonance spectroscopy at 72 hours and two weeks after MI. Treatment with Akt-MSCs spared phosphocreatine stores and significantly limited the increase in 2-DG uptake in the residual intact myocardium compared to the PBS or the MSC treated animals. Furthermore, Akt-MSC treated hearts had normal pH, whereas low pH was measured in the PBS and MSC groups. Correlative analysis indicated that functional recovery after MI was inversely related to the rate of 2-DG uptake.
Conclusion
We conclude that administration of MSCs overexpressing Akt at the time of infarction results in preservation of normal metabolism and pH in the surviving myocardium.
Keywords: metabolism, glucose uptake, paracrine effect, mesenchymal stem cell, myocardial infarction
Myocardial infarction (MI) in the absence of timely reperfusion leads to significant loss of cardiomyocytes 1,2. The reduction of cardiac muscle imposes an increased mechanical load on the remaining viable cardiomyocytes 3. The capacity of the non-infarcted tissue to undergo structural and metabolic remodeling and to compensate for the loss of cardiac tissue critically determines the subsequent hemodynamic course and the clinical outcome. Even though currently available therapeutic strategies are able to partially counteract the maladaptive remodeling processes and thus ameliorate the clinical prognosis, none of them can reverse the myocardial damage and fully restore the cardiac function to the pre-MI status. As a result, many patients surviving MI develop congestive heart failure and eventually succumb. Consequently, there is the urgency for new and more effective therapies to prevent and/or reverse post-MI ventricular remodeling.
Recently, the possibility of regenerating and/or repairing infarcted myocardium using cell-based therapies has received much attention 4. One of these strategies uses mesenchymal stem cells (MSCs). Adult bone marrow derived MSCs are multipotent stem cells that, given their ease of isolation, low immunogenicity, amenability to ex vivo expansion and genetic modification, are optimal candidates for cardiac cell therapy 5-8. Indeed, intramyocardial injection of MSCs has been shown to be an effective strategy to repair post-MI damage in experimental models 7-11. The mechanisms by which these cells exert their beneficial effects remain controversial. Several studies have reported evidence of de novo cardiomyogenesis from the donor cells as well as improved vascularization after injection of MSCs 7-11. Recently, we and others have suggested that the MSCs mediate their therapeutic effects primarily via paracrine actions 12-14. In particular, we demonstrated that MSCs release soluble factors that are cytoprotective 12. Furthermore, we have shown that MSCs genetically modified to overexpress the survival gene Akt1 (Akt-MSCs) exert an even greater cytoprotective effect than unmodified MSCs in both adult cardiomyocytes challenged with hypoxia and in intact hearts following infarction. In both a mouse and rat model of MI the salutary effects could be demonstrated within 72 hours, suggesting that Akt-MSCs promote early survival of ischemic myocytes 15-17.
Treating infarcted myocardium with MSCs may also influence metabolic processes involved in cardiac adaptation and repair within the myocytes of the surviving myocardium 18,19. So far, little has been reported on the metabolic characteristics of the surviving myocardium of hearts treated with stem cells. A recent study using a pig model of MI 20 has reported that the surviving myocardium of MSC-treated hearts demonstrated a partial improvement (30%) in the ratio of phosphocreatine (PCr), the energy reserve metabolite, to ATP, the energy metabolite required for cell function and viability. An improvement, or alternatively preservation, of this ratio is important because it indicates that the balance between the cost of contraction and ATP supply in the surviving myocardium is closer to normal. It is also known that the surviving myocardium undergoes metabolic remodeling of entire pathways for ATP supply. An early metabolic change that occurs in the surviving myocardium of infarcted hearts is a change in substrate preference away from free fatty acid oxidation toward increased glucose uptake and utilization needed for the synthesis of ATP 18,19,21. It remains unclear whether the metabolic shift toward increased glucose utilization is adaptive or maladaptive 22. While high glucose utilization yields increased ATP supply 23, some have suggested that the accumulation of protons from increased anaerobic glycolysis may exacerbate myocardial injury and depress cardiac function 24,25. There are no data available defining the cardioprotective consequences for Akt-modified MSCs on energetics of the surviving myocardium, and there is no information about the effect of modified or unmodified MSCs on metabolic remodeling of the surviving myocardium early post MI.
Thus, in the current study, we tested whether injecting either MSCs or Akt-MSCs in the peri-infarct zone of rat hearts at the time of MI may preserve ATP and PCr levels in the surviving myocardium. We also tested whether the surviving myocardium of hearts protected by supply of Akt-MSCs and MSCs demonstrated metabolic remodeling or whether the cytoprotection was sufficient to protect the surviving myocardium from metabolic remodeling. We assessed metabolic remodeling as glucose (2-deoxyglucose, 2-DG) uptake rate and pH homeostasis. [ATP] and [PCr], intracellular pH and glucose uptake rates were quantified using 31P nuclear magnetic resonance (31P NMR) spectroscopy of isolated infarcted rat hearts, which had been injected with either saline, control MSCs or Akt-MSCs one hour after coronary artery ligation. In order to determine whether any difference in metabolic remodeling was associated with myocardial contractile performance, we also measured global left ventricular (LV) contractile function in the same hearts simultaneously with the 31P NMR experiments. Finally, to determine whether any change was rapid, supporting a mechanism of action of preservation, or occurred only after some time, supporting a mechanism of repair, measurements were made at two time points, 72 hrs and two weeks post MI.
METHODS
Mesenchymal stem cell isolation and retroviral transduction
We isolated and expanded the MSCs from the bone marrow of adult Sprague Dawley male rats (Harlan World Headquarters, Indianapolis, Indiana) according to protocols reported previously 26. After two passages, the cells were transduced with monocistronic or bicistronic murine stem cell retrovirus encoding either the reporter gene green fluorescent protein (MSCs) or both the GFP and the Akt1 genes simultaneously (Akt-MSCs). The cells were transduced twice with 10 multiplicity of infection (MOI) of either virus for six hours in the presence of 8 μg/ml polybrene during a two-day period. The cells were then passed twice before being used for the experiments. Transduction efficiency was assessed by FACS analysis (Becton Dickinson FACS Vantage).
Myocardial infarction model
Female Sprague Dawley rats (180-200 grams of body weight) (from Harlan) were used in all studies. Before surgery, the animals were randomized into four groups. Three groups underwent coronary artery ligation; the fourth group served as a surgical sham control. The investigators responsible for surgery, cell injections, isolated heart experiments and data analysis were blinded to the treatment groups. Ligation of the left ascending coronary artery was performed essentially as previously described 27. Briefly, the animals were anesthetized by intraperitoneal injection (IP) of a mixture of xylazine (10 mg/kg) and ketamine (80 mg/kg). Under artificial ventilation with air, a left thoracotomy was performed and the left coronary artery was ligated with a silk suture approximately midway between the left atrium and the apex of the heart. Infarction was identified by the presence of ST elevation at the ECG tracing and by blanching of the ischemic myocardium. One hour after MI, 5×106 MSCs or Akt-MSCs re-suspended into 100 μL of phosphate buffered saline (PBS) were injected in 5 different sites in the border zone. An equivalent volume of PBS was injected in the control group. Sham animals were operated the same way, except that the ligature was left loose and no injection was performed. All animal procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act and were approved by the Harvard Standing Committee on Animals.
