Abstract
Recent changes in demographic patterns of drug use have resulted in the increased non-medical use of prescription opiates. These users are younger and more likely to be female, which has the potential for increasing rates of in utero exposure. Therefore, we developed a rat model that simulates a prescription opiate-dependent woman who becomes pregnant. Adult female Sprague-Dawley rats were treated for 30 days via oral gavage with ascending doses of oxycodone HCl up to a final dose of 15 mg/kg/day, which was maintained during breeding and gestation. Controls were treated with water. The adult male offspring of these treated dams were tested on the radial arm maze, the Morris water maze (with a short and a long intertrial interval), and a spatial T-maze. Prenatal oxycodone exposure led to a deficit in the radial arm maze characterized by a greater number of reference memory errors, especially in the beginning of testing. In contrast, in the T-maze, prenatal oxycodone-exposed rats learned the task as well as well as the prenatal water controls. However, they had a modest deficit in retention of the task when assessed 5 days after acquisition training ended. For the Morris water maze, the intertrial interval affected the pattern of learning. While there was no deficit when the training had a short intertrial interval, when there was a long intertrial interval, prenatal oxycodone-exposed rats had poorer acquisition. The spatial learning deficit was characterized by and increased latency to find and a greater distance traveled to the platform in the prenatal oxycodone-exposed rats. These data were corroborated by analysis of the behavioral search strategy, which showed a decreased use of spatial strategies and an increase in non-spatial strategies, especially wall-hugging, in prenatal oxycodone-exposed rats as compared to prenatal water control rats on day 2 of acquisition. These results indicate that prenatal oxycodone exposure consistently impairs learning and memory in a battery of spatial tasks.
Keywords: Morris water maze, Radial arm maze, T-maze, Pregnancy, Development, Opiate
1. Introduction
Oxycodone, a powerful opiate analgesic used for moderate to severe pain, can result in dependence, tolerance, and addiction [9]. While most illicit drug use is decreasing, recreational use of prescription drugs has increased in young adults age 18–25 and prescription drugs are used as often as marijuana as a drug of initiation in youths aged 12–17 [14]. The same report shows that women aged 15–17 who were pregnant had a higher rate of drug use (22.6%) than those who were not pregnant (13.3%). These data together demonstrate that there is now a growing risk for exposure to prescription opiates in utero.
There have been surprisingly few comprehensive studies examining the effects of prenatal opiate exposure on cognitive development in humans, and none to date with prescription opiates. Olofsson et al. [23] investigated children born to opiate dependent mothers (majority were methadone dependent) one to ten years after birth. Over half of the children displayed maladaptive behaviors including hyperactivity, aggression, and lack of concentration, and over 20% had moderate or sever delays in psycho-motor development. However, the authors noted that many of the children were reared in unstable households and had multiple shifts in their primary caregiver, so it was difficult to assess the degree to which early drug exposure vs. a chaotic environment contributed to the behavioral effects. A decade later Van Baar and de Graaf [34] examined cognitive measures in children exposed to opiates (heroin and methadone, alone or in combination with other illicit drugs), most of whom underwent neonatal withdrawal after birth. In comparison to a non-drug exposed reference group, non-verbal intelligence deficits were noted in children aged three to four and language and general intelligence deficits were notes in children aged four to six. The researchers attempted to control for background characteristics, but study attrition led to a lower incidence of being reared by both parents, a lower level of education of the mother, and a less stable home environment in the drug-exposed group. And more recently a study of heroin and methadone dependent mothers in a residential treatment facility in Switzerland reported lower performance IQ’s in the offspring at approximately five years compared to population norms [32]. Studies looking at poly-drug abuse including opiate exposure have noted similar cognitive deficits (e.g., [24]). As noted above, it has been difficult to control for the effects environmental influence in these studies, which strengthens the need for controlled animal studies. Thus, to better understand the deleterious effects on cognition later in life that accompany opiate exposure in the womb, we have developed a model of prenatal oxycodone exposure in rats.
Prenatal opiate exposure has been shown previously to have adverse effects on cognition when embryos are exposed in utero to classically studied opiates like heroin, morphine, and the methadone analogue, l-α-acetylmethadol (LAAM). For example, radial arm maze [31] and Morris water maze [40] deficits were observed after exposure to prenatal heroin. Prenatal morphine increased latency in the radial arm maze [30] and caused a deficit in long-, but not intermediate-term memory in the one-trial passive avoidance task paradigm in the chick [8]. However, no prenatal exposure studies on learning and memory have been performed using prescription opiates.
Further, some of the studies with the above-mentioned opiates have been performed with exposure beginning at some time during gestation: prenatal heroin during embryonic days 9–18 in rodents [31, 40], prenatal morphine during embryonic days 11–18 in rats [30], and embryonic days 12–16 in the chick [8]. Late gestation is a time frame critical for hippocampus and cortical development and these studies are important in showing that drug-induced deficits can occur during such exposure [3]. However, they do not model a woman taking an opiate chronically who becomes pregnant. Therefore, we developed a model in which prenatal opiates are administered before and during the entire gestational period. Previous work in our laboratory using this approach with prenatal exposure to LAAM found poor performance in acquisition of the radial arm maze, a spatial memory task [28]. Prenatal LAAM-exposed rats had more reference and working memory errors, but were able to acquire the task after five days of training. In this study, prenatal LAAM resulted in a decrease in the synaptic expression of brain-derived neurotrophic factor (BDNF), which has been implicated in playing a role in synaptic architecture in development [6], in plasticity associated with drug exposure and addiction [5], and in synaptic plasticity and learning and memory in the adult [4, 15]. While studies like the ones described above have provided a foundation for the effect of prenatal opiates on learning and memory, these studies have not addressed whether prescription opiates have similar effects. Additionally, few reports have described the effect of the same prenatal opiate on multiple spatial memory tasks.
