Abstract
We sought an optimal method for targeted delivery into dorsal root ganglia (DRGs) for experimental studies, in terms of precision of delivery and avoidance of behavioral disturbances. We examined three approaches for injection into rat DRGs: percutaneous injection without surgical exposure, injection after deep exposure, and injection following deep exposure and partial laminectomy. Coomassie blue and Fast Blue were injected into DRGs for validation. At necropsy, the spread of Coomassie blue and Fast Blue was investigated under stereomicroscope and fluorescent microscope, respectively. We found that percutaneous approach did not provide any successful DRG injections. Deep exposure prior to intraganglionic injection provided variable results, but intraganglionic injection after deep exposure plus partial laminectomy was successful in 100% of attempts. Our subsequent skeletal analysis showed that the anatomical location of DRG is not compatible with successful DRG injection without surgical exposure. Neither of the methods using surgical exposure caused behavioral disturbances. Based on these results we conclude that partial laminectomy offers the most precise method of injecting DRG and does not produce behavioral evidence of nerve damage. Intraganglionic injection after deep exposure alone is less predictable, while percutaneous approaches only allow injection in the peripheral nerve.
Keywords: Rat, Dorsal root ganglion, Injections, Neuropathic pain, Pain models
1. Introduction
Application of agents into the dorsal root ganglion (DRG) has been used in studies investigating pain mechanisms, drug toxicity, and gene transfer (Coronel et al., 2006; Glatzel et al., 2000; Han et al., 2004; Hu and Xing, 1998; Lu and Richardson, 1991; McGaraughty et al., 2006; Obata et al., 2002; Wang et al., 1998; Wu et al., 2007; Zhang et al., 2001). A common application of this approach is gene transfer to peripheral nerve system or to distinguish pharmacologic action of certain drugs on either the DRG or the spinal cord. A strong impetus for developing a suitable model of DRG injection is the potential advantage of therapy targeting the DRG, specifically the avoidance of systemic and CNS toxicity during treatment of segmental conditions. However, targeted DRG delivery in small animal subjects is a methodological challenge, as DRGs are enclosed within the intervertebral foramen and are therefore not easily accessible for intraganglionic injection. However, the permeability of the DRG capsule, unlike other components of the peripheral nervous system (Abram et al., 2006; Devor, 1999), allows ready transit of solution and agents into and out of the DRG.
Although the goals and principles of targeted delivery of agents into the DRG appear to be consistent across many of the published experimental protocols, there are very few validated approaches and no comparison studies. In experimental conditions, the fifth lumbar DRG (L5 DRG) is the most commonly injected among DRGs in rats. It should be also noted that lumbo-sacral nerve segmentation is different in mice (Rigaud et al., 2008). It is located in the L5–L6 intervertebral foramen and is covered dorsomedially by the articular processes. Surgical approaches have included exposure with removal of soft tissue alone or removal of vertebral lamina as well (Abram et al., 2006; Obata et al., 2004). Targeted DRG injection involving only soft tissue surgery requires advancing a needle into the intervertebral foramen for at least 5 mm. Other techniques involve administration of drugs into the DRG after laminectomy, which directly exposes the ganglion. Recently, a technique of direct drug administration into the DRG was described that does not involve surgery and is performed through the skin based on palpable landmarks (Ferrari et al., 2007). Here we describe our experience with these three methods of targeted DRG delivery. Our goal was to further develop methodology for targeted DRG delivery and to identify which technique is the most precise and reliable.
2. Materials and methods
2.1. Experimental animals
All experimental procedures and protocols followed the IASP Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals and were approved by the Ethical Committee of the University of Split School of Medicine or the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. A total of 37 Sprague–Dawley rats weighing 150−200 g were used in this study. In order to compare efficacy and precision of the three DRG injection methods, we randomly assigned rats into three groups: percutaneous DRG injection (n = 7), DRG injection after soft tissue removal (n = 10), and DRG injection after partial laminectomy (n = 10). For behavioral analysis, we compared these groups with control animals that received no intraganglionic injection or surgery (n = 10). After behavioral testing, rats were sacrificed on the first day after injection, their DRGs were harvested, and dimensions of DRGs were measured. Additionally, L5 DRGs were excised from 15 rats 17 days following spinal nerve ligation (SNL) for determination of their dimensions. The SNL is a frequent model for generation of neuropathic pain in rats (Chung et al., 1995) and it was performed as previously reported (Hogan et al., 2004).