Isolated perfused heart preparation and measurement of contractile performance
The animals were sacrificed either at 72 hours or two weeks after MI. The rats were heparinized (2,850 units/kg, IP) and then euthanized by injection of sodium pentobarbital (100 mg/kg, IP). The hearts were isolated and perfused in the Langendorff mode in a 20-mm glass NMR tube as described 28. Briefly, the heart was excised, arrested in ice-cold buffer, and connected via the aorta to the perfusion cannula. Retrograde perfusion was carried out at a constant coronary perfusion pressure of 75 mm Hg at 37°C with insulin-free Krebs-Henseleit buffer containing (mM concentration) NaCl 118, KCl 5.3, CaCl2 1.75, MgSO4 1.2, EDTA 0.5, NaHCO3 25, pyruvate 5 and glucose 5 prepared freshly and equilibrated with 95% O2/5% CO2, yielding a pH of 7.4. Right ventricular drainage was accomplished by incision of the pulmonary artery. The effluent from the Thebesian veins was drained by a thin polyethylene tube (PE-90) pierced through the apex of the LV. A water-filled balloon was inserted into the LV for recording of ventricular pressures and heart rate. The balloon was inflated to set LV end diastolic pressure (EDP) to ~10 mm Hg and the balloon volume was then held constant. Isovolumic contractile performance data were collected online at a sampling rate of 200 Hz using a commercially available data acquisition system (MacLab; ADInstruments Pty., Milford, MA, USA). LV developed pressure (DevP) [the difference between systolic pressure (SP) and EDP], the rate-pressure product (RPP) (product of DevP and HR), and minimum and maximum values within a beat of the first derivative of left ventricular pressure (+dP/dt and − dP/dt) were calculated off-line.
Measurement of [ATP], [PCr], inorganic phosphate [Pi] and pH
[ATP], [PCr] and [Pi] were measured in isolated perfused hearts by 31P NMR spectroscopy. The isolated perfused rat hearts were placed in a 20-mm NMR sample tube and inserted into a 1H/31P double-tuned probe situated in an 89-cm bore 9.4-Tesla superconducting magnet. 31P NMR spectra were collected without proton decoupling at a pulse width of 15 μs, pulse angle of 60°, recycle time of 2.3 s, and sweep width of 6,000 Hz using a GE-400 wide-bore spectrometer (Omega, General Electric, Fremont, California, USA). Single spectra were collected during four-minute periods and consisted of data averaged from 104 free induction decays. 31P NMR spectra were analyzed using 20-Hz exponential multiplication and zero and first-order phase corrections.
The resonance areas corresponding to ATP, PCr and Pi were fitted to Lorentzian function and calculated using a commercially available program (NMR1 software, New Methods Research Inc., Syracuse, New York, USA) 29 and corrected for saturation [ATP (1.0), PCr (1.2), Pi (1.15) and 2-DG-P(1.35, see below)]. To determine the cytosolic concentration of these metabolites, the absolute resonance areas corresponding to the [γ-P]ATP, PCr and Pi in the 31P NMR spectra were normalized by heart weight. Since the protein concentrations, determined according to the Lowry method (see below), were similar among the different groups (Sham: 0.185 ± 0.009; PBS: 0.188±0.007; MSC: 0.188±0.003 and Akt-MSC: 0.175±0.009 mg/mg wet weight), we made the assumption that the fractional volumes of intracellular water in the myocytes of all the hearts analyzed were similar and equal to values typical of well-perfused rodent hearts (0.48 μl/mg wet wt) 30. The mean of [γ-P]ATP peak areas of the 31P NMR spectrum for sham hearts obtained during baseline perfusion period was set to 10 mM 31. Changes in [ATP], [PCr] and [Pi] during the protocols were calculated by multiplying the ratio of their resonance peak areas to the mean of the [γ-P]ATP peak areas from the initial baseline spectrum by 10 mM. Intracellular pH was determined by comparing the chemical shift of the Pi peak and relative position of PCr in each spectrum to values from a standard curve.
Changes in [ATP] and its metabolites can be described by a single number, the free energy released from ATP hydrolysis (ΔG~ATP). It is calculated as: |ΔG~ATP | (kJ/mol) = |ΔG° + RT ln ([ADP][Pi]/[ATP])|, where ΔG° (−30.5 kJ/mol) is the value of ΔG~ATP under standard conditions of molarity, temperature, pH, and [Mg2+] 32, R is the gas constant (8.3 J/mol K), and T is the temperature in degrees Kelvin. [ATP] and [Pi] were obtained from NMR measurements; [ADP] was calculated from the creatine kinase equilibrium expression as described 33. Because ΔG~ATP is a negative number, we describe changes in ΔG~ATP in absolute values, denoted as |ΔG~ATP |.
Measurement of 2-DG uptake rate
Glucose uptake rate was also measured by 31P NMR as described 23,34. Hearts were perfused with an insulin-free buffer in which 5 mM 2-DG replaced 5 mM glucose. Upon entering the cell, 2-DG is phosphorylated by hexokinase to 2-DG phosphate (2-DG-P), which produces an easily detectable peak in 31P NMR spectra. The area of this peak is proportional to the amount of 2-DG-P accumulated and was calculated using the same software that we used to determine the other NMR-observable metabolite concentrations. Even though the phosphorylation of 2-DG leads to decreases in [PCr] and [ATP], our perfusion protocol was designed to ensure the linearity of 2-DG-P accumulation with time 23,24.
Measurement of the infarcted tissue and chamber weights
At the end of each of these experiments, the hearts were removed from the perfusion apparatus, immerged in ice-cold PBS and dissected under a stereomicroscope. The infarcted tissue, identified visually by its pallor as compared with the pink-red colour of the viable myocardium, was separated by the viable LV, blotted and weighed using an electronic scale. The remaining viable LV, the right ventricle and both atria were blotted and weighed. The tissue was then immediately frozen in liquid nitrogen and stored at −80 °C for subsequent biochemical assays.
Biochemical assays
~50 mg of ventricular tissue were homogenized for 10 seconds at 4 °C in potassium phosphate buffer containing 1 mM EDTA and 1 mM β-mercaptoethanol at pH 7.4 (final concentration of 5 mg wet wt ml−1). Aliquots were used for protein quantification assay and for total creatine content measurement 35. All reagents were purchased from Sigma Chemical Co. (St. Louis, Missouri, USA).