Many tasks have been used to assess spatial memory. Some of the most common include the Morris water maze, radial arm maze, and T-maze. The advantages of the radial arm maze and T-maze are that rats use natural foraging capabilities, as well as their exploratory instinct [16]. The disadvantages are that the rats must be food-restricted and this could lead to confounding differences in motivation, metabolism, or energy level that may affect the question to be studied. The rats must also learn the procedure of moving through a relatively non-natural maze and turning specific directions to find food, which involves procedural memory. Some of these disadvantages are overcome in the Morris water maze, which has been used extensively to study spatial memory [10, 19]. Rats are placed in a large circular tank of water with a platform submerged just beneath the surface of the water. They must use spatial cues within the room to find the location of the platform. Unlike the radial arm maze and T-maze, the rats do not have to be food-restricted and therefore are not metabolically challenged. However, the rats may undergo thermoregulatory stress in the water. The procedural learning component of the task is presumably less, because rats can naturally swim; however, procedural memory is still present. Therefore, multiple spatial memory tasks should ideally be used to determine differences potentially caused by drug treatment. Our hypothesis is that prenatal oxycodone will produce selective deficits in spatial learning and/or memory in three different spatial memory tasks, radial arm maze, T-maze, and the Morris water maze.
2. Material and Methods
2.1 Subjects
The subjected assessed were adult male offspring of dams treated with oxycodone prior to and during pregnancy (treatment and breeding described below). Testing was done in male offspring so that the behavioral parameters were not confounded by fluctuations in hormones from estrous cycling. To prevent littermate effects from confounding data interpretation, only one male per litter was assigned to each of the testing groups. The rats were 4–6 months of age at the time testing was initiated. The experimental subjects were group-housed in standard flat bottom plastic cages containing hardwood bedding. Temperature (22°C ± 1°C), humidity (40–50%), and 12 hr light:dark cycle remained constant throughout the experimentation. Food (Teklad, Harlan) and water were available ad libitum for all rats except as noted in the radial arm maze and T-maze procedures. The experimental protocol and animal husbandry procedures were approved by the Institutional Animal Care and Use Committee and comply with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, (publication number 85-23, revised 1985).
2.2 Prenatal drug treatment
The development of the prenatal oxycodone treatment paradigm is described in detail in [28]. Nulliparous female (64–70 days of age) Sprague-Dawley rats (Harlan, Indianapolis, IN) were treated with oxycodone HCl (Mallinckrodt, St. Louis, MO) or the water vehicle. Drug or vehicle was administered via a 7.6 cm 18-gauge oral gavage needle (Popper and Sons, New Hyde Park, New York) in a volume of 1 ml/kg. An ascending dosing procedure was used wherein doses of 10 mg/kg/day oxycodone were orally gavaged for 5 days. The dose was escalated by 0.5 mg/kg/day for 10 days to a final dose of 15 mg/kg/day, which was maintained for 15 days. After 28 days of treatment, the females were harem bred to proven breeder males (3 females: 1 male), with the males rotated daily. Treatment continued through breeding and gestation until parturition. Because the half-life of oxycodone is relatively short and drug distribution can be altered by pregnancy, throughout gestation dams were monitored for signs of opiate withdrawal. The presence of opiate withdrawal can confound data interpretation. Thus, withdrawal signs such as weight loss, diarrhea, and irritability were monitored daily. Pregnant dams were individually housed from gestational day 17 until parturition, when litters were culled to 10 pups. Pups were reared by their biological mothers, weaned at postnatal day 21, and housed with like-treated male subjects. Three separate breedings were conducted to generate sufficient subjects for the behavioral studies described below. The exposed offspring remain undisturbed until testing commenced at approximately 3 – 5 months of age.
2.3 Food restriction for radial arm maze and T-maze
The rats for the radial arm maze and T-maze studies were food restricted to 85% of their free-feeding body weight. There was no difference in the initial adult body weights between prenatal water and prenatal oxycodone-exposed rats, nor was their a difference in the amount of time that was needed to reach 85% of the free-feeding weight. Shortly before testing, rats were supplemented by approximately 0.5 g of a Maypo (Parsippany, NJ), a sweetened maple-flavored oatmeal cereal that served as the food reinforcer for the radial arm maze and T-maze tasks. During all phases of the radial arm maze and T-maze training, rats were maintained at 85–90% of their pre-testing body weight.
2.4 Radial arm maze
The radial arm maze was constructed of black painted wood finished with a polyurethane coating. The center platform was 58 cm in diameter with 8 arms (15 cm wide × 80 cm long) extending from the center. A 2.5 cm diameter hole at the end of each arm held a plastic disposable cup that contained the food reinforcer, a small drop of Maypo mash (approximately 0.01–0.025 mg).
Radial arm maze testing was performed in three phases: shaping, acquisition, and retention. Shaping was done with all of the arms baited with the food reinforcer. It was initially done in groups and then individually. During the social shaping, 3 rats were allowed to explore the baited maze simultaneously for 10 minutes to become acclimated to the apparatus. There were at least 2 days of social shaping. Food rewards eaten were recorded and used as criterion for exclusion. Rats were excluded if they did not eat at least one food reward in the social shaping trials (one prenatal water and two prenatal oxycodone rats failed to meet these criteria). For the individual shaping, each rat was allowed 3 min to explore the maze with all eight arms baited, such that the rats received experience in gaining a food reinforcement by completely traversing an arm.
Acquisition followed the shaping trials. During the training trials 4 of the 8 arms were baited with the food reinforcer. The pattern of baited arms was chosen to minimize non-spatial search strategies and to ensure that the difficulty level was similar for all rats. The same arms remained baited for all training and retention trials within each rat. There were 10 massed trials for each subject, each of which was preceded by a one-minute confinement in the center platform. The trial terminated when the rat entered all 4 baited arms or following a maximum latency of 180 seconds. Between trials the Maypo food reinforcer was replenished and the maze cleaned. The experimenter recorded the sequence of arm entries and trial latency.
Retention testing was conducted 7 days after the final acquisition trials. The rats received 3 trials as described under the acquisition procedure.
For acquisition and retention testing, the pattern of arms entries was analyzed for correct choices and types of errors. Reference memory errors were defined to be a visit to an arm that had never been baited. Working memory errors were defined as a revisit to an arm in which the food reward had previously been obtained within that trial.
2.5 T-maze
Hippocampus-dependent spatial memory was assessed using a standard T-maze. The maze was constructed of black painted wood finished with a polyurethane coating. The arms were 15 cm wide and × 80 cm long. They were enclosed with a wall that was 45 cm in height. A hole at the end of each arm held a 2.5 cm diameter plastic disposable cup that contained the Maypo food reinforcer.
