Abstract
Bortezomib (VELCADE) could sensitize certain human renal cell carcinoma (RCC) lines to the apoptotic effects of TRAIL. Analysis of seven human RCC demonstrated a clear increase in the sensitivity of four of the RCC to TRAIL cytotoxicity following bortezomib (5–20nM) treatment, while the remaining three remained resistant. Tumor cell death following sensitization had all the features of apoptosis. The enhanced antitumor activity of the bortezomib and TRAIL combination was confirmed in long-term (6 day) cancer cell outgrowth assays. The extent of proteasome inhibition by bortezomib in the various RCC was equivalent. Following bortezomib treatment, neither changes in the intracellular protein levels of various Bcl-2 and IAP family members, nor minor changes in expression of TRAIL receptors (DR4, DR5) correlated well with sensitization or resistance of RCC to TRAIL-mediated apoptosis. However, enhanced procaspase-8 activation following bortezomib pretreatment and subsequent TRAIL exposure was only observed in the sensitized RCC in both cell extracts and DISC immunoprecipitates. These data suggest that the molecular basis for bortezomib sensitization of RCC to TRAIL primarily involves early amplification of caspase-8 activity. In the absence of this increased caspase-8 activation, other bortezomib-induced changes are not sufficient to sensitize RCC to TRAIL-mediated apoptosis.
Keywords: Bortezomib, TRAIL, apoptosis, renal carcinoma, caspase-8
Introduction
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL or Apo2L) preferentially causes apoptosis in cancer cells rather than normal cells. Therefore there has been much interest in the use of TRAIL, or other TRAIL receptor agonists such as antibodies, as a novel approach to cancer therapy. Currently humanized agonist antibodies to TRAIL R1 (DR4), TRAIL R2 (DR5), and TRAIL itself, are in clinical trials in a wide variety of cancer patients (1). Encouragingly, these agents can be administered to cancer patients in the absence of major toxicities. Nonetheless, in vitro studies indicate that only a minority of cancer cell lines undergo apoptosis in response to TRAIL. This has prompted intense interest in the identification of compounds that can sensitize cancer cells to TRAIL.
Bortezomib, a proteasome inhibitor that has been approved for the therapy of multiple myeloma, has been investigated extensively for its therapeutic effects in many types of cancer (2). Earlier studies also suggested that bortezomib could dramatically sensitize not only multiple myeloma cells (3), but also a variety of human and mouse solid tumor cells to the apoptotic effects of TRAIL in vitro (4, 5). Subsequent studies confirmed that a combination of bortezomib with TRAIL could overcome the resistance to TRAIL cytotoxicity in vitro in a wide variety of cancer cells including leukemias and lymphomas (6, 7), prostate (8, 9), colon (5), bladder (8), thyroid (10), ovarian (11), lung (12), sarcoma (13), hepatoma (14, 15) and glioma (16). Encouragingly, normal cells still seem to remain relatively resistant to TRAIL, even in combination with bortezomib (14, 15). However, the molecular events responsible for the sensitizing effect of bortezomib on TRAIL-induced apoptosis in cancer cells remain controversial and unclear.
Bortezomib has been reported to affect levels of TRAIL receptors (12, 17), c-FLIP (4), NF-κB (18), p21 and p27 (8) and pAkt (19) and these changes have been implicated in promoting tumor cell apoptosis on subsequent exposure to TRAIL. In addition other studies suggested that the effects of bortezomib on members of the Bcl-2 family (20), particularly Bik and Bim (21), could also be crucial in promoting TRAIL apoptosis. Nonetheless, proteasome inhibition results in changes in the levels of many cellular proteins. The extent to which some of the observed changes are directly involved in amplifying TRAIL-mediated apoptosis has not been stringently addressed. In this study to investigate the molecular mechanisms whereby bortezomib sensitizes human RCC to TRAIL, we have performed a thorough molecular analysis of bortezomib sensitization using a panel of human RCC. We have used concentrations of bortezomib that could be realistically achieved in vivo. All these human RCC were quite resistant to TRAIL alone. Interestingly, some human RCC were dramatically sensitized to TRAIL-mediated apoptosis by bortezomib, whereas others remained resistant. This allowed us to systematically test which molecular changes induced by bortezomib were crucial for TRAIL sensitization, and attempt to distinguish these from changes that were merely a consequence of proteasome inhibition. Results show that a crucial mechanism of bortezomib-sensitization of human RCC to TRAIL-mediated apoptosis involves increased activation of caspase-8 in the death-inducing signaling complex (DISC).