2.2. DRG injection techniques
All surgical procedures were performed under inhalation anesthesia using 4% isoflurane in oxygen for induction and 2% for maintenance of anesthesia (Forane, Abbott laboratories Ltd., Queenborough, UK).
Percutaneous intranganglionic injections were performed as described by Ferrari et al. using a sharp 25-gauge needle 1.5 cm laterally to the vertebral column, approximately 0.5 cm caudal from a line passing the rostral borders of the iliac crests (Ferrari et al., 2007). To facilitate the penetration of the injecting needle through the skin, an initial puncture with a 19-gauge needle was made. The 25-gauge injecting needle was then introduced through the punctured skin, towards the intervertebral foramen between L5 and L6, until the tip touched the lateral region of the vertebrae. The injection was made after a paw flinch reflex was observed.
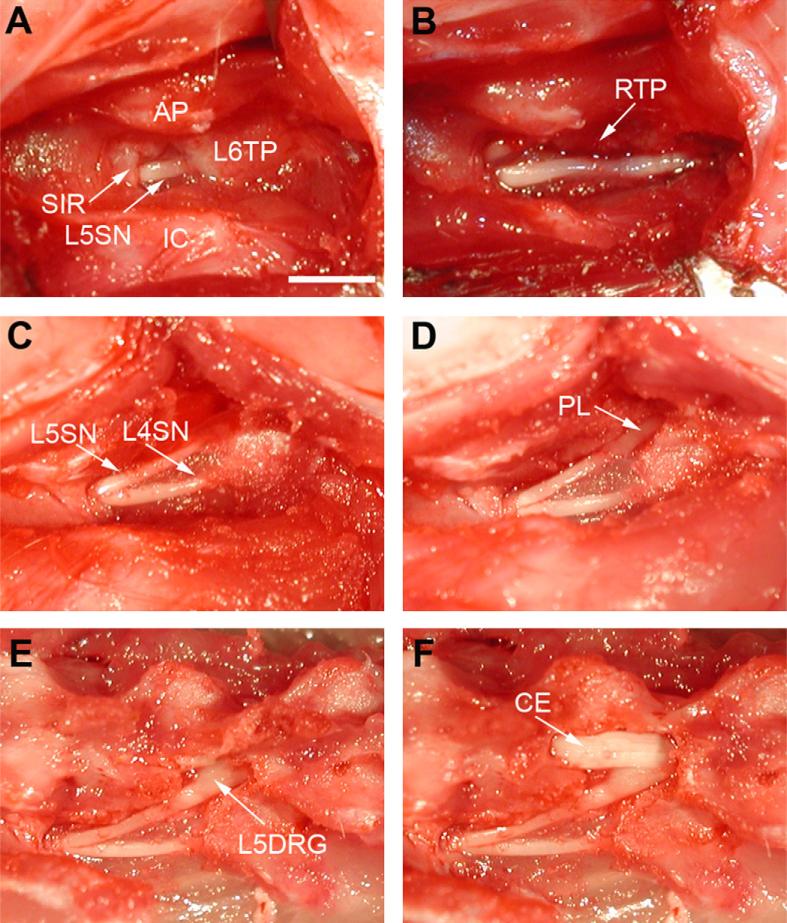
For intraganglionic injection with only soft tissue removal, the skin was incised, the right paravertebral region was exposed, and connective tissue and muscles were removed by iris scissors, thus exposing bony structures and the right L5 intervertebral foramen (Fig. 1A). A 29-gauge needle with a slightly bent beveled tip was then advanced 5 mm into the foramen and 4 μL of Coomassie blue or Fast Blue were injected over 10 s using a manual microsyringe pump with digital display (WPI, USA). This volume was used to proportionately reflect the volume injected in patients.
Fig. 1.
(A) Surgical approach used for DRG injection after soft tissue removal. (B and C) Surgery procedure used for injection following partial laminectomy. (D, E, and F) Exposure of the DRG preformed for validation. AP, articular processes; L6TP, L6 transverse process; SIR, sacroiliac rim; L5SN or L4SN, L5 or L4 spinal nerve; IC, iliac crest; RTP, remnants of the transverse process; PL, partial laminectomy area; L5DRG, L5 dorsal root ganglia; CE, cauda equina. Scale bar: 4 mm.