T-maze training consisted of four phases: shaping, preference testing, acquisition, and retention. Shaping trials were conducted as described for the radial arm maze. These trials permitted the rats to become acclimated to the apparatus and the food reinforcers.
Preference testing was conducted following shaping. To determine if the rat had a preference for the left or right arm, each rat was tested with both arms of the maze baited. There were 7 trials a day for 5 days. Responses were recorded as “left choice” or “right choice” if all of the body entered the left or right arm. Responses were recorded as “no choice” if the rat did not enter either arm. Rats were excluded from further testing if they had more than 50% “no choices” during preference testing (one prenatal water and one prenatal oxycodone) or if they did not eat the reward at the end of the arm at least once in preference testing phase (one prenatal water). The number of left and right entries was summed and a side preference assigned based on the side that the rat chose most often.
Acquisition testing was conducted for 10 days. The non-preferred side was baited with the food reward on each trial. Each rat was given 6 trials per day with a maximal latency of 60 sec per trial. To minimize the effect of working memory, the time between trials was limited to a maximum of 10 sec. Responses were recorded as “correct” or “incorrect choice” if all of the body entered the correct or incorrect arm. Responses were recorded as “no choice” if the rat did not enter either arm. Latency to choice was also recorded.
Retention was tested 5 days after the last day of acquisition in the same manner as the acquisition trial.
2.6 Morris water maze
A clear, Plexiglas platform (15 × 15 cm) was placed in the middle of a quadrant of a 61 × 183 cm circular tank made of galvanized aluminum. The water level was 2 cm above the level of platform. The water temperature was kept at room temperature (19 – 20.5 °C; average water temperatures for the two experiments were 19.5 and 19.64 °C). Water maze training involved three phases: shaping, acquisition, and retention. Shaping was conducted by placing the rats on the escape platform for 10 sec before Trial 1 on the first day of testing.
Acquisition training was conducted for 7 days, with 4 trials each day. Trials were initiated by placing the rats gently into water facing the outer portion of the maze. On the first day of testing the rats were placed at the North position. On the remaining trials (and subsequent days) the rats were started from quasi-randomly selected positions, such that the starting point was always one of the positions farthest from the platform and the two farthest or two closest start locations were not done in succession. The sequence was consistent between rats within each day. Different sequences were used each day, except that the same sequence was used on day 1, day 7, and the retention day to allow for direct data comparison. Rats were allowed 60 sec to find platform and then guided to the platform if it was not found. The rats remained on the platform for approximately 10 sec after finding it or being guided to it. They were taken out of the maze and placed in a drying cage with heated lamp for a minimum of 5 min between trials.
Intertrial Interval
Two separate water maze experiments were conducted that differed by only one parameter, the intertrial interval. For the first experiment, the interval between all four trials in each training day was 40–60 min (long intertrial interval). For the second experiment, the intertrial interval was 15–30 min (short intertrial interval).
Retention testing was conducted for one day 7 days after the conclusion of the acquisition training. The retention test consisted of 4 trials for each rat similar to a single acquisition day. The data were analyzed as for acquisition.
Data were recorded with the SMART v.2.5.12 video tracking system (San Diego Instruments, San Diego, CA). Latency and distance traveled to find the platform, and the type of search strategy used were analyzed. For behavioral strategy analysis, each trial was placed in one of the following categories according to the dominant strategy used: 1) spatial strategies, which included direct search, focused search, and intermittent circles or 2) non-spatial strategies of random, chaining, and wall hugging (see Supplemental Figure 1 for an example of tracks from the different strategies). These categories were based on the reports of [1, 2, 13].
2.7 Statistical analysis
Acquisition of Morris water maze, radial arm maze, and T-maze was analyzed with repeated measures analysis of variance (RM-ANOVA) using Trial Day as the repeated measure and Prenatal Treatment as the between subjects measure. Retention analyses were performed as paired or unpaired t-tests, depending on the type of comparison. The paired t-test for the T-maze retention experiment was one-tailed because the radial arm maze data previously indicated that spatial performance was impaired. Mean +/− SEM is reported for all parametric tests. Behavioral strategy in the Morris water maze was analyzed by contingency analysis using total spatial versus total non-spatial strategies. The most common two spatial and most common two non-spatial strategies were also analyzed by Chi-Square contingency analysis. Frequency data are reported for all contingency analyses.
3. Results
3.1 Pregnancy and neonatal measures
There were 3 breedings that generated the pups for the studies described herein. Reproductive and developmental outcomes for these breedings indicated that oxycodone treatment was well tolerated by the dams and successful breeding was unaffected. There was no effect of oxycodone treatment on number of females that became pregnant and sustained pregnancy, in gestation length, weight gain during pregnancy, or litter size, which indicated no severe toxicity due to oxycodone. In all of the breedings, prenatal oxycodone pups had a lower birth weight (approximately 10%). The body weight remained lower than prenatal controls as they underwent opiate withdrawal across the first few days. However, body weight differences resolved by the end of the first postnatal week. Our studies revealed that neonatal withdrawal activated the hypothalamic-pituitary-adrenal (HPA) axis as shown by a three-fold elevation of corticosterone levels on postnatal day 1, which was completely recovered by postnatal day 2. Behavioral indices of spontaneous and precipitated withdrawal were noted, with pups having increased abdominal stretching, rolling, and twisting, especially when withdrawal was precipitated by injection of the opiate antagonist naloxone. The reproductive and developmental outcomes are described in more detail in [25].
3.2 Radial arm maze
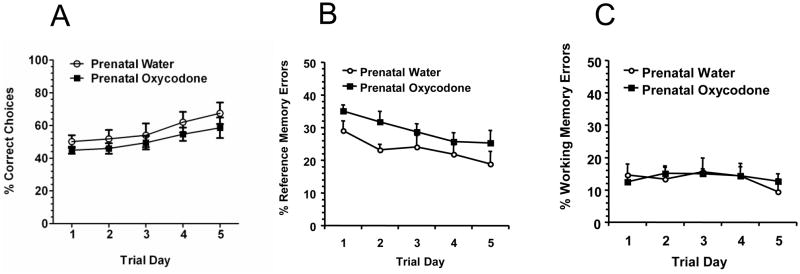
Prenatal oxycodone exposure did not affect the total number of arms visited (data not shown) or affect the percentage of correct choices made by rats in the radial arm maze (Figure 1A). Although both groups of rats increased the percentage of correct choices over trial days (Trial Day effect: F4,44 = 6.17, p < 0.0006), there was no difference between prenatal drug treatments or interaction between trial day and prenatal drug treatment. However, the profile of errors was different across prenatal treatments. For the percentage of reference memory errors, prenatal oxycodone-exposed rats had more reference memory errors during acquisition (Prenatal Drug effect: F1,11 = 4.63, p = 0.05; Figure 1B). In addition, there was an overall effect of Trial Day, with both groups decreasing across time (F4,44 = 4.74, p < 0.003). Working memory errors (Figure 1C) remained stable across the 5 days of acquisition, with no difference between prenatal treatments and no trial day effect or interactions.