Results
Bortezomib Sensitization of RCC lines to TRAIL
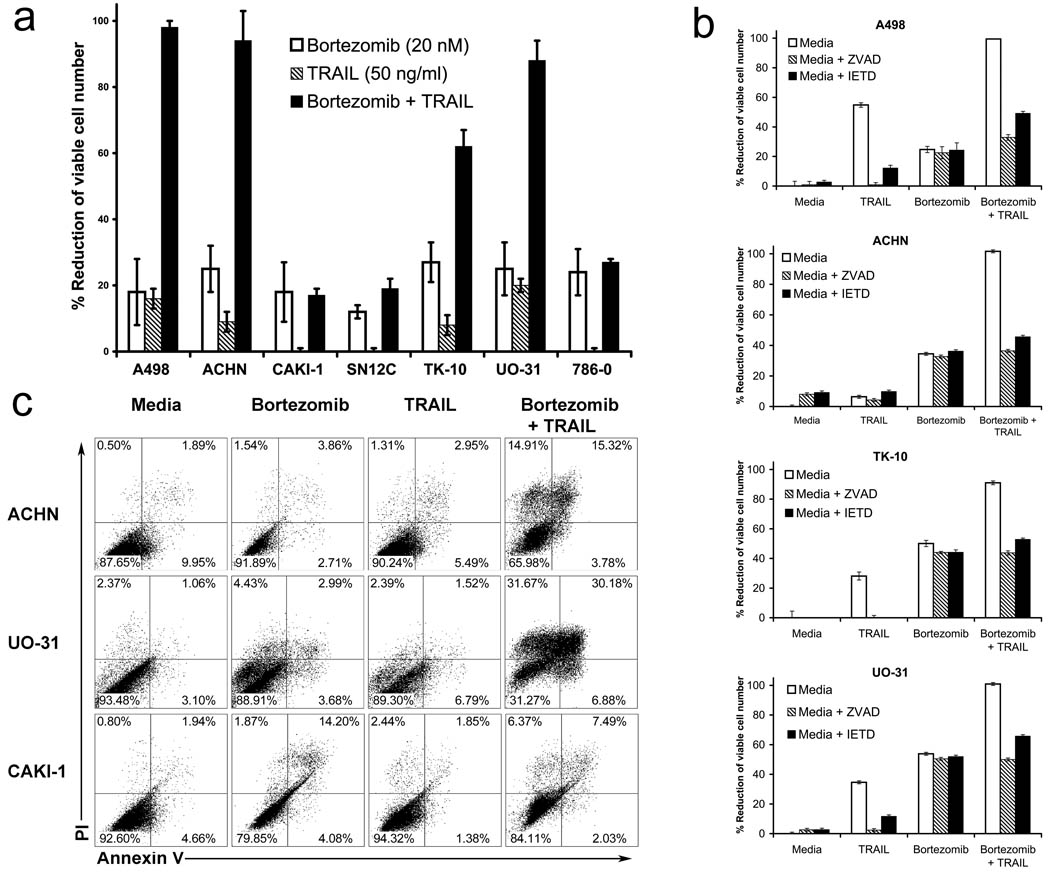
The sensitivity of a panel of human RCC to TRAIL in the presence or absence of bortezomib was tested after overnight incubation. Bortezomib alone resulted in a 20–45% % reduction in the number of viable cells for these RCC.. TRAIL treatment alone had little effect on viable cell number, whereas the combination of bortezomib and TRAIL treatment for A498, ACHN, TK-10 and UO-31 resulted in a 60–95 % reduction in the number of viable cells (Figure 1a). Therefore, for these RCC, bortezomib treatment dramatically sensitized cells to the effects of TRAIL. By contrast, CAKI-1, SN12C and 786-0 were not sensitized to TRAIL by bortezomib, and the combination resulted in a reduction in viable cell number no different to that induced by the single agents alone. Thus, we consider these lines to be resistant to bortezomib sensitization for TRAIL cytotoxicity. Interestingly bortezomib sensitized these RCC to FasL-mediated apoptosis in a similar manner (Supplemental Figure 1). Furthermore for the resistant RCC, bortezomib failed to sensitize over a range of TRAIL concentrations (Supplemental Figure 2). The pan-caspase inhibitor zVAD-FMK and the more caspase-8 specific zIETD-FMK both abolished the reduction of cell number that occurred in response to bortezomib plus TRAIL (Figure 1b). This suggested that the combination enhanced cell death by apoptosis. Also since zVAD-FMK had no effect on the reduction of cell number observed in response to bortezomib alone, this suggested that the effects of bortezomib on RCC were predominantly cytostatic rather than cytotoxic. This was confirmed by annexin V and propidium iodide (PI) staining of cells (Figure 1c). However, the combination of bortezomib and TRAIL did not further increase the number of apoptotic cells in the resistant CAKI-1 cells.
Figure 1.
Bortezomib sensitizes some human renal cancer cell lines to caspase dependent apoptosis by rhTRAIL. (a) MTS growth inhibition assay (b) Annexin V and propidium iodide (PI) staining; Cells were treated overnight with 20 nM Bortezomib, and then 8 hours with 100 ng/ml rhTRAIL (c) Cells were treated for 2 hours with 20 uM zVAD or zIETD-FMK, and then for 2 hours with 20 nM Bortezomib followed by an overnight treatment with 50 ng/ml rhTRAIL.
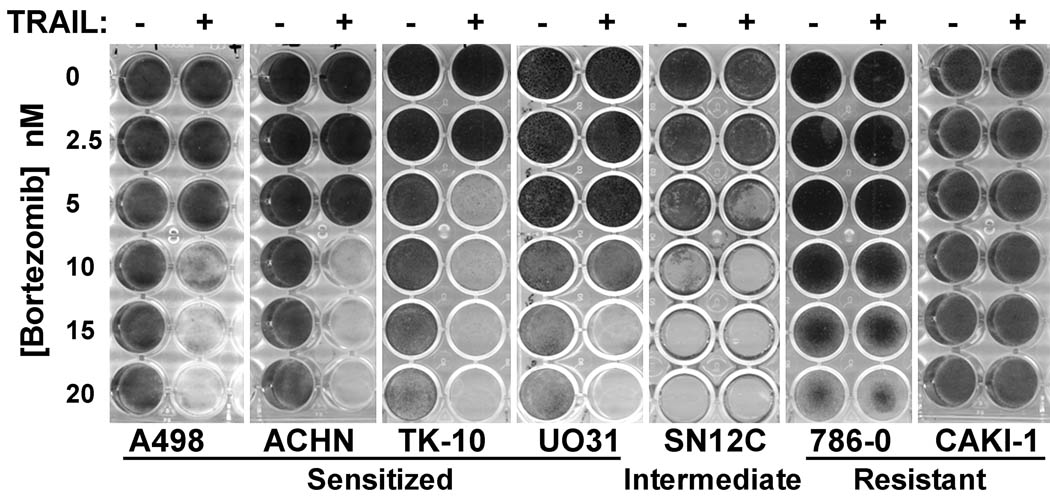
Nonetheless, it remained important to determine whether bortezomib sensitized some RCC to TRAIL-mediated apoptosis independent of any proapoptotic effects of bortezomib alone. Indeed, we have observed that the cytotoxic effects of bortezomib as a single agent may not become apparent until 48–72 hrs after exposure to the drug. However, using longer term cell survival assays following removal of the drugs after overnight exposure (Figure 2), we observed that the combination of bortezomib and TRAIL resulted in an almost 100% reduction of cell number 6 days later, for at least one concentration of bortezomib (between 5–20 nM) for A498, ACHN, TK-10 and UO-31. The transient exposure of the cells to bortezomib in this assay did initially reduce cell number due to the cytostatic effects of the drug, but most of the RCC recovered and then started proliferating. As seen in the shorter term assays, 786-0 and CAKI-1 were highly resistant to the combination treatment, so in this case the combination of bortezomib and TRAIL did not result in increased loss of cells due to apoptosis. SN12C was highly sensitive to bortezomib alone even at quite low doses of the drug. This only became apparent in the longer term cell survival assay. This makes it difficult to determine whether there is a combined effect of bortezomib and TRAIL in reducing cell survival for SN12C.
Figure 2.
Long-term cell survival assays also demonstrate bortezomib sensitization of RCC to TRAIL apoptosis. RCC were treated overnight with bortezomib and rhTRAIL alone or in combination. The following morning drugs were washed out and replaced with fresh media. After 6 days cells were fixed with methanol and stained with crystal violet.