Statistical analysis
All results are reported as mean ± standard error (SE) and the data were analyzed with a one-way or two-way ANOVA followed by Bonferroni all pair-wise multiple comparison test. Probability (p) values less than 0.05 were considered statistically significant.
RESULTS
The MSCs were separated from the hematopoietic cells based on their preferential attachment to polystyrene surface and their identity was verified testing specific cell surface markers (data not shown) 26. The efficiency of the retroviral transduction was approximately 90% for both viruses (data not shown), as previously reported 26.
Infarct weights and cardiac performance
We have previously reported that injecting Akt-MSCs into the peri-infarct region at the time of occlusion preserved more myocardium than untreated MSCs, resulting in smaller infarct size and better contractile performance of the remaining viable myocardium, both in rats and in mice 12,15,16. The hearts studied here displayed the same pattern, making them suitable for studying the surviving myocardium. In the current study, we grossly estimated cardiac damage by weighing infarcted tissue at both 72 hours and two weeks after MI (Table 1). The results showed that Akt-MSC treatment significantly limited the amount of damaged myocardium and confirmed our previous results on infarct size as determined by histological analysis of paraffin-embedded and freshly stained heart sections 12,15,16,26. Most importantly, administration of Akt-MSCs was accompanied by better parameters of both systolic and diastolic performance (Table 2). At 72 hrs post-infarction, DevP in the Akt-MSC hearts was 43% higher compared with the untreated infarct hearts (p<0.05) and 26% higher relative to the MSC treated hearts (p<0.05). In Akt-MSC hearts, the rate of tension development, +dP/dt, was higher compared with both the untreated (+46%, p<0.05) and the MSC (+39%, p<0.05) treated infarcted hearts. The rate of relaxation, −dP/dt, in the Akt-MSC injected hearts was 53% and 50% higher than in the untreated (p<0.05) and the MSC (p<0.05) treated infarcted hearts, respectively. These differences persisted for at least two weeks. Remarkably, for Akt-MSC hearts, systolic performance was only 12 % (72 hr) to 17 % (2 week) lower compared with sham-operated hearts while diastolic performance remained normal. In contrast, for unmodified MSC-treated and untreated infarcted hearts, markers of both systolic and diastolic performance were significantly lower than for sham-operated hearts.
Table 1.
Anatomical paremeters in sham, untreated (PBS), control MSC treated (MSC), and Akt-MSC treated animals at 72 hours and 2 weeks after myocardial infarction
| Sham (n=9) |
PBS (n=7) |
MSC (n=7) |
Akt-MSC (n=9) |
|
|---|---|---|---|---|
| 72 hours | ||||
| Body weight (g) | 211 ± 7 | 209 ± 6 | 216± 5 | 203 ± 2 |
| Heart weight (mg) | 628 ± 8 | 652 ± 2 | 620 ± 6 | 609 ± 3 |
| LV weight (mg) | 446 ± 5 | 449 ± 7 | 440 ± 5 | 429 ± 5 |
| Infarcted tissue weight (mg) | - | 89 ± 4 | 71 ± 4* | 42 ± 5*# |
| 2 weeks | ||||
| Body weight (g) | 215 ± 3 | 214 ± 5 | 213 ± 4 | 210 ± 3 |
| Heart weight (mg) | 693 ± 13 | 695 ± 11 | 695 ± 9 | 660 ± 13 |
| LV weight (mg) | 503 ± 13 | 498 ± 6 | 503 ± 10 | 466 ± 14 |
| Infarcted tissue weight (mg) | - | 96 ± 6 | 71 ± 5* | 40 ± 7*# |
Values expressed as average ± SE
p<0.05 vs PBS
p<0.05 vs MSC.
Abbreviations: Akt-MSC, MSCs overexpressing Akt1; LV, left ventricular; MSC, mesenchymal stem cells; PBS, phosphate-buffered saline.
Table 2.
Left ventricular function in sham, untreated (PBS), control MSC treated (MSC), and Akt-MSC treated animals at 72 hours and 2 weeks after myocardial infarction
| Sham (n=9) |
PBS (n=7) |
MSC (n=7) |
Akt-MSC (n=9) |
|
|---|---|---|---|---|
| 72 hours | ||||
| DevP (mmHg) | 98 ± 2 | 60 ± 5* | 68 ± 3* | 86 ± 3*#† |
| RPP (mmHg/min) | 29,131 ± 733 | 18,487 ± 1,588* | 21,661 ± 1,159* | 25,656 ± 948*#† |
| +dP/dt (mmHg/min) | 3,027 ± 63 | 1,893 ± 93* | 1,988 ± 93* | 2,760 ± 114#† |
| −dP/dt (mmHg/min) | 2,008 ± 72 | 1,254 ± 65* | 1,280 ± 89* | 1,919 ± 71#† |
| 2 weeks | ||||
| DevP (mmHg) | 102 ± 4 | 55 ± 6* | 61 ± 2* | 84 ± 5*#† |
| RPP (mmHg/min) | 31,148 ± 2.080 | 18,675 ± 2,927* | 18,790 ± 1,280* | 25,089 ± 2,131*#† |
| +dP/dt (mmHg/min) | 2,894 ± 69 | 1,866 ± 172* | 1,824 ± 66* | 2,406 ± 135*#† |
| −dP/dt (mmHg/min) | 1,868 ± 233 | 1,195 ± 167* | 1,094 ± 103* | 1,712 ± 170#† |
Values expressed as average ± SE
p<0.05 vs sham
p<0.05 vs PBS
p<0.05 vs control MSC.
Abbreviations: Akt-MSC, MSCs overexpressing Akt1; DevP, LV developed pressure; þdP/dt, maximum values within a beat of the first derivative of left ventricular pressure; −dP/dt, minimum values within a beat of the first derivative of left ventricular pressure; MSC, mesenchymal stem cells; PBS, phosphate-buffered saline; RPP, rate-pressure product.