The third intraganglionic injection method included additional removal of laminar bone. After connective tissue removal, the trans-verse process of L5 and the marginal laminar rim caudal to the L5 ganglion were removed using a small rongeur (Fig. 1B and C). After the caudal part of ganglion was exposed, the intraganglionic injection was performed under direct visualization. For this technique, we used a 29-gauge needle with a slightly bent tip that allowed a more favorable position for intraganglionic injection than a straight needle or micropipette. All injection procedures were performed by one investigator (LP).
2.3. Behavior
To test possible development of neuropathic pain behavior following intraganglionic injection procedures, a validated test of mechanical hyperalgesia was performed as previously described (Hogan et al., 2004). The testing was performed before intraganglionic injection and 24 h after intraganglionic injection. The rats were placed in plastic boxes with a wire mesh floor. A 22-gauge needle was pressed against the plantar skin of hind paws such that the skin was dimpled but not injured. With each touch, rats respond with either a sudden withdrawal of very small amplitude, or a complex, sustained response that included lifting of the foot accompanied with shaking, grooming, licking or vocalization (Hogan et al., 2004).
2.4. Validation
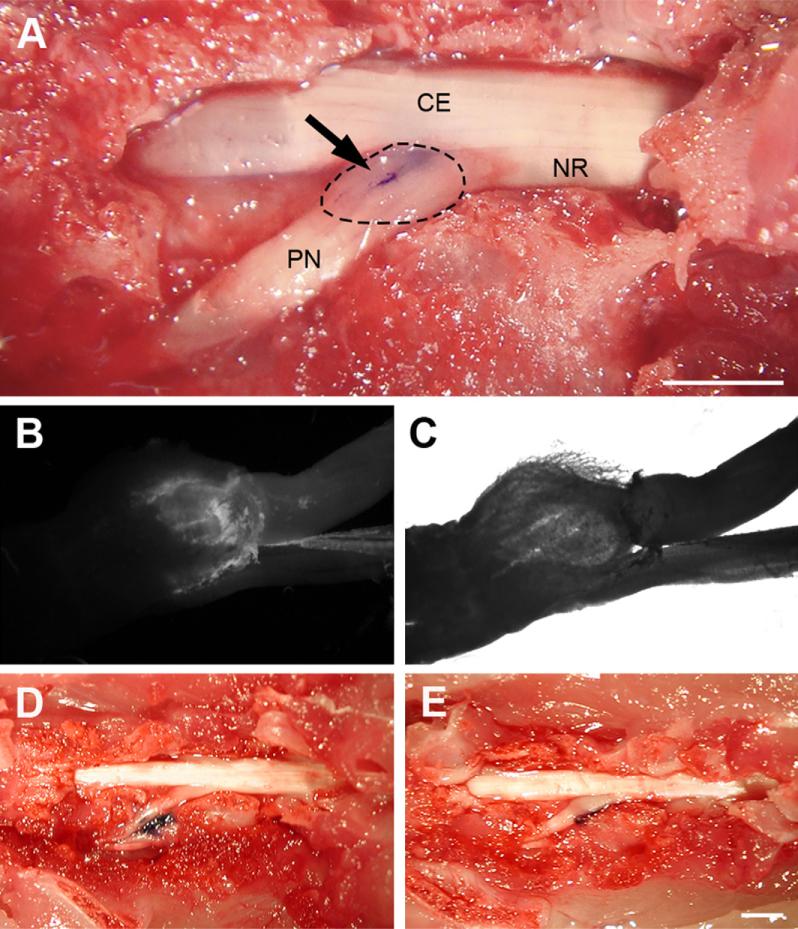
After behavioral examinations, the spine, injected DRG and all neighboring DRGs were exposed by removal of the covering tissue and bone (Fig. 1D, E and F). Coomassie blue staining was readily visible and photographs were taken with a digital camera. In each group, one rat was injected with Fast Blue. Upon tissue harvest, these DRGs were examined by fluorescence microscopy using a UV filter for Fast Blue, and photographs were made with a digital camera. Fast blue is a retrograde neuronal tracer that is transported only from the nerve terminal to the cell body. Therefore, fluorescence found in the DRG but not in the spinal nerve (Fig. 2B and C) indicates an injection into the DRG, whereas dye in the spinal nerve indicates original needle placement there. Theoretically, pressure from the injection could spread dye from the DRG to the spinal nerve in the absence of active transport. However, we did not observe a single case of dye spreading from DRG into spinal nerve after successful intraganglionic injection.