Proteasome inhibition in various human RCC
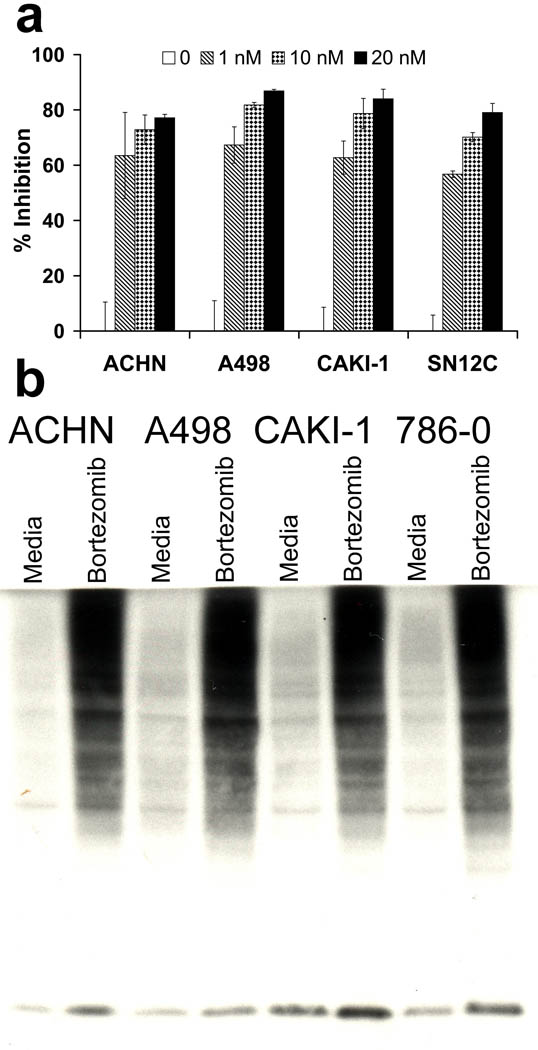
One possible explanation for the differences in the effects of bortezomib on TRAIL sensitization for different RCC could be differential effects of this inhibitor on proteasome enzyme activity. However, assays on proteasome activity using either a luminescence-based assay for proteasome enzyme activity (Figure 3a), or increases in ubiquitinated proteins as assessed by western blotting (Figure 3b), suggested that the proteasome activity was inhibited to a similar degree in all RCC, irrespective of whether they were sensitized or remained resistant to TRAIL apoptosis. Since the proteasome inhibition appeared constant, it seemed that specific changes that occurred downstream of this inhibition were most likely to account for sensitization or resistance to TRAIL-mediated apoptosis.
Figure 3.
The proteasome was inhibited equally in all RCC tested. (a) Cells were treated overnight with 1, 10 or 20 nM Bortezomib and then assayed with the Proteo-glo assay (Promega). (b) Cells were treated overnight with 20 nM Bortezomib; cell lysates were used for western blotting for ubiquitinated proteins.
Effect of bortezomib on TRAIL DISC components
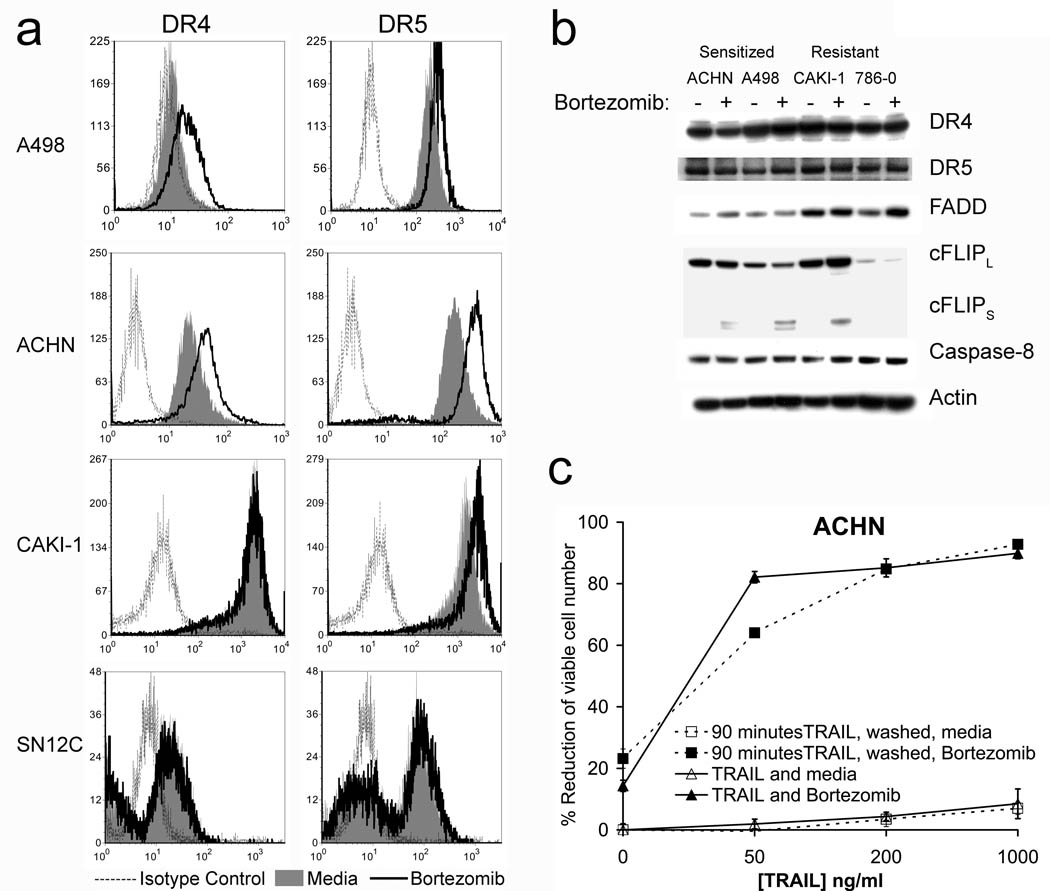
One possible effect of bortezomib, that might sensitize cells to TRAIL apoptosis, could be on altering the level of cell surface expression of TRAIL receptors, or components of the TRAIL death-inducing signaling complex. We therefore assessed the effects of bortzomib on cell surface expression of various TRAIL receptors on some of our panel of human RCC (Figure 4a). Interestingly slight increases in DR4 expression were observed only on sensitized cells (ACHN, A498). However, the resistant CAKI-1 cells had high endogenous cell surface levels of DR4 expression. The expression of DR4 and DR5 by SN12C cells was not affected by bortezomib treatment. Levels of DR5 were increased not only in sensitized A498 and ACHN but also in the resistant CAKI-1. Therefore, there was no clear correlation between the cell surface expression of DR4 and DR5 and bortezomib sensitization of RCC to TRAIL. Cell surface levels of the decoy receptors DcR1 and DcR2 did not change in any of the RCC following bortezomib treatment (data not shown).
Figure 4.
Bortezomib causes an increase in the surface expression of some death receptors and cFLIPs in some RCC lines. (a) Death receptor expression on tumor cell lines was analyzed after overnight treatment with 20nM bortezomib or media alone by flow cytometry using anti-TRAIL Receptor-1 to 4 Flow Cytometry Set (Axxora). (b) DISC protein expression was measured by western blotting of cell lysates. (c) MTS assay; Cells were treated with TRAIL for 90 minutes, and then washed three times with media to remove unbound TRAIL. Media was replaced in wells and bortezomib was added for an overnight treatment. For the control plate cells were washed with media then TRAIL and/or bortezomib were added for the overnight incubation.