Myocardial high-energy phosphate content
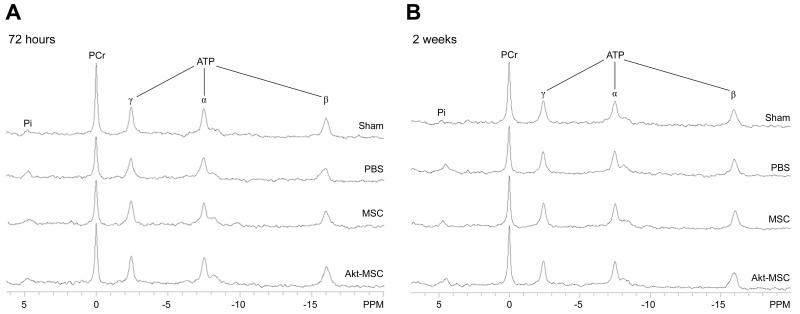
To test whether the energetics of the surviving myocardium was preserved in Akt-MSC treated hearts, we measured the concentrations of the high energy phosphate metabolites, ATP, PCr, and Pi, and calculated the free energy of ATP hydrolysis, ΔG~ATP, kJ mol−1, for hearts isolated both at 72 hr and two weeks post infarction (Fig. 1, Table 3). As the representative 31P NMR spectra demonstrate (Fig. 1), no differences in [ATP] and [Pi] were observed among the four groups at 72 hours after MI. However, [PCr], a sensitive marker of increased cost of contraction relative to ATP supply, was significantly decreased in both the untreated and the MSC treated hearts compared with sham-operated hearts. In contrast, [PCr] was preserved in the surviving tissue of hearts treated with Akt-MSCs. Small differences in [ADP] among groups (data not shown) were not large enough to alter ΔG~ATP. Two weeks after MI, [ATP] and [Pi] remained normal in all groups and [PCr] returned to normal levels in both the untreated and MSC treated hearts. As a result, at the two-week time point, there were no longer differences in [PCr] among the four groups.
Figure 1.
Representative 31P NMR spectra from sham, untreated (PBS), control MSC-treated, and Akt-MSC-treated heart at baseline. For each spectrum, peaks from left to right represent Pi, PCr, and γ-, α and β-phosphates of ATP. (A): Note that at 72 hours the PCr peak is decreased in the PBS and MSC groups compared with both the sham and the Akt-MSC-treated hearts. (B): After 2 weeks, the PCr peak recovered both in the PBS and control MSC groups to baseline levels. Abbreviations: Akt-MSC, MSCs overexpressing Akt1; MSC, mesenchymal stem cells; PBS, phosphate-buffered saline; PCr, phosphocreatine; Pi, inorganic phosphate.
Table 3.
Energetics in sham, untreated (PBS), control MSC treated (MSC), and Akt-MSC treated animals at 72 hours and 2 weeks after myocardial infarction
| Sham (n=9) |
PBS (n=7) |
MSC (n=7) |
Akt-MSC (n=9) |
|
|---|---|---|---|---|
| 72 hours | ||||
| ATP (mM) | 10.0 ± 0.7 | 9.9 ± 0.7 | 9.6 ± 0.7 | 10.0 ± 1.0 |
| PCr (mM) | 17.4 ± 1.5 | 15.1 ± 1.8* | 15.3 ± 0.6* | 16.5 ± 1.3 |
| Pi (mM) | 2.1 ± 1.1 | 1.9 ± 0.6 | 2.5 ± 0.9 | 1.7 ± 0.8 |
| |ΔG~ATP| (kJ/mo1) | 61.7 ± 0.5 | 61.1 ± 0.6 | 60.3 ± 0.4 | 61.9 ± 0.6 |
| 2 weeks | ||||
| ATP (mM) | 10.0 ± 0.4 | 10.5 ± 0.4 | 10.1 ± 0.4 | 10.1 ± 0.4 |
| PCr (mM) | 18.4 ± 0.8 | 19.2 ± 0.8 | 18.7 ± 0.5 | 19.1 ± 0.6 |
| Pi (mM) | 1.4 ± 0.5 | 1.1 ± 0.1 | 1.5 ± 0.4 | 0.9 ± 0.4 |
| |ΔG~ATP| (kJ/mo1) | 62.7 ± 0.5 | 64.1 ± 1.7 | 62.4 ± 0.7 | 65.4 ± 1.1 |
Values expressed as average ± SE
p<0.05 vs sham.
Abbreviations: Akt-MSC, MSCs overexpressing Akt1; |ΔG~ATP|, absolute values of free energy released from ATP hydrolysis;
MSC, mesenchymal stem cells; PBS, phosphate-buffered saline; PCr, phosphocreatine; Pi, inorganic phosphate.
Intracellular pH
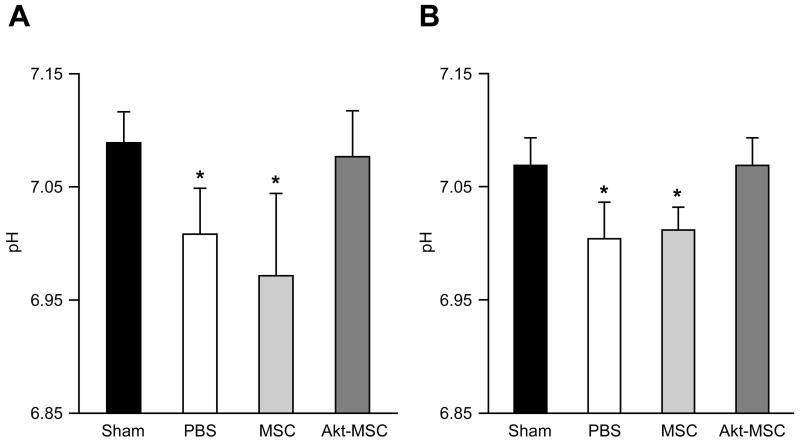
Fig 2 shows values for intracellular pH in the surviving myocardium measured at 72 hrs (Fig. 2A) and two weeks (Fig. 2B) following MI. While the decreases are small, pH in both the untreated and the MSC treated hearts was significantly decreased at 72 hrs compared with the sham (p<0.05). In contrast, pH did not decrease in the Akt-MSC treated hearts. The decrease in pH in the untreated and control MSC treated hearts persisted at two weeks after MI, whereas in the Akt-MSC hearts the pH remained normal.
Figure 2.
Intracellular pH.
(A): 72 hours after myocardial infarction, the pH was significantly reduced in untreated (phosphate-buffered saline [PBS]) and control MSC-treated animals compared with sham, whereas it was normal in the Akt-MSC group. (B): At 2 weeks, the pH in the viable myocardium of the PBS and MSC animals was still significantly lower compared with both sham and Akt-MSC groups. *, p < .05 versus sham and Akt-MSC. Abbreviations: Akt-MSC, MSCs overexpressing Akt1; MSC, mesenchymal stem cells; PBS, phosphate-buffered saline.