Fig. 2.
Examples of Coomassie blue and Fast Blue spread following injection into the L5 DRG. (A) Result of DRG injection following partial laminectomy; (arrow) trace of Coomassie blue dye; DRG is indicated by the dotted line, NR: nerve root, PN: peripheral nerve, CE: cauda equina. (B and C) Traces of Fast Blue dye under fluorescent illumination (B) and in bright field (C). (D) The result of percutaneous injection into DRG. Coomassie blue trace is located around peripheral nerve approximately 4 mm from L5 DRG. (E) Injection after removal of only soft tissue; Coomassie blue was located lateral to the L5 DRG.
After photographing, the vertebral columns were removed, boiled in water for 15 min to remove soft tissue, and then transferred to 30% hydrogen peroxide solution for 30 min. After mechanical cleaning, vertebral skeletons were photographed as described before (Kosta et al., 2008). Schematic drawings of the spine were made according to the photographs, and the position of L5 DRG was depicted.
3. Results
One hour after the intraganglionic injection, all animals were awake and without any evidence of motor deficits. At 24 h after intraganglionic injection, animals showed no significant differences in hyperalgesia responses. In the control group and in the rats that received percutaneous injection or intraganglionic injection after soft tissue removal, there were no hyperalgesic responses. In the rats that received intraganglionic injection following minimal laminectomy, two rats exhibited hyperalgesic behavior (1 and 3 responses out of 10 needle applications).
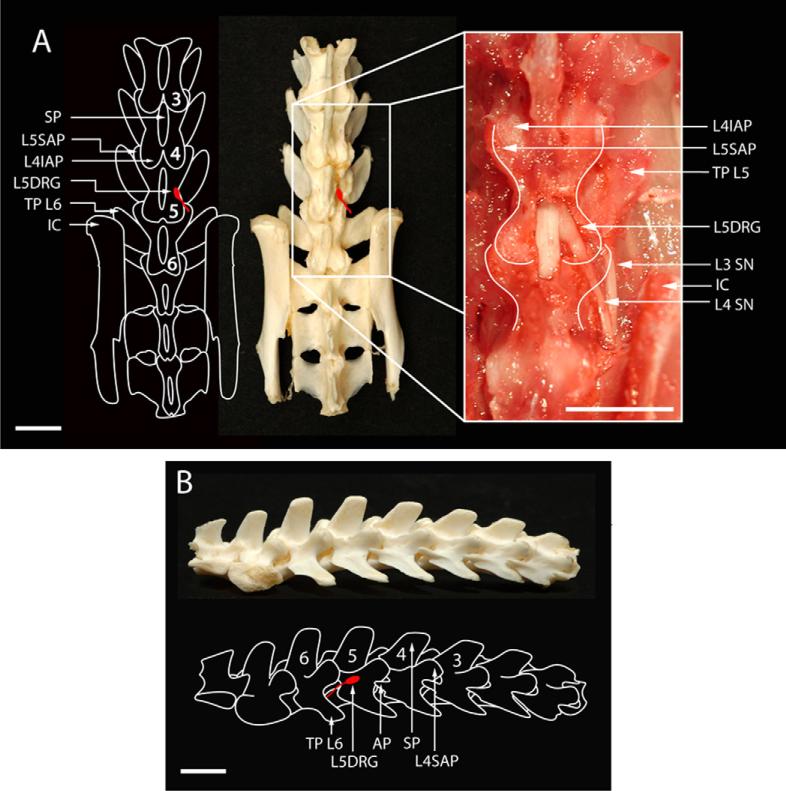
With the percutaneous DRG injection method, we were unable to inject solution into the vicinity of the L5 DRG due to interference from the bone, even during repeated manipulations of the needle and forcible advancement movements of the needle. We also tried to replicate this DRG access using clean spines as models (Fig. 3A and B). The authors describe that after reaching the space between the transverse process of the fifth and sixth vertebrae, “delicate movements of the needle were made until the bone resistance was diminished” (Ferrari et al., 2007). This description might indicate that perforation of the bone covering the DRG may have been necessary, but this was not possible even on the skeletonized spine models, due to the thickness of the bone. Thus, all injections using the Ferrari method were unsuccessful. Although a paw flinch reflex was observed in each case, staining showed injection into the spinal nerve rather than the DRG in all rats in this experimental group (7/7; 0% success rate for DRG injection). The average location of the injection site was 5 mm from DRG. Analysis of the isolated spines (Fig. 3A and B) demonstrated the L5 DRG is enclosed by bone and that direct access to this ganglion from a posterolateral angle is blocked by laminar bone.