The DISC is important for proximal signaling events in TRAIL-mediated apoptosis. We therefore analyzed changes in cellular levels of DISC components in sensitized and resistant RCC following bortezomib treatment (Figure 4b). No major changes in the cellular levels of DR4, DR5, FADD, cFLIPL or caspase-8 were observed following western blotting in either sensitized or resistant RCC following bortezomib treatment, while increases in cFLIPS were observed in both sensitized and resistant cells. Thus, bortezomib did not produce any changes in the overall levels of DISC components that could be directly correlated to TRAIL sensitization by bortezomib. Recently a wash kill technique has been described (15), to determine the relevance of the small increases in DR4 or DR5 cell surface expression that often occur in cells following bortezomib treatment on TRAIL apoptosis. We still observed similar reductions of cell number in ACHN cells even if TRAIL was removed by extensive washing of the cells prior to the addition of bortezomib. Thus the bortezomib-induced increases in DR4 or DR5 cell surface expression were not crucial for the sensitization of RCC to TRAIL (Figure 4c). It therefore seems likely that while this increase in receptor number may play some slight additional modifying role in further increasing apoptosis, it is not essential for bortezomib’s sensitizing effects to TRAIL.
Bortezomib-induced changes on proteins involved in apoptosis
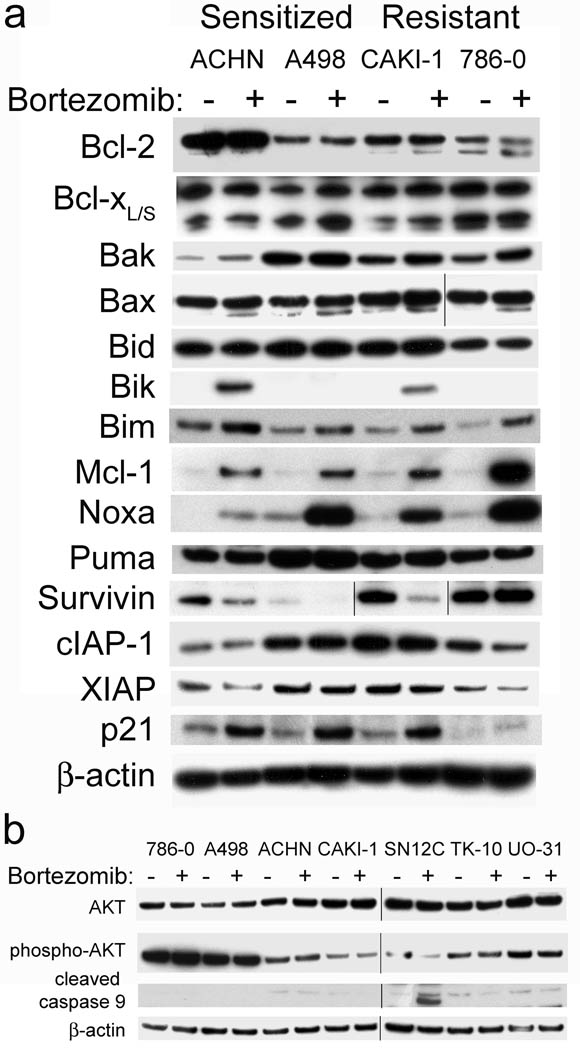
A large number of proteins are well known to influence apoptosis triggered by both the extrinsic and intrinsic apoptosis signaling pathways. We therefore assessed how bortezomib affected levels of these proteins by western blotting in RCC that could be sensitized or remained resistant to TRAIL apoptosis. Concerning Bcl-2 family proteins, no changes in expression of Bcl-2, Bcl-xL, Bak, Bax, Bid or Puma were observed in either sensitized or resistant RCC (Figure 5a). Cellular levels of Mcl-1, Noxa and Bim increased in all RCC, both sensitized and resistant. Bik increases following bortezomib treatment were only detected in 2 of the RCC, one sensitized and one resistant. Overall there was no direct correlation between any changes in levels of Bcl-2 family members and sensitization to TRAIL apoptosis. Concerning the IAP family, no changes in IAP-1 or XIAP levels occurred following bortezomib treatment. Survivin decreased in both the sensitized ACHN and A498 and the resistant CAKI-1, making it unlikely that decreases in survivin alone were crucial for TRAIL sensitization. Levels of p21 increased in all RCC tested. It has also been proposed that bortezomib effects on Akt phosphorylation or caspase activation could help promote TRAIL-mediated apoptosis. However, we did not observe any effect of sensitizing doses of bortezomib on Akt phosphorylation in either sensitized or resistant cells. Furthermore, at these concentrations of bortezomib we did not see activation of caspase-9 in most of the RCC (Figure 5b). This would be consistent with the fact that the tested concentrations of bortezomib were not directly triggering any apoptosis in these cell lines. Interestingly, the SN12C line was the exception, where bortezomib alone caused caspase-9 activation consistent with the cytotoxic effects of bortezomib observed with this cell line in long term growth assays (Figure 2). Since we observed no obvious changes in downstream apoptotic signaling that correlated with sensitization to TRAIL apoptosis, we then focused on determining if bortezomib induced any changes in the activation of procaspase-8.
Figure 5.
Bortezomib caused changes in expression of proteins related to apoptosis in some RCC cell lines. (a) Western blot analysis was performed on both pro and anti-apoptotic proteins. (b) Western blot analysis was performed on Akt and caspase-9.