Figure 1.
Prenatal oxycodone had no effect on overall percentage of correct choices in the radial arm maze (Panel A), but the error profile was different, such that percentage of reference memory errors(B) but not working memory errors (C) was higher in prenatal oxycodone treated rats compared to prenatal water treated rats. Open circles depict Prenatal Water and dark squares depict Prenatal Oxycodone. N=6 for Prenatal Water and N=7 for Prenatal Oxycodone.
There was no effect of prenatal treatment in the retention test conducted 7 days after the last day of acquisition. The percentage of correct choices (Prenatal Water = 45.11 ± 4.76 and Prenatal Oxycodone = 43.26 ± 4.58), reference errors (Prenatal Water = 27.63 ± 4.29 and Prenatal Oxycodone = 29.38 ± 4.13), and working errors (Prenatal Water = 23.45 ± 6.76 and Prenatal Oxycodone = 16.16 ± 3.65) were similar for both prenatal treatment groups.
3.3 T-maze
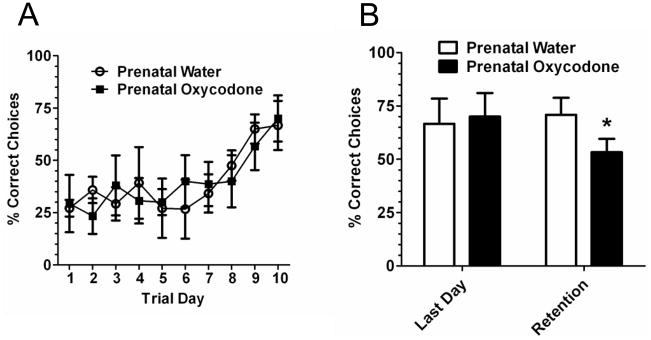
For the acquisition of the T-maze the rats were trained to go against their preferred side. Thus, it took 9–10 days to see evidence of learning (Figure 2A; Trial Day effect: F9,63 = 6.14, p < 0.0001), when the percentage of correct choices increased to approximately 70%. Similar results were observed with latency to make a choice (data not shown). There was no effect of prenatal oxycodone exposure on acquisition of the T-maze task. Retention testing was conducted 5 days after training. The data were first analyzed for a prenatal treatment effect or interaction on the percentage of correct choices on the retention, which were not significant. We then compared the rat’s performance on the last day of training to their retention trial. While the prenatal water exposed rats had a similar percentage of correct choices on the last day of acquisition and the retention day, prenatal oxycodone rats had approximately 20% fewer correct choices in the retention trial compared to the last day of acquisition (t4 = 2.24, p < 0.05).
Figure 2.
Prenatal oxycodone had no effect on acquisition of a spatial T-maze task (Panel A). It did however affect retention 5 days after acquisition (Panel B). The percent correct choices decreased across days in the prenatal oxycodone group, while there was no change for the prenatal water-exposed subjects (*p < 0.05; paired t-test vs. last day of acquisition). Open circles or bars depict Prenatal Water and dark squares depict or bars depict Prenatal Oxycodone. N=4 for Prenatal Water and N=5 for Prenatal Oxycodone.
3.4 Morris water maze – long intertrial interval
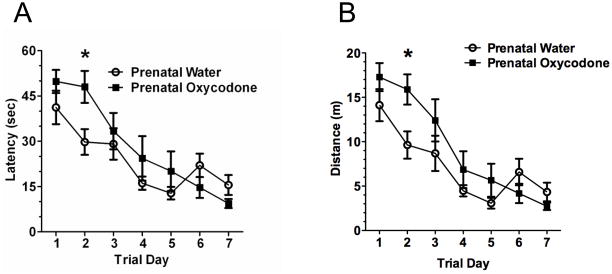
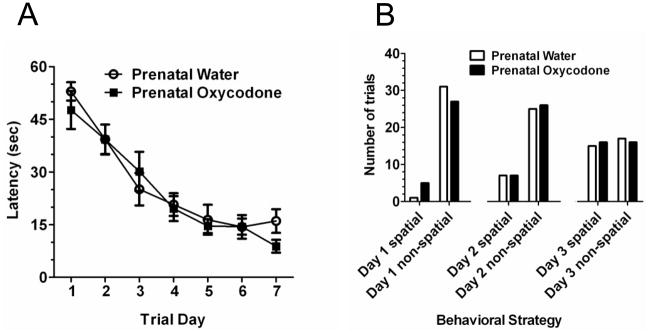
A sharp decrease in latency (Figure 3A) and distance traveled (Figure 3B) over trial days was found (Trial Day effect: F6,84 = 18.51, p < 0.0001 and F6,84 = 22.28, p < 0.0001 for latency and distance respectively). However, the rate of acquisition was not the same between prenatal treatment groups (F6,84 = 2.36, p < 0.04 and F6,84 = 2.31, p < 0.05; Prenatal Drug × Trial Day interaction for latency and distance respectively). Prenatal oxycodone-exposed subjects had a deficit in Morris water maze performance most evident on trial day 2 when the latency to locate and the distance traveled to the escape platform were over 60% greater than prenatal water-exposed subjects. Individual t-tests for each trial day indicated a significant prenatal treatment difference only on day 2 (t14 = 2.69, p < 0.02 and t14 = 2.72, p < 0.02 for latency and distance respectively). Indeed, inspection of the graphs reveals a 10 sec lower latency and over 4 meters less distance traveled for the prenatal water-exposed rats on day 2 compared to the prenatal oxycodone-exposed rats. However, the difference in latency and distance was transient, as performance of prenatal oxycodone-exposed rats was similar to prenatal water-exposed rats by day 3 of training.