Fig. 3.
Schematic drawings and photographs depicting the position of the L5 DRG and critical landmarks within the rat lumbosacral vertebral column. (A) Anteroposterior position demonstrates L5 DRG position (L5DRG), iliac crest (IC), spinous processes (SP), and superior (SAP) and inferior (ISP) articular processes. White lines in magnified inset show vertebral borders. (B) Lumbosacral vertebral column from the lateral view. Scale bar: 2 mm.
Injecting DRG after soft tissue removal and advancement of the needle into the intervertebral foramen was technically challenging. Overall, only in the last three of the ten rat intraganglionic injection series (30%) did the dye reach the DRG, whereupon dye was observed only in the lateral part of the DRG. In the first seven rats, dye was found only in the spinal nerve. These findings indicate that this is a technically demanding technique that requires acquisition of a certain level of skill before success can be achieved. Following partial laminectomy, the DRG was successfully injected in all 10 rats (100%), as validated with both uniformly distributed Coomassie blue and Fast Blue (Fig. 2).
Dimensions of the L5 DRG were determined in order to understand the size of the target of intraganglionic injection. Ligation of the spinal nerve just distal to the DRG is often used as a model of neuropathic pain (Chung et al., 1995), so we additionally examined any influence this might have on DRG dimensions. As there was no effect of this injury, data from ligated nerves (n =15) and unoperated controls (n = 41) were pooled. The DRGs weighed 2.14±0.10 mg (average±S.E.M.), and were 2.31±0.41 mm long and 1.42±0.03 mm wide.
4. Discussion
In this study, we compared three methods of targeted DRG delivery, including percutaneous injection, intraganglionic injection after soft tissue removal and advancement of the needle into intervertebral foramen, and intraganglionic injection after laminar bone removal and exposure of the caudal pole of DRG. All these methods have been described in published reports and our aim was to test the precision of those methods so that we might recommend the optimal approach for experiments involving DRG drug delivery. Our results show that intraganglionic injection after partial laminectomy results in consistently successful injection into the DRG, as validated both with Coomassie blue and Fast Blue dyes. Removal of soft tissue but no bone, with blind insertion of the needle through the intervertebral foramen, is more challenging and less successful. Although it may take longer to achieve consistently successful results, the less traumatic approach of leaving the bone intact may have an advantage in creating less trauma and inflammation. However, we have previously shown that laminectomy alone does not cause significant behavioral disturbances or pain-related behavior in rats during a 2-week postsurgical period (Kosta et al., 2008). We have also found that injection per se (e.g. saline) into spinal nerve or DRG does not cause neuroinflammation (Puljak et al., in press). Thus, this approach using direct exposure of the DRG offers the greatest accuracy and produces minimal unintended effects.
Until recently, all described methods of DRG targeted delivery involved more or less extensive surgery to enable a close approach to the DRG with an injecting needle. A new method of DRG delivery has been recently described in which injection is performed through the skin without surgical exposure (Ferrari et al., 2007). In that report, the authors present images showing injection to the spinal nerve, while the DRG lies dorsomedially from the injected material (Ferrari et al., 2007). Even after repeated efforts, we were unable to inject within the DRG, but rather managed only to inject into the spinal nerve. Our anatomic analysis shows that skeletal elements surrounding the DRG interfere with this simple percutaneous DRG injection. The Ferrari group described validation of intraganglionic injection by injection of radiolabeled solution, so a possible explanation for their result is including a part of injected L5 spinal nerve together with the DRG in the sample.