Bortezomib effects on procaspase-8 activation
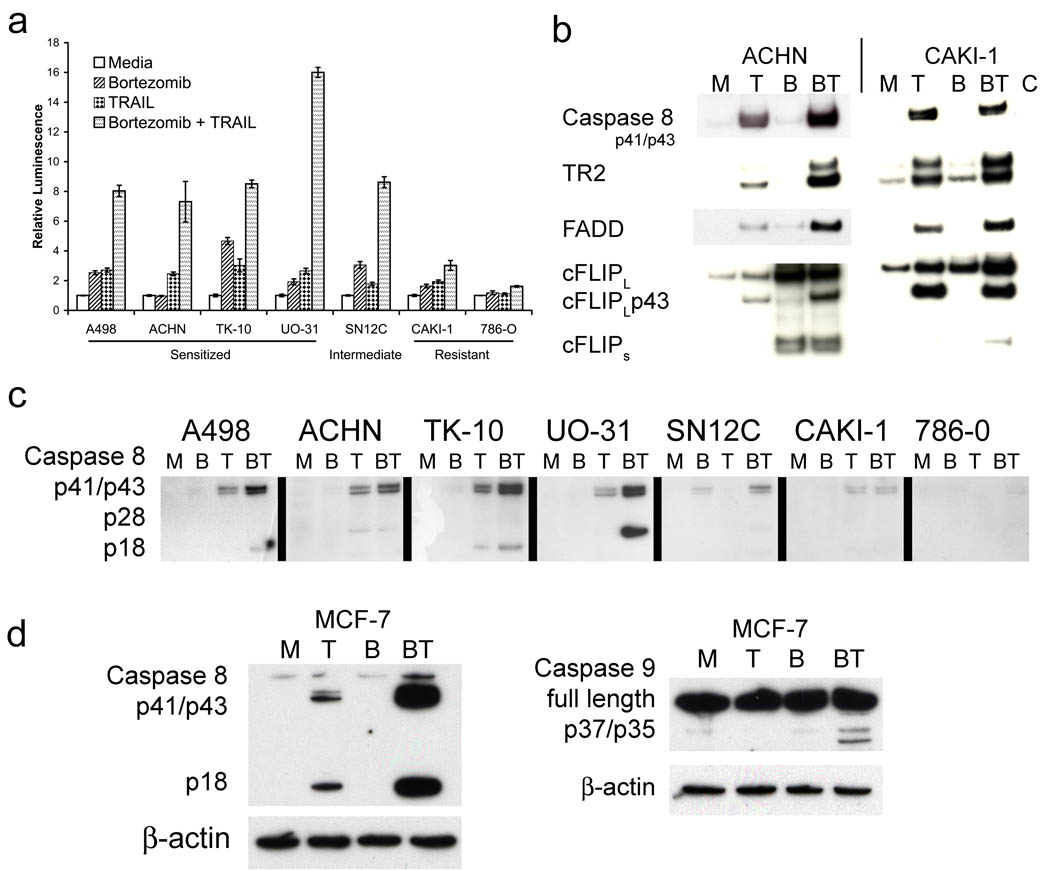
Measuring caspase-8 activity using a synthetic substrate assay (Figure 6a), the combination of bortezomib and TRAIL resulted in increased caspase-8 activation in all the sensitized cells (ACHN, A498, UO-31, TK-10) over and above that of either bortezomib or TRAIL alone. However, for the resistant CAKI-1 and 786-0 no such enhancement of caspase-8 activation was observed. In addition, western blotting using an antibody that only recognized the cleaved forms of procaspase-8 showed increased enzyme processing following TRAIL exposure in response to bortezomib in the sensitized ACHN, A498, UO-31 and TK-10, but not in the resistant CAKI-1 or 786-0 (Figure 6c). Interestingly, no processing of procaspase-8 in response to bortezomib alone was observed in any of the RCC with the exception of SN12C. This illustrates that for most RCC the sensitizing effect of bortezomib to TRAIL can occur in the absence of any direct apoptotic effects produced by the bortezomib alone. Since caspases can be activated during the process of apoptosis, it was necessary to clarify whether observed caspase-8 activation following bortezomib treatment was due to enhanced proximal processing of procaspase-8, or merely a consequence of ongoing apoptosis. Using the MCF-7 breast cancer cell line that is resistant to apoptosis due to a lack of caspase-3, we observed that bortezomib treatment still dramatically enhanced caspase-8 activation. A slight increase in activation of caspase-9 was also observed (Figure 6d). No apoptosis was observed in MCF-7 cells in response to bortezomib and TRAIL treatment (data not shown). Thus bortezomib treatment still increased caspase-8 activation even in the absence of subsequent apoptosis.
Figure 6.
Bortezomib causes increased caspase 8 activation at the DISC. (a) Caspase 8 activation was measured using the Caspase-Glo® 8 Assay System (Promega). (b) Cells were treated overnight with 20 nM Bortezomib followed by 60 minute treatment with biotinylated rhTRAIL. Lysates were immunoprecipitated with strepavidin coated magnetic beads and western blot analysis was performed on eluates for DISC components. (c) Cells were treated overnight with 20 nM bortezomib followed by 60 minutes with rhTRAIL. Lysates were prepared and analyzed by western blot. (d) MCF-7 cells were treated overnight with 20 nM bortezomib followed by 2 hours with rh TRAIL and lysates were prepared and analyzed by western blotting.
Since, of all the molecular parameters that we had assessed, only increased activation of caspase-8 activity correlated with the sensitizing effects of bortezomib, we examined the DISC components following ligand-induced immunoprecipitation from the bortezomib-sensitized ACHN and resistant CAKI-1 (Figure 6b). Higher levels of processed caspase-8 were associated with the ACHN DISC following pretreatment of the cells with bortezomib whereas for the CAKI-1 DISC no effect of bortezomib was observed. In the DISC of both cell lines there seemed to be somewhat higher levels of DR5 following bortezomib in both sensitized and resistant RCC. We failed to detect DR4 in our DISC immunoprecipitates from either ACHN or CAKI-1 cells. A more pronounced increase in FADD at the DISC following bortezomib treatment of ACHN, as opposed to CAKI-1 cells, was observed. The levels of cFLIP in the immunoprecipitates were somewhat difficult to determine, due to what seemed to be non-specific antibody binding in the region where full length cFLIP should be detected. However, levels of the cleaved fragment of cFLIP (p43) were increased in ACHN following bortezomib treatment, whereas no change was seen with CAKI-1. This would also be consistent with an increased caspase-8 activity at the ACHN DISC. Increased cFLIPS was associated with DISC immunoprecipitates particularly with ACHN cells; however this also could have been due to non-specific binding events rather than TRAIL-mediated recruitment since it was also present in DISC immunoprecipitates of ACHN treated with bortezomib alone. Overall the data suggest that bortezomib treatment in sensitized RCC promotes a more efficient DISC formation that results in enhanced activation of caspase-8. This increase in proximal apoptotic signaling then results in apoptosis of these cells. By contrast, in the resistant RCC lines no enhanced caspase-8 activation was observed following bortezomib treatment.
Discussion
Bortezomib can sensitize certain human RCC to TRAIL-mediated apoptosis, whereas others remain resistant. We have previously shown that all these RCC can be sensitized to TRAIL-mediated apoptosis by cycloheximide (22), thus the TRAIL apoptotic signaling pathway is intact. Consequently, the ability of bortezomib to sensitize RCC cells to TRAIL at the concentrations we used is selective. Previous studies have suggested that increases in the surface expression of the TRAIL death receptors (particularly DR5 or TRAIL-R2) could play an important role in sensitization of cancer cells to TRAIL-apoptosis following proteasome inhibition (12, 17). From our data we conclude that increases in cell surface expression of TRAIL death receptors in response to bortezomib are not absolutely required for sensitizing RCC to TRAIL apoptosis. A number of other studies have also concluded that changes in cell surface levels of DR5 do not play an essential role in the effects of bortezomib in sensitizing cancer cells to TRAIL (15, 23).
Concerning human RCC, we could find no direct evidence for an absolute requirement for the intrinsic apoptotic signaling pathway in bortezomib sensitization to TRAIL apoptosis. No obvious changes in cellular levels of most Bcl-2 family members were observed. Overall it was noteworthy that all the bortezomib-induced changes to Bcl-2 family members (Bik and Mcl-1) as well as changes in survivin and p21 that occurred in the sensitized ACHN cells were paralleled in Caki-1 cells that remained TRAIL resistant. Furthermore, neither the over expression of Bcl-2 nor Bcl-xL had any influence on bortezomib sensitization of ACHN to TRAIL (data not shown). In addition, Mcl-1 increased in all RCC tested. Mcl-1 is an antiapoptotic molecule that efficiently blocks the intrinsic or mitochondrial apoptosis pathway during TRAIL-mediated apoptosis (24), yet its increase in response to bortezomib in RCC does not block TRAIL apoptosis. Indeed others have reported that Mcl-1 increases following bortezomib treatment can limit the TRAIL sensitizing activity of bortezomib (25). Therefore it is very unlikely that effects of bortezomib on the intrinsic signaling pathway are required to promote TRAIL-mediated apoptosis in human RCC.