Figure 3.
Prenatal oxycodone treatment impaired acquisition of the Morris water maze in the long intertrial interval version (40–60 min) of the Morris water maze. This is seen by increased latency (Panel A) and increased distance traveled (Panel B) to find the hidden platform. The effect was greatest on day 2 (*p < 0.05 vs. Prenatal Water). Open circles depict Prenatal Water and dark squares depict Prenatal Oxycodone. N=8 for both groups.
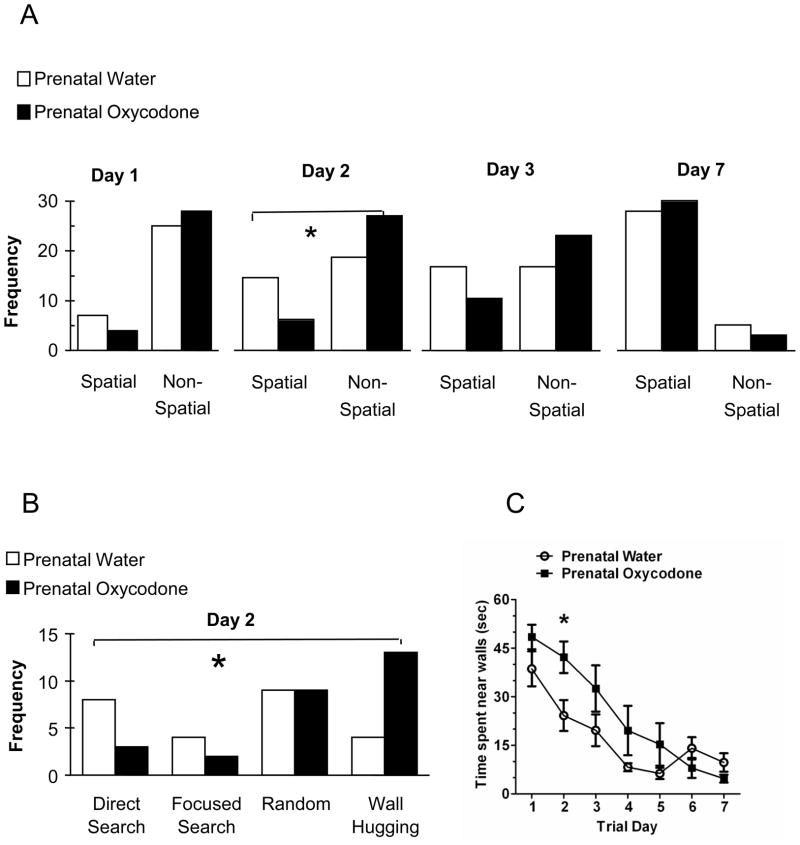
The behavioral strategy used in the maze was analyzed across days. The initial analyses divided the search strategies into categories: spatial and non-spatial. Because prenatal oxycodone treatment affected latency and distance traveled on day 2, we were particularly interested in the use of search strategies on this day. Thus, we analyzed the number of trials in which spatial vs. non-spatial search strategies were used on day 1, 2 and 3 and on the final day of testing, day 7 (Figure 4A). On day 1 both prenatal treatment groups relied heavily on non-spatial search strategies. On day 2, the prenatal water treated used spatial strategies to a greater degree than did the prenatal oxycodone exposed rats (χ21 = 4.66, p < 0.04). We examined the day 2 data in more detail, analyzing the specific strategies employed (Figure 4B). Prenatal oxycodone-exposed rats had fewer trials using a direct search or focused search strategy and more trials where the wall hugging strategy was used (χ23 = 7.64, p = 0.05). By day 3 of training however, the profile of the search strategies used across trials was not different between the prenatal drug-exposed groups and by day 7 the majority of trials employed a spatial search strategy for both prenatal treatment groups. Examples of the various types of search strategies can be seen in Supplementary Figure 1.
Figure 4.
Panel A depicts the frequency of trials where a spatial or a non-spatial search strategy was used on days 1, 2, 3, and 7 (last day) of the long intertrial interval version (40–60 min) of the Morris water maze. On test day 1, there no difference in the type of search strategies used as a consequence of prenatal drug exposure. However on test day 2, prenatal oxycodone-exposed rats had fewer trials where they used a spatial search strategy as compared to prenatal water-exposed rats. By test day 3, the search strategy profiles were no longer different between prenatal treatment groups and this continued for the remainder of testing (day 7 is depicted for comparison). Panel B depicts the breakdown of the top two spatial (direct search and focused search) and non-spatial (random and wall hugging) strategies on day 2 of testing. Prenatal oxycodone-exposed rats had fewer trials where they used a spatial search strategy as compared to prenatal water-exposed rats. This was particularly evident for the direct search spatial search strategy (decreased) and the non-spatial wall hugging strategy (increased). Panel C depicts the wall hugging strategy represented as the amount of time spent in within the outer 25% of the maze circumference. Prenatal oxycodone-exposed rats spent more time near the walls of the maze as compared to prenatal water-exposed rats. Open bars and symbols depict Prenatal Water and dark bars and symbols depict Prenatal Oxycodone. N=8 for both groups. (*p < 0.05).
We examined the wall hugging search pattern in more detail by measuring the amount of time spent swimming near the walls (defined as within the outer 25% of the maze circumference). Prenatal oxycodone exposure increased the amount of time the rats spent swimming near the walls (Figure 4C). The pattern was similar to latency and distance. The time spent near walls decreased over trial days (F6,84 = 23.64, p < 0.0001) and there was a significant interaction between Trial Day and Prenatal Drug Treatment (F6,84 = 2.63, p < 0.03). Individual t-tests were conducted for each trial day. As with the other measures, there was a significant effect of prenatal oxycodone exposure on day 2 (t14 = 2.64, p < 0.02).
Retention testing was conducted 7 days after the final day of testing. The data were analyzed for latency to find the platform on the first trial as a consequence of prenatal treatment (unpaired t-test). There was no difference in latency between the rats exposed prenatally to water as compared to oxycodone. Both groups had good retention of the platform location (Prenatal water = 12.50 sec ± 2.56 and Prenatal Oxycodone = 20.63 sec ± 5.07). In addition, we compared the latency to find the platform on first trial of the last day of acquisition to the latency on the first trial of retention (repeated measures ANOVA). Note that the start location for the first trial was the same on both days. There was no effect of prenatal treatment, no effect of test phase and no prenatal treatment × test phase interaction. A third set of analyses compared the search strategies utilized on the retention trials. There was no difference between the groups with respect to the use of spatial vs. non-spatial strategies or specific search strategies within each category. The majority of the trials for both groups utilized a direct search spatial strategy.