Substantial methodological diversity characterizes published reports of DRG injection, probably due to difficulties with which investigators are faced when trying to access this structure. Intraganglionic injections have been performed with Hamilton needles of various diameters (Coronel et al., 2006; Glatzel et al., 2000; Obata et al., 2002; Wu et al., 2007), L-shaped needles (Hu and Xing, 1998), glass micropipettes (Han et al., 2004; Lu and Richardson, 1991; Wang et al., 1998) or polyurethane catheter (McGaraughty et al., 2006), while some authors do not specify needle gauge (Xu et al., 2003). Delivery systems also vary from those using stereotaxic frame (Glatzel et al., 2000), micromanipulators (Han et al., 2004)or micropumps syringe injectors (Coronel et al., 2006). Authors inject volumes ranging from 0.2 to 5.0 μL in rats weighing from 150 to 400 g (Ferrari et al., 2007; Lu and Richardson, 1991; McGaraughty et al., 2006; Obata et al., 2002; Wang et al., 1998; Xu et al., 2003), or 4 μLinmice (Glatzel et al., 2000). Bolus topical intraganglionic injection of as much as 50 μL has been performed in the rat DRG (Zhang et al., 2001).
Only a few articles describe validation of DRG injections. To confirm accurate intraganglionic injection, investigators have measured radioactivity from radiolabeled injectate (Ferrari et al., 2007), observed local anesthetic blockade of transmitted neuronal activity (McGaraughty et al., 2006), identified a transient twitch of hind leg muscles from needle contact with the nerve or DRG (Ferrari et al., 2007; Hu and Xing, 1998), or labeled neuronal cell bodies and fibers in the DRG by injecting fluorescein or IB4-HRP (Abram et al., 2006; Ferrari et al., 2007; Hu and Xing, 1998; Wang et al., 1998). For sustained administration of pharmacological agents, DRG perfusion via catheter, sometimes aided with an osmotic pump, has been employed (Dobretsov et al., 2001; Zhang et al., 2001; Zhou et al., 1999, 2000). Full validity testing of any of these techniques requires that the dye have the same permeability and diffusing properties as the planned injectate, such as local anesthetic, virus or other drug.
In conclusion, this multiplicity of alternative techniques has evolved to achieve specific experimental needs, but may also indicate a general inadequacy of the various methods. Our observation of the variability in results using approaches in which the DRG is not exposed indicates that anatomic validation should be a routine component of experiments using DRG injection.
Acknowledgement
This work was supported by the Ministry of Science, Education, and Sports of the Republic of Croatia (grants numbers 2160528 and 216-2160528-0522).
Footnotes
Conflict of interest
None.
References
- Abram SE, Yi J, Fuchs A, Hogan QH. Permeability of injured and intact peripheral nerves and dorsal root ganglia. Anesthesiology. 2006;105:146–53. doi: 10.1097/00000542-200607000-00024. [DOI] [PubMed] [Google Scholar]
- Chung JM, Choi Y, Yoon YW, Na HS. Effects of age on behavioral signs of neuropathic pain in an experimental rat model. Neurosci Lett. 1995;183:54–7. doi: 10.1016/0304-3940(94)11113-w. [DOI] [PubMed] [Google Scholar]
- Coronel MF, Musolino PL, Villar MJ. Selective migration and engraftment of bone marrow mesenchymal stem cells in rat lumbar dorsal root ganglia after sciatic nerve constriction. Neurosci Lett. 2006;405:5–9. doi: 10.1016/j.neulet.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain. 1999;6(Suppl.):S27–35. doi: 10.1016/S0304-3959(99)00135-9. [DOI] [PubMed] [Google Scholar]
- Dobretsov M, Hastings SL, Stimers JR, Zhang JM. Mechanical hyperalgesia in rats with chronic perfusion of lumbar dorsal root ganglion with hyperglycemic solution. J Neurosci Methods. 2001;110:9–15. doi: 10.1016/s0165-0270(01)00410-1. [DOI] [PubMed] [Google Scholar]