In contrast to our findings with RCC, a number of publications using other human cancer cell lines have demonstrated that bortezomib sensitization of cells to TRAIL apoptosis involves effects on Bcl-2 family members (21, 26, 27). However caution should be used when invoking a contribution of signaling via the intrinsic apoptotic pathway for bortezomib sensitization to TRAIL. In some of these studies quite high concentrations of bortezomib (50–1000nM) were used to show the sensitizing effect. In our experience bortezomib cytoxicity may only become apparent for most cancer cells 48–72 hours after exposure to the drug. Therefore we feel it is crucial that survival of the cancer cells should be assessed 5–6 days after removal of bortezomib and TRAIL. Otherwise the increased cytotoxic effects of the bortezomib and TRAIL combination observed at early time points might just reflect an acceleration of apoptosis in cells already destined to die.
Bortezomib has also been proposed to overcome TRAIL resistance in hepatoma cells through inhibition of the PI3K/Akt pathway (28). For human RCC we did not observe any effect of bortezomib on levels of pAkt at the concentrations we employed. Also, inhibitors of NF-κB that did not block proteasome activity were unable to sensitize RCC to TRAIL (data not shown). This would be consistent with other studies suggesting that inhibition of NF-κB activation is not required for bortezomib sensitization of RCC to TRAIL apoptosis (29). The presence or absence of mutations in p53 in the RCC did not correlate with the effects of bortezomib on TRAIL sensitization (data not shown), suggesting a lack of p53 involvement in the apoptotic signaling in this instance.
For RCC the amplification of the caspase-8 signal in response to TRAIL following bortezomib treatment is the one molecular change that always correlated with enhanced apoptosis. This does not rule out the possibility that other changes in apoptosis proteins induced by bortezomib may further amplify apopototic signaling. Indeed, the relative importance of such additional changes may vary in a cell type specific manner. However for RCC, the increased caspase-8 activation seems to be the crucial event. In contrast to the studies with hepatoma and glioma cells (15, 16), and previous findings from our laboratory and others (4, 30), we did not consistently observe a major reduction in cellular levels of cFLIPL following bortezomib treatment of RCC. However, the TRAIL DISC contained a significantly increased level of caspase-8 and FADD in bortezomib-sensitized RCC. In resistant RCC there were no obvious effects of bortezomib on the composition of the DISC following exposure to TRAIL. Our data in agreement with others (14–16) suggests that a crucial component of bortezomib sensitization of cancer cells to TRAIL apoptosis involves increased generation of caspase-8 at the DISC. The molecular basis for this increased activity of caspase-8 in RCC previously exposed to bortezomib is not yet known. Recent findings suggest that the glycosylation state of the TRAIL receptors (31) and ubiquitination of caspase-8 (32) can influence the strength of the apoptotic signal. Therefore, the effects of bortezomib on these processes are worthy of further investigation.
Although numerous studies have demonstrated that bortezomib can sensitize tumor cells to TRAIL apoptosis in vitro, few studies have attempted to extend this to the in vivo setting. We have shown improved therapeutic efficacy in the absence of toxicity in a mouse model of renal cancer when bortezomib is combined with an agonist antibody to mouse DR5 (33). Furthermore, combinations of bortezomib together with NK cells have also shown some therapeutic benefits in vivo in the same mouse renal cancer model (34). More recently therapeutic benefit of the bortezomib and TRAIL combination was observed in a xenograft model of human prostate cancer (35). These studies need to be extended. Since bortezomib has many side effects, and may be somewhat immunosuppressive in vivo (36), identification of the molecular mechanism(s) whereby it sensitizes tumor cells to TRAIL could be very beneficial. This may allow for the development of tumor biomarkers for prior identification of specific tumors that are most likely to respond to TRAIL-based therapies. Furthermore, this could also allow for the identification of components of the apoptotic pathway that could be targeted using agents more specific, and maybe more potent and less toxic than proteasome inhibitors.
Materials and Methods
Reagents and cell lines
Antibodies were purchased from the following companies: Anti-FLIP Dave-2 (Kamiya Biomedical, Seattle, WA), Anti-FLIP NF6 (Axxora, San Diego, CA), anti-cIAP-1 AF818 and anti-Survivin AF886 (R&D Systems, Minneapolis, MN), anti-β-actin AC74 and anti-DR5 D3938 (Sigma, St. Louis, MO), anti-DR4 32A242 (Stratagene, La Jolla, CA), anti-FADD 1F7 (Millipore, Billerica, MA), anti-bax sc-493, anti-bik sc1710, anti-ubiquitin sc8017 and anti-Bcl-xS/L sc-1041 (Santa Cruz, CA), anti-bcl-2 PC68 (EMD Chemicals Inc., San Diego, CA), and anti-Noxa 114C307 (EMD Chemicals Inc., San Diego, CA), anti-AKT, anti-phoso-AKT (Ser473) (D9E), anti-bid (2002),anti-Bim C34C5, anti-Puma (4976), anti-cleaved caspase-8 18C8, and anti-cleaved caspase-9 (Asp315) (Cell Signaling, Danvers, MA), anti-Mcl-2 559027, anti-XIAP 610716, p21 SX118 and anti-bak G317-2 (BD Biosciences, San Jose, CA), horseradish peroxidase-conjugated (HRP) anti-goat (Santa Cruz), HRP-anti-rabbit and HRP-anti-mouse (Thermo Fisher Scientific Inc., Rockford, IL), HRP-anti-rat (Cell Signaling, and rhTRAIL (Peprotech, Rocky Hill, NJ). Cell lines were obtained from the Developmental Therapeutics Program Tumor Repository (NCI-Frederick).
Flow Cytometric Analysis
TRAIL receptors 1 – 4 expression on tumor cell lines were analyzed after overnight treatment with 20nM Bortezomib (Millennium Pharmaceuticals, Cambridge, MA) or media alone by flow cytometry using anti-TRAIL receptor-1 to 4 Flow Cytometry Set (Axxora). Biotin-labeled polyclonal goat anti-mouse (Alexis) secondary was used followed by Phycoerythrin-labeled Streptavidin (BD Biosciences, Mountain View, CA). Apoptosis was measured using the TACS Annexin V-FITC Apoptosis Detection Kit (R&D systems). Flow cytometry analysis was performed on a FACScan using CellQuest software (BD Biosciences), figures were prepared using FCS 3 Express (De Novo Software).