Glucose uptake
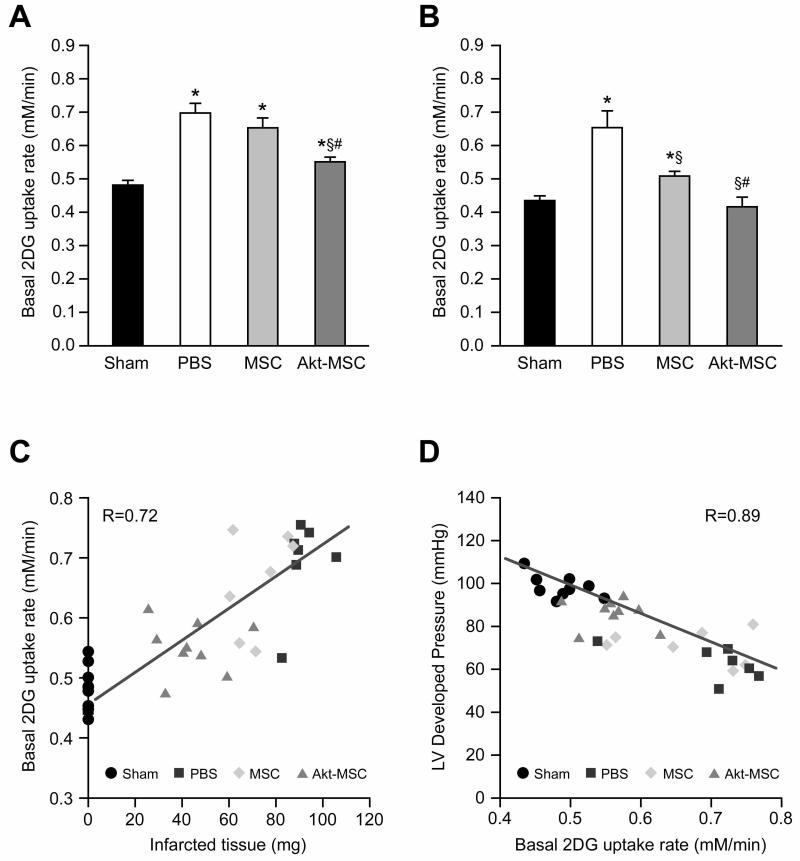
To determine whether the surviving myocardium demonstrated evidence of metabolic remodeling by increasing basal glucose uptake, we measured insulin-independent 2-DG uptake rates in all four groups at both 72 hr (Fig. 3A) and two weeks (Fig. 3B) post MI. At 72 hrs, 2-DG uptake was increased in both the untreated and MSC treated hearts compared with the sham-operated hearts (+43% and +36% respectively, p<0.05). The 2-DG glucose uptake was also increased in the Akt-MSC treated hearts (+14% vs. sham, p<0.05), but the uptake rate was significantly lower than in both untreated and MSC treated hearts (p<0.05). Two weeks after MI, the 2-DG uptake rate remained elevated in the untreated hearts (+49%; p<0.05), fell by ~half after administration of MSC (+18%; p<0.05) and returned to normal levels in the Akt-MSC treated hearts. We found a strong positive correlation (r = 0.72) between the rate of glucose uptake and infarct weight (Fig. 3C) and a strong negative correlation (r = 0.89) between 2-DG uptake and LV developed pressure (Fig. 3D).
Figure 3.
Baseline 2-DG uptake rate. (A): 72 hours after myocardial infarction, the 2-DG uptake rate was significantly increased in untreated (PBS) and control MSC-treated animals; also in the Akt-MSC group the 2-DG uptake rate was increased compared with the sham group, but it was significantly lower than in the PBS and MSC groups. (B): At 2 weeks, the 2-DG uptake rate was normalized in the Akt-MSC-treated hearts, whereas it was still significantly higher in both the PBS and MSC groups when compared with the sham. Statistics: *, p < .05 versus sham; §, p < .05 versus PBS group; #, p < .05 versus control MSC group. (C): Scatterplot showing direct correlation between 2-DG uptake rate and wet weight of the infarcted tissue. (D): The LV developed pressure was plotted against 2-DG uptake and the result was a significant inverse correlation. Abbreviations: Akt-MSC, MSCs overexpressing Akt1; 2-DG, 2-deoxy-glucose; LV, left ventricular; MSC, mesenchymal stem cells; PBS, phosphate-buffered saline.
Enzyme activities in the viable cardiac tissue
We measured the activity of the major glycolytic and mitochondrial oxidative enzymes in the viable myocardium of the various groups at 2 weeks after infarction (Table 4). No significant differences were found among groups in the content of glycolytic enzymes glyceraldehyde phosphate dehydrogenase, phosphofructokinase or lactate dehydrogenase, or the content of oxidative enzymes citrate synthase and cytochrome oxidase. The activity of creatine kinase, the cytosolic enzyme required for PCr generation, also did not differ significantly between the four groups (Table 4).
Table 4.
Enzyme quantification in the viable tissue at 2 weeks
| Sham (n=9) |
PBS (n=7) |
MSC (n=7) |
Akt-MSC (n=9) |
|
|---|---|---|---|---|
| CK (IU/mg) | 6.14 ± 0.18 | 5.86 ± 0.11 | 5.48 ± 0.22 | 5.57 ± 0.21 |
| CS (IU/mg) | 1.06 ± 0.05 | 1.03 ± 0.06 | 1.05 ± 0.03 | 1.01 ± 0.02 |
| COX (IU/mg) | 117 ± 6 | 114 ± 4 | 124 ± 4 | 123 ± 2 |
| GAPDH (IU/mg) | 0.7 ± 0.04 | 0.72 ± 0.13 | 0.76 ± 0.06 | 0.7 ± 0.07 |
| PKF (IU/mg) | 0.22 ± 0.01 | 0.22 ± 0.02 | 0.22 ± 0.03 | 0.2 ± 0.01 |
| LDH (IU/mg) | 1.1 ± 0.05 | 1 ± 0.06 | 1.05 ± 0.07 | 1.1 ± 0.01 |
CK: creatine kinase; CS: citrate synthase; COX: cytochrome oxidase; GAPDH: glyceraldehyde phosphate dehydrogenase; PFK: phosphofructokinase; LDH: lactate dehydrogenase. Values expressed as average ± SE.
DISCUSSION
We have reported that transplantating MSCs to the peri-infarct region promotes early survival of ischemic myocytes and that MSCs overexpressing the survival gene Akt1 exert even greater cytoprotection 12,15,26. In the current study, we tested whether the surviving myocardium of hearts protected by supply of MSCs and Akt-MSCs demonstrate metabolic remodeling characteristic of surviving myocardium post infarction, or whether the cytoprotection is sufficient to protect the surviving myocardium from metabolic remodeling. Three important aspects of cardiac metabolism were measured: high-energy phosphate content, intracellular pH and insulin-independent glucose uptake rates. To determine whether any change was rapid, supporting a mechanism of action for MSCs of preservation, or required time to develop, supporting a mechanism of repair, measurements were made at 72 hrs and two weeks. We found that the surviving myocardium of hearts treated with Akt-MSCs had normal high-energy phosphate content and normal pH at 72 hrs post-infarction, and that the small increase in basal glucose (assessed as 2-DG) uptake rate observed at 72 hrs normalized by two weeks post-infarction. In contrast, hearts treated with control MSCs demonstrated a transient decrease in [PCr], a small but persistent fall in pH and persistent increase in glucose (as 2-DG) uptake rate similar to untreated infarcted hearts. These results suggest that transplanting MSCs overexpressing Akt at the time of infarction leads to preservation of normal metabolism in the surviving myocardium.