3.5 Morris water maze – short intertrial interval
The Morris water maze task was also performed in a separate group of rats using a shorter intertrial interval. Both prenatal treatment groups of rats learned the task (Figure 7A), as shown by a significant difference across Trial Days (F6,84 = 39.40, p < 0.0001) for the latency measure. In contrast to the experiment with the longer intertrial interval above, prenatal oxycodone-exposed rats did not differ from the prenatal water-exposed rats on any of the testing days. There was also no difference in the search strategies across the prenatal treatment groups on days 1–3, whether a 2 category spatial vs. non-spatial analysis was conducted (Figure 7B), or multiple categories were assessed (data not shown).
Retention testing was conducted 7 days after the final day of testing. The data were analyzed in a similar fashion as the data from the long intertrial interval-testing paradigm. There was no difference in latency to find the platform on the first trial between the rats exposed prenatally to water as compared to oxycodone (Prenatal water = 15.13 sec ± 3.97 and Prenatal Oxycodone = 22.38 sec ± 6.47). There was no effect of prenatal treatment, no effect of test phase and no prenatal treatment × test phase interaction when we compared the latency to find the platform on the first trial of the last day of acquisition as compared to the latency on the first trial of retention. Likewise, prenatal treatment did not affect the search strategies used on the retention trials, where the majority of the trials were using a spatial search strategy.
4. Discussion
The findings in this study indicate that prenatal oxycodone caused deficits in all three of the tasks used to assess spatial learning or memory. This is the first study to describe learning and memory deficits due to prenatal exposure to a prescription opiate using multiple tasks in a single study. The deficits in acquisition using the radial arm maze and Morris water maze were robust, yet transient. While there was no deficit on the first day in each of these two tasks, there was a profound difference between prenatal oxycodone- and prenatal water-exposed rats on day 2 that diminished by day 3. These differences consisted of an increase in reference, but not working memory errors in the radial arm maze and an increase in latency and distance traveled to find the platform in the water maze.
The spatial learning deficit of increased latency to find and distance traveled to the platform in the water maze was corroborated by analysis of behavioral search strategy, which showed a decreased use of spatial strategies and an increase in non-spatial strategies compared to prenatal water control rats on day 2 of acquisition. Altered use of spatial search strategies in the water maze has been reported in previous studies, with scopolamine administration [1] and prenatal restraint stress [39] in rats. In addition, decreased use of spatial search strategies were found in an Alzheimer’s disease transgenic mouse model [13] and a cAMP response element-binding protein knockout mouse [2]. The present study is the first study to show a decrease in use of a spatial search strategy in the water maze induced by prenatal drug exposure.
The main difference in water maze search strategy in the prenatal oxycodone-exposed rats was an increase on day 2 of non-spatial search strategies, particularly wall hugging. This thigmotaxic behavior is often indicative of enhanced stress- or anxiety-like behavior. It is unclear why a long, but not short, interval conditions would produce this pattern. Increased thigmotaxic behavior in the water maze has been associated with altered motivation in a mouse model of Alzheimer’s disease [26], non-cued and strategy shifting (non-mnemonic) learning in rats with lesions to the mediodorsal thalamic nucleus [11] and anxiety in rats that had prior exposure to cocaine [18] or lacked a thyroid hormone receptor [37]. There are also reports that anxiety-like behavior and performance in the water maze are inversely related [17, 20, 29]. However, it should be noted that there are a number of models of developmental drug exposure that induce deficits in the Morris maze in the absence of enhanced anxiety [36, 38]. We have previously demonstrated altered stress responding and enhanced acoustic startle (an anxiety behavior) in rats that were exposed in utero to the opiate LAAM [12]. And anxiety was speculated to play a role in revealing a prenatal opiate-induced effect on radial arm maze performance [30], in which prenatal morphine exposure caused an increase in latency to complete the radial arm maze in male rats. In the present study anxiety-like behavior may have also played a role in the poorer performance of the prenatal oxycodone-exposed pups as well, although we did not have any specific measures for the radial arm maze.
In contrast to the radial arm maze and water maze, prenatal oxycodone had no effect on acquisition of the standard T-maze. Instead, there was a modest deficiency in retention of the task 5 days after acquisition. One explanation is that the T-maze is a simpler task. The effects of prenatal oxycodone on brain structure and/or function may lead to acquisition deficits in tasks with a greater cognitive load (i.e., radial arm maze and Morris water maze with a long intertrial interval). Although the retention deficit was small in the T-maze, it does suggest that retention indices may be more sensitive than acquisition measures for tasks with less cognitive demand. The task parameters of the T-maze were such that the working memory component in solving the task was minimized, i.e., the intertrial interval was less than 10 sec. Since the intertrial interval affected the acquisition of the Morris water maze, further direct assessment of working memory and prefrontal cortical function after prenatal oxycodone exposure is warranted.
The deficits that we are reporting in the present study are less severe than those reported in studies with other prenatal opiate paradigms. For example, prenatal heroin exposure on gestational days 9–15 in mice caused a deficit manifest as increased number of entries in the radial arm maze (15–35% greater throughout all six days of testing compared to control) [31]. Also, prenatal heroin on gestational days 9–18 in mice caused an approximate 1.5–2.5-fold increase in latency to find the platform in the water maze across all five days of testing [40]. The effect of prenatal morphine administered on GD11–18 showed a dramatic increase in the latency to solve the radial arm maze (approximately 60%–100% higher) on the first day of testing [30]. In our prenatal oxycodone study, these differences are approximately a 55% increase in reference memory errors in the radial arm maze and 65% and 70% increases in latency and distance, respectively, in the water maze on day 2 of acquisition only. The difference in the magnitude of the effects may be related to the timing of the exposure and withdrawal and/or to the specific opiate examined. In the above studies, opiate exposure began at some point during pregnancy rather than before and throughout pregnancy as in the present prenatal oxycodone study. Therefore, it is possible that a later, more sudden prenatal opiate exposure is more detrimental to the development of learning and memory systems such as hippocampus and cortical structures, which begins during this time frame [3]. In contrast, continuous exposure may allow compensatory mechanisms to attenuate, although not eliminate, developmental damage that may occur at this sensitive time of development. This maybe especially relevant with respect to the timing of the withdrawal that invariably occurs following chronic exposure. Another very important difference between the above-mentioned studies and the present study is the use of the prescription opiate oxycodone vs. heroin and morphine. Oxycodone has different pharmacological properties than heroin and morphine, including the presence of active metabolites that increase the duration of action in rodent, especially in females. Females have higher blood levels of the parent compound and the active metabolite oxymorphone as compared to males. With a 10-mg/kg oral dose, the elimination half-life of oxycodone is approximately 4 hr in female rats [7]. A number of reports have suggested that oxycodone has more κ-opioid receptor activity than does morphine [e.g., 21, 22, 25].