Growth inhibition assay
RCC (5×103) were plated, incubated overnight, treated with 20 nM Bortezomib for two hours, then treated with rhTRAIL (100 ng/ml) and incubated overnight. Growth inhibition was measured using CellTiter 96 AQueous Non-Radioactive Cell Proliferation (MTS) Assay (Promega, Madison, WI). In some experiments the caspase inhibitors zVAD-FMK (Axxora) or zIETD-FMK (BD Biosciences) were added 2 hours prior to the addition of bortezomib. For TRAIL wash kill experiments, TRAIL was added to cells for 90 minutes, all wells were washed three times with 200 µl media to remove unbound TRAIL. Wells were refilled with fresh media and Bortezomib was added for an overnight treatment.
Long-term cell survival assays
RCC (5×104) were plated in 24 well plates, incubated overnight, treated with bortezomib for 2 hrs, then with rhTRAIL (1000 ng/ml), and incubated overnight. Treatments and dead cells were washed off and replaced with fresh media. Surviving cells were cultured for an additional 6 days, then fixed with methanol and stained with a 0.1% crystal violet solution.
Caspase 8 activity assays
Cells were plated in white 96 well plates at 1×104 cells/well, incubated 24 hrs, and then treated overnight with 20 nM bortezomib or media. 500 ng/ml rhTRAIL was added for 60 minutes prior to the addition of Caspase-8 glo reagent (Promega, Madison, WI) containing MG-132 and readings were taken 1 hour after the addition of substrate. Duplicate samples were run in standard 96 well plates to measure growth inhibition. Luminescence values were then adjusted based on cell number, and 1 luminescence unit was defined as the value obtained for untreated cells.
Proteasome Inhibition Assays
Cells were plated in white 96 well plates at 1×104 cells/well, incubated 24 hrs, and then treated overnight with 1, 10 or 20 nM bortezomib or media. Cells were assayed using the Proteasome-Glo™ Chymotrypsin-Like Cell-Based Assay (Promega, Madison, WI) according to the manufactures protocol. Duplicate samples were run in standard 96 well plates to measure growth inhibition.
Immunoprecipitation and immunoblotting
rhTRAIL (Peprotech, Rocky Hill, NJ) was biotinylated using PlatinumLink Antibody Labeling Kit (Kreatech Diagnostics, Amsterdam, The Netherlands). For immunoprecipitation cells were treated overnight with either 20 nM bortezomib or media treated with or without 500 ng/ml biotinylated TRAIL for 60 minutes; washed in cold PBS and lysed in IP DISC lysis buffer (30 mM Tris-HCL pH 7.5, 150 mM NaCl, 10% glycerol, 1% Triton X-100) supplemented with Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific Inc.) and 20 µM zVAD-FMK (BioMol, Plymouth Meeting, PA). Samples were equalized for protein concentration and incubated with MagnaBind Streptavidin resin (Thermo Fisher Scientific Inc.) overnight at 4 °C with end over end rotation, washed three times in IP DISC lysis buffer and eluted in 75 µl 2× loading buffer (NuPAGE® LDS Sample Buffer and NuPAGE® Reducing Agent, Invitrogen, Carlsbad, CA). Samples for immunoblot only were lysed in lysis buffer (50 mM Tris-Cl pH 8.0, 0.5% Triton X-100, 300 mM NaCl, and 5 mM EDTA) supplemented with Halt Protease Inhibitor Cocktail and 20 µM zVAD-FMK. Western blotting was performed as previously described (22).
Supplementary Material
Acknowledgements
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contracts NO1-CO-12400 and HSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. This Research was supported [in part] by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
We thank Dr. Rachel de Kluyver for helpful discussions, and Evan Lowery and Candace Thompson for technical assistance.
Abbreviations
- TRAIL
TNF-related apoptosis-inducing ligand
- cFLIP
cellular FLICE-inhibitory protein
- IAP
inhibitor of apoptosis protein
- FADD
Fas-associated death domain
- DISC
death-inducing signaling complex
- RCC
renal cell carcinoma
Footnotes
Conflict of interest: None of the authors have any competing financial interests in relation to the work described.
References
- 1.Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118:1979–1990. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–421. doi: 10.1016/s1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 3.Mitsiades CS, Treon SP, Mitsiades N, et al. TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: therapeutic applications. Blood. 2001;98:795–804. doi: 10.1182/blood.v98.3.795. [DOI] [PubMed] [Google Scholar]
- 4.Sayers TJ, Brooks AD, Koh CY, et al. The proteasome inhibitor PS-341 sensitizes neoplastic cells to TRAIL-mediated apoptosis by reducing levels of c-FLIP. Blood. 2003;102:303–310. doi: 10.1182/blood-2002-09-2975. [DOI] [PubMed] [Google Scholar]
- 5.Johnson TR, Stone K, Nikrad M, et al. The proteasome inhibitor PS-341 overcomes TRAIL resistance in Bax and caspase 9-negative or Bcl-xL overexpressing cells. Oncogene. 2003;22:4953–4963. doi: 10.1038/sj.onc.1206656. [DOI] [PubMed] [Google Scholar]
- 6.Nencioni A, Wille L, Dal Bello G, et al. Cooperative cytotoxicity of proteasome inhibitors and tumor necrosis factor-related apoptosis-inducing ligand in chemoresistant Bcl-2-overexpressing cells. Clin Cancer Res. 2005;11:4259–4265. doi: 10.1158/1078-0432.CCR-04-2496. [DOI] [PubMed] [Google Scholar]
- 7.Conticello C, Adamo L, Vicari L, et al. Antitumor activity of bortezomib alone and in combination with TRAIL in human acute myeloid leukemia. Acta Haematol. 2008;120:19–30. doi: 10.1159/000151511. [DOI] [PubMed] [Google Scholar]
- 8.Lashinger LM, Zhu K, Williams SA, et al. Bortezomib abolishes tumor necrosis factor-related apoptosis-inducing ligand resistance via a p21-dependent mechanism in human bladder and prostate cancer cells. Cancer Res. 2005;65:4902–4908. doi: 10.1158/0008-5472.CAN-04-3701. [DOI] [PubMed] [Google Scholar]
- 9.An J, Sun YP, Adams J, et al. Drug interactions between the proteasome inhibitor bortezomib and cytotoxic chemotherapy, tumor necrosis factor (TNF) alpha, and TNF-related apoptosis-inducing ligand in prostate cancer. Clin Cancer Res. 2003;9:4537–4545. [PubMed] [Google Scholar]
- 10.Conticello C, Adamo L, Giuffrida R, et al. Proteasome inhibitors synergize with tumor necrosis factor-related apoptosis-induced ligand to induce anaplastic thyroid carcinoma cell death. J Clin Endocrinol Metab. 2007;92:1938–1942. doi: 10.1210/jc.2006-2157. [DOI] [PubMed] [Google Scholar]