The untreated infarcted hearts
Metabolic abnormalities are a hallmark of MI 18,19,24,36-38. In the ischemic myocardium, ATP utilization exceeds ATP synthesis rates; as a consequence, [PCr] rapidly falls, followed by loss of [ATP] 21. For myocytes that go on to infarction either by apoptosis and/or necrosis, neither PCr nor ATP recover 24. For cardiomyocytes that recover from ischemia, PCr and ATP are rapidly re-synthesized; the extent of recovery depends on the duration and magnitude of the low-flow state 39,40. The increased mechanical load in the surviving myocardium caused by loss of myocytes also leads to molecular remodeling that is characterized by an up-regulation of ATP-synthesizing pathways and down-regulation in ATP-consuming pathways. Increased glucose uptake is one early measure of this remodeling 21,22,41,42. This remodeling is thought to be mediated in part by AMP-dependent protein kinase, activated by elevated levels of AMP secondary to a lower ratio of PCr to creatine 43. Thus, the transient decrease in [PCr] with normal [ATP] and unchanged [total creatine] observed here for the surviving myocardium in untreated infarcted hearts indicates a mismatch in ATP supply and demand at 72 hrs that normalizes by two weeks post-infarction 24,36-38. The increase in glucose uptake and decrease in intracellular pH (see below) for the untreated infarcted hearts was observed as early as 72 hrs post MI and persisted at two weeks following MI, indicative of metabolic remodeling.
The control MSC treated infarcted hearts
A recent report characterizing the border zone of the surviving myocardium of a swine model of MI treated with unmodified bone-marrow derived stem cells can be compared to our results for control MSC treated infarcted rat hearts 20. Four weeks following permanent occlusion in the pig heart, PCr/ATP in the border zone of the infarct fell from normal values of 2.0 to 1.2; absolute values of PCr and ATP were not measured. Hearts treated with stem cells demonstrated partial improvements in PCr/ATP (from 1.2 to 1.5), regional contractile performance and global LV ejection fraction; infarct sizes in untreated (13% by area) and treated (10-11%) hearts were similar. We also observed an improvement in PCr/ATP between 72 hrs to two weeks post MI in surviving myocardium of MSC-treated rat hearts, from 1.5 to 1.9. However, we observed the same improvement in untreated infarcted hearts, suggesting that the change in PCr/ATP in MSC-treated hearts was not mediated by MSCs. MSC-specific effect on post MI rat hearts was a partial recovery of glucose uptake rate at two weeks post MI (p<0.05), while neither systolic nor diastolic performance improved.
The Akt-MSC treated hearts
In contrast to hearts injected with unmodified MSCs, the surviving myocardium of hearts treated at the time of infarction with Akt-MSCs had normal [PCr] and [ATP] as early as 72 hrs post-MI. In addition, intracellular pH remained normal and glucose uptake rate was only transiently elevated. Even by 72 hrs, systolic contractile performance was only minimally affected and diastolic performance was preserved. This metabolic and functional profile is consistent with protection, rather than repair, of ischemic myocytes by mechanisms operating within the first few hours of Akt-MSC supply. The damage occurring after coronary occlusion is a dynamic process and after the initial loss of cardiomyocytes in the ischemic region in the first few hours after MI, the infarcted area expands, largely due to apoptosis of cells at the infarct border 44,45. Thus, early interventions capable of arresting apoptosis in the infarct border zone would be expected to limit infarct expansion and infarct size 3,46,47. Our current observation that treating MI hearts with Akt-MSCs, but not untreated MSCs, leads to preservation of normal levels of both [PCr] and [ATP] as early as 72 hrs post-infarction and to only a small transient increase in glucose uptake rate in the surviving myocardium may partially explain the salutary effects of cell therapy with Akt-MSCs in myocardial recovery after MI. Cardiac metabolism may be influenced both by cell-to-cell contact between the MSCs and the ischemic but viable cardiomyocytes present at the border zone of the infarct and/or by paracrine factors secreted by the transplanted cells and able to positively influence the cardiomyocyte metabolism 48-50. In either case, there are plausible explanations to the fact that Akt-MSCs are more effective than control MSCs in preserving cardiac metabolism. Indeed, the overexpression of Akt may have several important effects on MSCs 15,26. We have shown that Akt can reduce the number of MSCs dying after their injection by activating survival pathways 26. The higher number of Akt-MSC engrafted might account for their pronounced effect on cardiac metabolism compared with the GFP-MSCs. Furthermore, Akt can also increase the production and/or release of secreted factors by the MSCs 15,17 which would explain, in addition to the higher number of cells engrafted, the differences between GFP-MSCs and Akt-MSCs if the main mechanism mediating the metabolic effects was through paracrine factors. For example, we have shown 15 that the production of IGF-I and VEGF is increased in MSCs overexpressing Akt and both these factors have been described to influence cardiac metabolism 51,52.
pH
The finding that intracellular pH was lower in surviving myocardium of untreated and control MSC treated hearts, but not of Akt-MSC treated hearts, at both 72 hrs and two weeks post MI was unexpected. Using the same non-invasive method to determine intracellular pH used here, it has been shown that recovery of intracellular pH in surviving myocardium following even long durations of ischemia is rapid and complete, within four minutes39. Furthermore, normal pH values have been reported for surviving myocardium in a rat model eight weeks post MI 24. It is possible that differences as small as that observed here, ~0.1 units, could have been missed. Indeed, pH during reperfusion following 60 minutes of total ischemia in the ferret heart tended to fall by this amount, but did not reach statistical significance 39. The differences in pH observed here are in the range of pH values shown to increase apoptosis 53 and to negatively influence cardiac contractility 54. It is noteworthy that the pH in the viable myocardium of Akt-MSC treatment was normal, and that apoptosis was lower and contractile performance higher in these hearts compared with untreated and MSC-treated hearts 12,15. Possible mechanisms that could lead to acidosis of the surviving myocardium of untreated and MSC-treated hearts are many, and include increased ATP turnover (consistent with lower [PCr]), increased anaerobic metabolism (consistent with higher glucose uptake rates) and altered Na+/H+ exchange.