The deficits in learning and memory using the model described herein indicate that problems in formation and/or storage of memories can occur in offspring due to prenatal oxycodone exposure. Previous studies have shown alterations in glutamatergic neurotransmission [33, 41, 42] and hippocampal cholinergic function [35] after prenatal morphine or heroin exposure. In our prior report examining prenatal LAAM effects on radial arm maze performance, we found reduced levels of the neurotrophic factor BDNF in the synaptic fraction of hippocampal samples [28]. Prenatal oxycodone exposure may also lead to similar disruptions in brain neurochemistry or architecture. Considering the increased use and abuse of the prescription opiates like oxycodone and the potential for a growing population of exposed infants, understanding the similarities and differences in the long-term consequences of exposure to a variety of opiates is an important research endeavor.
Supplementary Material
Figure 5.
In the Morris water maze version with a shorter intertrial interval (15–30 min), there was no effect of prenatal oxycodone exposure. There was no difference in latency to find the platform (Panel A), nor a difference in the use of spatial strategies across the test days 1–3. Open circles or bars depict Prenatal Water and dark squares depict or bars depict Prenatal Oxycodone. N=8 for both groups.
Acknowledgments
This research was supported, in part, by the National Institute on Drug Abuse, National Institutes of Health (Grant DA018181) and the Board of Regents, State of Louisiana (LEQSF(2005-08)-RD-A-19). The authors wish to thank Ms. Sylvie Mullins for her technical assistance with some these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Baldi E, Lorenzini CA, Bucherelli C. Task solving by procedural strategies in the Morris water maze. Physiol Behav. 2003;78:785–793. doi: 10.1016/s0031-9384(03)00064-7. [DOI] [PubMed] [Google Scholar]
- 2.Balschun D, Wolfer DP, Gass P, Mantamadiotis T, Welzl H, Schutz G, Frey JU, Lipp HP. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J Neurosci. 2003;23:6304–6314. doi: 10.1523/JNEUROSCI.23-15-06304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer SA. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 4.Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Bolanos CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. NeuroMolecular Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- 6.Chan JP, Unger TJ, Byrnes J, Rios M. Examination of behavioral deficits triggered by targeting BDNF in fetal or postnatal brains of mice. Neuroscience. 2006;142:49–58. doi: 10.1016/j.neuroscience.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Chan S, Edwards SR, Wyse BD, Smith MT. Sex differences in the pharmacokinetics, oxidative metabolism and oral bioavailability of oxycodone in the Sprague-Dawley rat. Clin Exp Pharmacol Physiol. 2008;35:295–302. doi: 10.1111/j.1440-1681.2007.04821.x. [DOI] [PubMed] [Google Scholar]
- 8.Che Y, Sun H, Tan H, Peng Y, Zeng T, Ma Y. The effect of prenatal morphine exposure on memory consolidation in the chick. Neurosci Lett. 2005;380:300–304. doi: 10.1016/j.neulet.2005.01.061. [DOI] [PubMed] [Google Scholar]
- 9.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 10.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 11.Dolleman-van der Weel MJ, Morris RG, Witter MP. Neurotoxic lesions of the thalamic reuniens or mediodorsal nucleus in rats affect non-mnemonic aspects of watermaze learning. Brain Struct Funct. 2009;213:329–342. doi: 10.1007/s00429-008-0200-6. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton KL, Harris AC, Gewirtz JC, Sparber SB, Schrott LM. HPA axis dysregulation following prenatal opiate exposure and postnatal withdrawal. Neurotoxicol Teratol. 2005;27:95–103. doi: 10.1016/j.ntt.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Janus C. Search strategies used by APP transgenic mice during navigation in the Morris water maze. Learn Mem. 2004;11:337–346. doi: 10.1101/lm.70104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on adolescent drug use: overview of key findings 2007. NIH Publication No 08-6418. National Institute on Drug Abuse: Bethesda MD; 2008. [Google Scholar]
- 15.Ma YL, Wang HL, Wu HC, Wei CL, Lee EH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82:957–67. doi: 10.1016/s0306-4522(97)00325-4. [DOI] [PubMed] [Google Scholar]
- 16.Macphail EM. Cognitive function in mammals: the evolutionary perspective. Cog Brain Res. 1996;3:279–290. doi: 10.1016/0926-6410(96)00013-4. [DOI] [PubMed] [Google Scholar]
- 17.Malleret G, Hen R, Guillou JL, Segu L, Buhot MC. 5-HT1B receptor knock-out mice exhibit increased exploratory activity and enhanced spatial memory performance in the Morris water maze. J Neurosci. 1999;19:6157–6168. doi: 10.1523/JNEUROSCI.19-14-06157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendez IA, Montgomery KS, LaSarge CL, Simon NW, Bizon JL, Setlow B. Long-term effects of prior cocaine exposure on Morris water maze performance. Neurobiol Learn Mem. 2008;89:185–191. doi: 10.1016/j.nlm.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 20.Moseley AE, Williams MT, Schaefer TL, Bohanan CS, Neumann JC, Behbehani MM, Vorhees CV, Lingrel JB. Deficiency in Na, K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci. 2007;27:616–626. doi: 10.1523/JNEUROSCI.4464-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen CK, Ross FB, Lotfipour S, Saini KS, Edwards SR, Smith MT. Oxycodone and morphine have distinctly different pharmacological profiles: radioligand binding and behavioural studies in two rat models of neuropathic pain. Pain. 2007;132:289–300. doi: 10.1016/j.pain.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen CK, Ross FB, Smith MT. Incomplete, asymmetric, and route-dependent cross-tolerance between oxycodone and morphine in the Dark Agouti rat. JPET. 2000;295:91–99. [PubMed] [Google Scholar]