- 11.Saulle E, Petronelli A, Pasquini L, et al. Proteasome inhibitors sensitize ovarian cancer cells to TRAIL induced apoptosis. Apoptosis. 2007;12:635–655. doi: 10.1007/s10495-006-0025-9. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Yue P, Chen S, et al. The proteasome inhibitor PS-341 (bortezomib) up-regulates DR5 expression leading to induction of apoptosis and enhancement of TRAIL-induced apoptosis despite up-regulation of c-FLIP and survivin expression in human NSCLC cells. Cancer Res. 2007;67:4981–4988. doi: 10.1158/0008-5472.CAN-06-4274. [DOI] [PubMed] [Google Scholar]
- 13.Lu G, Punj V, Chaudhary PM. Proteasome inhibitor Bortezomib induces cell cycle arrest and apoptosis in cell lines derived from Ewing's sarcoma family of tumors and synergizes with TRAIL. Cancer Biol Ther. 2008;7:603–608. doi: 10.4161/cbt.7.4.5564. [DOI] [PubMed] [Google Scholar]
- 14.Ganten TM, Koschny R, Haas TL, et al. Proteasome inhibition sensitizes hepatocellular carcinoma cells, but not human hepatocytes, to TRAIL. Hepatology. 2005;42:588–597. doi: 10.1002/hep.20807. [DOI] [PubMed] [Google Scholar]
- 15.Koschny R, Ganten TM, Sykora J, et al. TRAIL/bortezomib cotreatment is potentially hepatotoxic but induces cancer-specific apoptosis within a therapeutic window. Hepatology. 2007;45:649–658. doi: 10.1002/hep.21555. [DOI] [PubMed] [Google Scholar]
- 16.Koschny R, Holland H, Sykora J, et al. Bortezomib sensitizes primary human astrocytoma cells of WHO grades I to IV for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Clin Cancer Res. 2007;13:3403–3412. doi: 10.1158/1078-0432.CCR-07-0251. [DOI] [PubMed] [Google Scholar]
- 17.Kandasamy K, Kraft AS. Proteasome inhibitor PS-341 (VELCADE) induces stabilization of the TRAIL receptor DR5 mRNA through the 3'-untranslated region. Mol Cancer Ther. 2008;7:1091–1100. doi: 10.1158/1535-7163.MCT-07-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanbolooki S, Nawrocki ST, Arumugam T, et al. Nuclear factor-kappaB maintains TRAIL resistance in human pancreatic cancer cells. Mol Cancer Ther. 2006;5:2251–2260. doi: 10.1158/1535-7163.MCT-06-0075. [DOI] [PubMed] [Google Scholar]
- 19.Chen KF, Yeh PY, Hsu C, et al. Bortezomib overcomes tumor necrosis factor-related apoptosis-inducing ligand resistance in hepatocellular carcinoma cells in part through the inhibition of the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2009;284:11121–11133. doi: 10.1074/jbc.M806268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fennell DA, Chacko A, Mutti L. BCL-2 family regulation by the 20S proteasome inhibitor bortezomib. Oncogene. 2008;27:1189–1197. doi: 10.1038/sj.onc.1210744. [DOI] [PubMed] [Google Scholar]
- 21.Nikrad M, Johnson T, Puthalalath H, et al. The proteasome inhibitor bortezomib sensitizes cells to killing by death receptor ligand TRAIL via BH3-only proteins Bik and Bim. Mol Cancer Ther. 2005;4:443–449. doi: 10.1158/1535-7163.MCT-04-0260. [DOI] [PubMed] [Google Scholar]
- 22.Brooks AD, Sayers TJ. Reduction of the antiapoptotic protein cFLIP enhances the susceptibility of human renal cancer cells to TRAIL apoptosis. Cancer Immunol Immunother. 2005;54:499–505. doi: 10.1007/s00262-004-0595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luster TA, Carrell JA, McCormick K, Sun D, Humphreys R. Mapatumumab and lexatumumab induce apoptosis in TRAIL-R1 and TRAIL-R2 antibody-resistant NSCLC cell lines when treated in combination with bortezomib. Mol Cancer Ther. 2009;8:292–302. doi: 10.1158/1535-7163.MCT-08-0918. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Ricci MS, El-Deiry WS. Mcl-1: a gateway to TRAIL sensitization. Cancer Res. 2008;68:2062–2064. doi: 10.1158/0008-5472.CAN-07-6278. [DOI] [PubMed] [Google Scholar]
- 25.Nencioni A, Hua F, Dillon CP, et al. Evidence for a protective role of Mcl-1 in proteasome inhibitor-induced apoptosis. Blood. 2005;105:3255–3262. doi: 10.1182/blood-2004-10-3984. [DOI] [PubMed] [Google Scholar]
- 26.Liu FT, Agrawal SG, Gribben JG, et al. Bortezomib blocks Bax degradation in malignant B cells during treatment with TRAIL. Blood. 2008;111:2797–2805. doi: 10.1182/blood-2007-08-110445. [DOI] [PubMed] [Google Scholar]
- 27.Zhu H, Guo W, Zhang L, et al. Proteasome Inhibitors-Mediated TRAIL Resensitization and Bik Accumulation. Cancer Biol Ther. 2005;4:781–786. doi: 10.4161/cbt.4.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen KF, Yeh PY, Yeh KH, et al. Down-regulation of phospho-Akt is a major molecular determinant of bortezomib-induced apoptosis in hepatocellular carcinoma cells. Cancer Res. 2008;68:6698–6707. doi: 10.1158/0008-5472.CAN-08-0257. [DOI] [PubMed] [Google Scholar]
- 29.Pawlowski JE, Nesterov A, Scheinman RI, Johnson TR, Kraft AS. NF-kappa B does not modulate sensitivity of renal carcinoma cells to TNF alpha-related apoptosis-inducing ligand (TRAIL) Anticancer Res. 2000;20:4243–4255. [PubMed] [Google Scholar]
- 30.Kabore AF, Sun J, Hu X, et al. The TRAIL apoptotic pathway mediates proteasome inhibitor induced apoptosis in primary chronic lymphocytic leukemia cells. Apoptosis. 2006;11:1175–1193. doi: 10.1007/s10495-006-8048-9. [DOI] [PubMed] [Google Scholar]
- 31.Wagner KW, Punnoose EA, Januario T, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 32.Jin Z, Li Y, Pitti R, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–735. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Shanker A, Brooks AD, Tristan CA, et al. Treating metastatic solid tumors with bortezomib and a tumor necrosis factor-related apoptosis-inducing ligand receptor agonist antibody. J Natl Cancer Inst. 2008;100:649–662. doi: 10.1093/jnci/djn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundqvist A, Yokoyama H, Smith A, Berg M, Childs R. Bortezomib treatment and regulatory T-cell depletion enhance the antitumor effects of adoptively infused NK cells. Blood. 2009;113:6120–6127. doi: 10.1182/blood-2008-11-190421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christian PA, Thorpe JA, Schwarze SR. Velcade sensitizes prostate cancer cells to TRAIL induced apoptosis and suppresses tumor growth in vivo. Cancer Biol Ther. 2009;8:73–80. doi: 10.4161/cbt.8.1.7132. [DOI] [PubMed] [Google Scholar]
- 36.Sun K, Welniak LA, Panoskaltsis-Mortari A, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc Natl Acad Sci U S A. 2004;101:8120–8125. doi: 10.1073/pnas.0401563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.