- Ferrari LF, Cunha FQ, Parada CA, Ferreira SH. A novel technique to perform direct intraganglionar injections in rats. J Neurosci Methods. 2007;159:236–43. doi: 10.1016/j.jneumeth.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Glatzel M, Flechsig E, Navarro B, Klein MA, Paterna JC, Bueler H, et al. Adenoviral and adeno-associated viral transfer of genes to the peripheral nervous system. Proc Natl Acad Sci U S A. 2000;97:442–7. doi: 10.1073/pnas.97.1.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han PJ, Shukla S, Subramanian PS, Hoffman PN. Cyclic AMP elevates tubulin expression without increasing intrinsic axon growth capacity. Exp Neurol. 2004;189:293–302. doi: 10.1016/j.expneurol.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101:476–87. doi: 10.1097/00000542-200408000-00030. [DOI] [PubMed] [Google Scholar]
- Hu SJ, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain. 1998;77:15–23. doi: 10.1016/S0304-3959(98)00067-0. [DOI] [PubMed] [Google Scholar]
- Kosta V, Kojundzic SL, Sapunar LC, Sapunar D. The extent of laminectomy affects pain-related behavior in a rat model of neuropathic pain. Eur J Pain. 2008 doi: 10.1016/j.ejpain.2008.04.012. doi:10.1016/j.ejpain.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Lu X, Richardson PM. Inflammation near the nerve cell body enhances axonal regeneration. J Neurosci. 1991;11:972–8. doi: 10.1523/JNEUROSCI.11-04-00972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Faltynek CR, Jarvis MF. Systemic and site-specific effects of A-425619, a selective TRPV1 receptor antagonist, on wide dynamic range neurons in CFA-treated and uninjured rats. J Neurophysiol. 2006;95:18–25. doi: 10.1152/jn.00560.2005. [DOI] [PubMed] [Google Scholar]
- Obata K, Tsujino H, Yamanaka H, Yi D, Fukuoka T, Hashimoto N, et al. Expression of neurotrophic factors in the dorsal root ganglion in a rat model of lumbar disc herniation. Pain. 2002;99:121–32. doi: 10.1016/s0304-3959(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Dai Y, Mizushima T, Fukuoka T, Tokunaga A, et al. Activation of extracellular signal-regulated protein kinase in the dorsal root ganglion following inflammation near the nerve cell body. Neuroscience. 2004;126:1011–21. doi: 10.1016/j.neuroscience.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Puljak L, Lovric Kojundzic S, Hogan Q, Sapunar D. Lidocaine injection into the rat dorsal root ganglion causes neuroinflammation. Anesth Analg. doi: 10.1213/ane.0b013e318193873e. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud M, Gemes G, Barabas ME, Chernoff DI, Abram SE, Stucky CL, et al. Species and strain differences in rodent sciatic nerve anatomy: implications for studies of neuropathic pain. Pain. 2008;136:188–201. doi: 10.1016/j.pain.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HF, Robertson B, Grant G. Anterograde transport of horseradish-peroxidase conjugated isolectin B4 from Griffonia simplicifolia I in spinal primary sensory neurons of the rat. Brain Res. 1998;811:34–9. doi: 10.1016/s0006-8993(98)00916-0. [DOI] [PubMed] [Google Scholar]
- Wu D, Zhang Y, Bo X, Huang W, Xiao F, Zhang X, et al. Actions of neuropoietic cytokines and cyclic AMP in regenerative conditioning of rat primary sensory neurons. Exp Neurol. 2007;204:66–76. doi: 10.1016/j.expneurol.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Xu Y, Gu Y, Wu P, Li GW, Huang LY. Efficiencies of transgene expression in nociceptive neurons through different routes of delivery of adeno-associated viral vectors. Hum Gene Ther. 2003;14:897–906. doi: 10.1089/104303403765701187. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Homma Y, Ackerman WE, Brull SJ. Topical application of acidic bupivacaine to the lumbar ganglion induces mechanical hyperalgesia in the rat. Anesth Analg. 2001;93:466–71. doi: 10.1097/00000539-200108000-00045. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Deng YS, Chie E, Xue Q, Zhong JH, McLachlan EM, et al. Satellite-cell-derived nerve growth factor and neurotrophin-3 are involved in noradrenergic sprouting in the dorsal root ganglia following peripheral nerve injury in the rat. Eur J Neurosci. 1999;11:1711–22. doi: 10.1046/j.1460-9568.1999.00589.x. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Deng YS, Xian CJ, Zhong JH. Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. Eur J Neurosci. 2000;12:100–5. doi: 10.1046/j.1460-9568.2000.00884.x. [DOI] [PubMed] [Google Scholar]