- 23.Oloffson M, Buckley W, Andersen GE, Friis-Hansen B. Investigation of 89 children born by drug-dependent mothers. II. Follow-up 1–10 years after birth. Acta Paediat. 1983;72:407–410. doi: 10.1111/j.1651-2227.1983.tb09737.x. [DOI] [PubMed] [Google Scholar]
- 24.Pulsifer MB, Radonovich K, Belcher HM, Butz AM. Intelligence and school readiness in children with prenatal drug exposure. Child Neuropsychol. 2004;10:89–101. doi: 10.1080/09297040490911104. [DOI] [PubMed] [Google Scholar]
- 25.Ross FB, Smith MT. The intrinsic antinociceptive effects of oxycodone appear to be kappa-opioid receptor mediated. Pain. 1997;73:151–157. doi: 10.1016/S0304-3959(97)00093-6. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt U, Hiemke C, Fahrenholz F, Schroeder A. Over-expression of two different forms of the alpha-secretase ADAM10 affects learning and memory in mice. Behav Brain Res. 2006;175:278–284. doi: 10.1016/j.bbr.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Schrott LM, Batra V, Franklin LM, Johnson GS, Bouso E, et al. Developmental characterization of in utero oxycodone exposure in the rat. Submitted. [Google Scholar]
- 28.Schrott LM, Franklin LM, Serrano PA. Prenatal opiate exposure impairs radial arm maze performance and reduces levels of BDNF precursor following training. Brain Res. 2008;1198:132–140. doi: 10.1016/j.brainres.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skelton MR, Ponniah S, Wang DZ, Doetschman T, Vorhees CV, Pallen CJ. Protein tyrosine phosphatase alpha (PTP alpha) knockout mice show deficits in Morris water maze learning, decreased locomotor activity, and decreases in anxiety. Brain Res. 2003;984:1–10. doi: 10.1016/s0006-8993(03)02839-7. [DOI] [PubMed] [Google Scholar]
- 30.Slamberová R, Schindler CJ, Pometlová M, Urkuti C, Purow-Sokol JA, Vathy I. Prenatal morphine exposure differentially alters learning and memory in male and female rats. Physiol Behav. 2001;73:93–103. doi: 10.1016/s0031-9384(01)00469-3. [DOI] [PubMed] [Google Scholar]
- 31.Steingart RA, Silverman WF, Barron S, Slotkin TA, Awad Y, Yanai J. Neural grafting reverses prenatal drug-induced alterations in hippocampal PKC and related behavioral deficits. Dev Brain Res. 2000;125:9–19. doi: 10.1016/s0165-3806(00)00123-1. [DOI] [PubMed] [Google Scholar]
- 32.Steinhausen H-C, Blattmann B, Pfund F. Developmental outcomes in children with intrauterine exposure to substances. Eur Addict Res. 2007;13:94–100. doi: 10.1159/000097939. [DOI] [PubMed] [Google Scholar]
- 33.Tao PL, Yeh GC, Su CH, Wu YH. Co-administration of dextromethorphan during pregnancy and throughout lactation significantly decreases the adverse effects associated with chronic morphine administration in rat offspring. Life Sci. 2001;69:2439–2450. doi: 10.1016/s0024-3205(01)01316-9. [DOI] [PubMed] [Google Scholar]
- 34.van Baar A, de Graaff BM. Cognitive development at preschool-age of infants of drug-dependent mothers. Develop Med Child Neurol. 1994;36:1063–1075. doi: 10.1111/j.1469-8749.1994.tb11809.x. [DOI] [PubMed] [Google Scholar]
- 35.Vatury O, Barg J, Slotkin TA, Yanai J. Altered localization of choline transporter sites in the mouse hippocampus after prenatal heroin exposure. Brain Res Bull. 2004;63:25–32. doi: 10.1016/j.brainresbull.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Vorhees CV, Schaefer TL, Skelton MR, Grace CE, Herring NR, Williams MT. (+/−) 3,4-Methylenedioxymethamphetamine (MDMA) dose-dependently impairs spatial learning in the morris water maze after exposure of rats to different five-day intervals from birth to postnatal day twenty. Develop Neurosci. 2009;31:107–120. doi: 10.1159/000207499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilcoxon JS, Nadolski GJ, Samarut J, Chassande O, Redei EE. Behavioral inhibition and impaired spatial learning and memory in hypothyroid mice lacking thyroid hormone receptor alpha. Behav Brain Res. 2007;177:109–116. doi: 10.1016/j.bbr.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology. 2003;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- 39.Wu J, Song TB, Li YJ, He KS, Ge L, Wang LR. Prenatal restraint stress impairs learning and memory and hippocampal PKCbeta1 expression and translocation in offspring rats. Brain Res. 2007;1141:205–213. doi: 10.1016/j.brainres.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 40.Yanai J, Steingart RA, Snapir N, Gvaryahu G, Rozenboim I, Katz A. The relationship between neural alterations and behavioral deficits after prenatal exposure to heroin. Ann N Y Acad Sci. 2000;914:402–411. doi: 10.1111/j.1749-6632.2000.tb05214.x. [DOI] [PubMed] [Google Scholar]
- 41.Yang SN, Huang LT, Wang CL, Chen WF, Yang CH, Lin SZ, Lai MC, Chen SJ, Tao PL. Prenatal administration of morphine decreases CREBSerine-133 phosphorylation and synaptic plasticity range mediated by glutamatergic transmission in the hippocampal CA1 area of cognitive-deficient rat offspring. Hippocampus. 2003;13:915–921. doi: 10.1002/hipo.10137. [DOI] [PubMed] [Google Scholar]
- 42.Yang SN, Liu CA, Chung MY, Huang HC, Yeh GC, Wong CS, Lin WW, Yang CH, Tao PL. Alterations of postsynaptic density proteins in the hippocampus of rat offspring from the morphine-addicted mother: beneficial effect of dextromethorphan. Hippocampus. 2006;16:521–530. doi: 10.1002/hipo.20179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.