In summary, in the present study we show that intramyocardial injection of Akt-MSCs maintains normal metabolism in the surviving myocardium for up to two weeks post MI. The effects on cardiac metabolism are demonstrated by the sparing of PCr, a highly labile metabolite; by maintaining normal rate of glucose (as 2-DG) uptake; and by maintaining normal cytosolic pH. In contrast, injecting control MSCs failed to protect the surviving myocardium in these ways. We conclude that administration of Akt-MSCs prevents cardiac metabolic remodeling after myocardial infarction. To our knowledge, this is the first time that transplanted Akt-MSCs have been reported to exert positive effects on cardiac metabolism after MI. Our results are also of value in the general context of cardiac metabolism studies since this is the first time that high energy phosphate compounds and 2-DG uptake rate of infarcted rat hearts have been evaluated early following permanent occlusion. Finally, our findings are also important from a clinical perspective. We suggest that cardiac metabolism, that can be investigated in humans using non-invasive methods, may serve as an end-point in clinical trials testing stem cell therapy treatment of myocardial infarction.
Acknowledgments
This work was supported in part by National Institutes of Health Grants HL 072010, HL 073219, HL 058516, HL 35610 (to V.J.D.) and HL 052320 and HL 063985 (to J.S.I) and gifts from the Edna Mandel Foundation (to V.J.D.). M.G. is supported by the Fondazione IRCCS Policlinico San Matteo Pavia, Italy and by grants from the Ministero Italiano dell’Università e della Ricerca (MIUR – Programma “Rientro dei cervelli”), from the Ministero Italiano della Sanità, from the Fondazione Cariplo, and from the Fondazione Banca del Monte di Lombardia. L.G.M. was Canada Research Chair in Molecular Cardiology and New Investigator of the Heart and Stroke Foundation of Canada.
REFERENCES
- 1.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–94. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 2.Anversa P, Olivetti G, Capasso JM. Cellular basis of ventricular remodeling after myocardial infarction. Am J Cardiol. 1991;68:7D–16D. doi: 10.1016/0002-9149(91)90256-k. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA. Left ventricular remodeling after acute myocardial infarction. Annu Rev Med. 1995;46:455–66. doi: 10.1146/annurev.med.46.1.455. [DOI] [PubMed] [Google Scholar]
- 4.Melo LG, Pachori AS, Kong D, Gnecchi M, Wang K, Pratt RE, Dzau VJ. Molecular and cell-based therapies for protection, rescue, and repair of ischemic myocardium: reasons for cautious optimism. Circulation. 2004;109:2386–93. doi: 10.1161/01.CIR.0000128597.37025.00. [DOI] [PubMed] [Google Scholar]
- 5.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 6.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–6. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 7.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–8. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 8.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 9.Wang JS, Shum-Tim D, Chedrawy E, Chiu RC. The coronary delivery of marrow stromal cells for myocardial regeneration: pathophysiologic and therapeutic implications. J Thorac Cardiovasc Surg. 2001;122:699–705. doi: 10.1067/mtc.2001.116317. [DOI] [PubMed] [Google Scholar]
- 10.Tomita S, Mickle DA, Weisel RD, Jia ZQ, Tumiati LC, Allidina Y, Liu P, Li RK. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J Thorac Cardiovasc Surg. 2002;123:1132–40. doi: 10.1067/mtc.2002.120716. [DOI] [PubMed] [Google Scholar]
- 11.Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Herve P, Etievent JP, Kantelip JP. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003;108(Suppl 1):II253–8. doi: 10.1161/01.cir.0000089186.09692.fa. [DOI] [PubMed] [Google Scholar]
- 12.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 13.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–21. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 15.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb J. 2006;20:661–9. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 16.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–50. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–8. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation. 1999;99:578–88. doi: 10.1161/01.cir.99.4.578. [DOI] [PubMed] [Google Scholar]
- 19.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 20.Zeng L, Hu Q, Wang X, Mansoor A, Lee J, Feygin J, Zhang G, Suntharalingam P, Boozer S, Mhashilkar A, Panetta CJ, Swingen C, Deans R, From AH, Bache RJ, Verfaillie CM, Zhang J. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007;115:1866–75. doi: 10.1161/CIRCULATIONAHA.106.659730. [DOI] [PubMed] [Google Scholar]
- 21.Cave AC, Ingwall JS, Friedrich J, Liao R, Saupe KW, Apstein CS, Eberli FR. ATP synthesis during low-flow ischemia: influence of increased glycolytic substrate. Circulation. 2000;101:2090–6. doi: 10.1161/01.cir.101.17.2090. [DOI] [PubMed] [Google Scholar]
- 22.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007;12:331–43. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 23.Luptak I, Shen M, He H, Hirshman MF, Musi N, Goodyear LJ, Yan J, Wakimoto H, Morita H, Arad M, Seidman CE, Seidman JG, Ingwall JS, Balschi JA, Tian R. Aberrant activation of AMP-activated protein kinase remodels metabolic network in favor of cardiac glycogen storage. J Clin Invest. 2007;117:1432–9. doi: 10.1172/JCI30658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neubauer S, Horn M, Naumann A, Tian R, Hu K, Laser M, Friedrich J, Gaudron P, Schnackerz K, Ingwall JS, et al. Impairment of energy metabolism in intact residual myocardium of rat hearts with chronic myocardial infarction. J Clin Invest. 1995;95:1092–100. doi: 10.1172/JCI117756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Depre C, Taegtmeyer H. Metabolic aspects of programmed cell survival and cell death in the heart. Cardiovasc Res. 2000;45:538–48. doi: 10.1016/s0008-6363(99)00266-7. [DOI] [PubMed] [Google Scholar]
- 26.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 27.Friedrich J, Apstein CS, Ingwall JS. P nuclear magnetic resonance spectroscopic imaging of regions of remodeled myocardium in the infarcted rat heart. Circulation. 1995;92:3527–38. doi: 10.1161/01.cir.92.12.3527. [DOI] [PubMed] [Google Scholar]
- 28.Chu G, Luo W, Slack JP, Tilgmann C, Sweet WE, Spindler M, Saupe KW, Boivin GP, Moravec CS, Matlib MA, Grupp IL, Ingwall JS, Kranias EG. Compensatory mechanisms associated with the hyperdynamic function of phospholamban-deficient mouse hearts. Circ Res. 1996;79:1064–76. doi: 10.1161/01.res.79.6.1064. [DOI] [PubMed] [Google Scholar]
- 29.Ingwall JS. Phosphorus nuclear magnetic resonance spectroscopy of cardiac and skeletal muscles. Am J Physiol. 1982;242:H729–44. doi: 10.1152/ajpheart.1982.242.5.H729. [DOI] [PubMed] [Google Scholar]
- 30.Polimeni PI, Buraczewski SI. Expansion of extracellular tracer spaces in the isolated heart perfused with crystalloid solutions: expansion of extracellular space, trans-sarcolemmal leakage, or both? J Mol Cell Cardiol. 1988;20:15–22. doi: 10.1016/s0022-2828(88)80175-5. [DOI] [PubMed] [Google Scholar]
- 31.Bak MI, Ingwall JS. NMR-invisible ATP in heart: fact or fiction? Am J Physiol. 1992;262:E943–7. doi: 10.1152/ajpendo.1992.262.6.E943. [DOI] [PubMed] [Google Scholar]
- 32.Gibbs C. The cytoplasmic phosphorylation potential. Its possible role in the control of myocardial respiration and cardiac contractility. J Mol Cell Cardiol. 1985;17:727–31. doi: 10.1016/s0022-2828(85)80034-1. [DOI] [PubMed] [Google Scholar]
- 33.Saupe KW, Spindler M, Tian R, Ingwall JS. Impaired cardiac energetics in mice lacking muscle-specific isoenzymes of creatine kinase. Circ Res. 1998;82:898–907. doi: 10.1161/01.res.82.8.898. [DOI] [PubMed] [Google Scholar]
- 34.Nascimben L, Ingwall JS, Lorell BH, Pinz I, Schultz V, Tornheim K, Tian R. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension. 2004;44:662–7. doi: 10.1161/01.HYP.0000144292.69599.0c. [DOI] [PubMed] [Google Scholar]
- 35.Kammermeier H. Microassay of free and total creatine from tissue extracts by combination of chromatographic and fluorometric methods. Anal Biochem. 1973;56:341–5. doi: 10.1016/0003-2697(73)90199-1. [DOI] [PubMed] [Google Scholar]
- 36.Ingwall JS, Kramer MF, Fifer MA, Lorell BH, Shemin R, Grossman W, Allen PD. The creatine kinase system in normal and diseased human myocardium. N Engl J Med. 1985;313:1050–4. doi: 10.1056/NEJM198510243131704. [DOI] [PubMed] [Google Scholar]
- 37.Murakami Y, Zhang J, Eijgelshoven MH, Chen W, Carlyle WC, Zhang Y, Gong G, Bache RJ. Myocardial creatine kinase kinetics in hearts with postinfarction left ventricular remodeling. Am J Physiol. 1999;276:H892–900. doi: 10.1152/ajpheart.1999.276.3.H892. [DOI] [PubMed] [Google Scholar]
- 38.Omerovic E, Bollano E, Basetti M, Kujacic V, Waagstein L, Hjalmarson A, Waagstein F, Soussi B. Bioenergetic, functional and morphological consequences of postinfarct cardiac remodeling in the rat. J Mol Cell Cardiol. 1999;31:1685–95. doi: 10.1006/jmcc.1999.1004. [DOI] [PubMed] [Google Scholar]
- 39.Neubauer S, Hamman BL, Perry SB, Bittl JA, Ingwall JS. Velocity of the creatine kinase reaction decreases in postischemic myocardium: a 31P-NMR magnetization transfer study of the isolated ferret heart. Circ Res. 1988;63:1–15. doi: 10.1161/01.res.63.1.1. [DOI] [PubMed] [Google Scholar]
- 40.DeBoer LW, Rude RE, Kloner RA, Ingwall JS, Maroko PR, Davis MA, Braunwald E. A flow- and time-dependent index of ischemic injury after experimental coronary occlusion and reperfusion. Proc Natl Acad Sci U S A. 1983;80:5784–8. doi: 10.1073/pnas.80.18.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apstein CS. Increased glycolytic substrate protection improves ischemic cardiac dysfunction and reduces injury. Am Heart J. 2000;139:S107–14. doi: 10.1067/mhj.2000.103920. [DOI] [PubMed] [Google Scholar]
- 42.Eberli FR, Weinberg EO, Grice WN, Horowitz GL, Apstein CS. Protective effect of increased glycolytic substrate against systolic and diastolic dysfunction and increased coronary resistance from prolonged global underperfusion and reperfusion in isolated rabbit hearts perfused with erythrocyte suspensions. Circ Res. 1991;68:466–81. doi: 10.1161/01.res.68.2.466. [DOI] [PubMed] [Google Scholar]
- 43.Young LH. AMP-activated protein kinase conducts the ischemic stress response orchestra. Circulation. 2008;117:832–40. doi: 10.1161/CIRCULATIONAHA.107.713115. [DOI] [PubMed] [Google Scholar]
- 44.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996;74:86–107. [PubMed] [Google Scholar]
- 45.Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, Gambert SR, Cigola E, Anversa P. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol. 1996;28:2005–16. doi: 10.1006/jmcc.1996.0193. [DOI] [PubMed] [Google Scholar]
- 46.Pfeffer MA, Braunwald E. Ventricular enlargement following infarction is a modifiable process. Am J Cardiol. 1991;68:127D–131D. doi: 10.1016/0002-9149(91)90270-u. [DOI] [PubMed] [Google Scholar]
- 47.Gill C, Mestril R, Samali A. Losing heart: the role of apoptosis in heart disease--a novel therapeutic target? Faseb J. 2002;16:135–46. doi: 10.1096/fj.01-0629com. [DOI] [PubMed] [Google Scholar]
- 48.Guarda E, Myers PR, Brilla CG, Tyagi SC, Weber KT. Endothelial cell induced modulation of cardiac fibroblast collagen metabolism. Cardiovasc Res. 1993;27:1004–8. doi: 10.1093/cvr/27.6.1004. [DOI] [PubMed] [Google Scholar]
- 49.Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev. 2003;83:59–115. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
- 50.Lawrence TS, Beers WH, Gilula NB. Transmission of hormonal stimulation by cell-to-cell communication. Nature. 1978;272:501–6. doi: 10.1038/272501a0. [DOI] [PubMed] [Google Scholar]
- 51.Karpanen T, Bry M, Ollila HM, Seppanen-Laakso T, Liimatta E, Leskinen H, Kivela R, Helkamaa T, Merentie M, Jeltsch M, Paavonen K, Andersson LC, Mervaala E, Hassinen IE, Yla-Herttuala S, Oresic M, Alitalo K. Overexpression of vascular endothelial growth factor-B in mouse heart alters cardiac lipid metabolism and induces myocardial hypertrophy. Circ Res. 2008;103:1018–26. doi: 10.1161/CIRCRESAHA.108.178459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren J, Samson WK, Sowers JR. Insulin-like growth factor I as a cardiac hormone: physiological and pathophysiological implications in heart disease. J Mol Cell Cardiol. 1999;31:2049–61. doi: 10.1006/jmcc.1999.1036. [DOI] [PubMed] [Google Scholar]
- 53.Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat Cell Biol. 2000;2:318–25. doi: 10.1038/35014006. [DOI] [PubMed] [Google Scholar]
- 54.Orchard CH, Kentish JC. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol. 1990;258:C967–81. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]