Abstract
Muscle weakness ensues when serum testosterone declines with age in men. Testosterone’s female counterpart, estrogen, has also been implicated in age-related strength loss but these results are less conclusive. Our working hypothesis is that estrogens do benefit muscle strength, and that the underlying mechanism involves estrogen receptors to improve muscle quality more so than quantity.
Keywords: aging, skeletal muscle, hormone replacement therapy, 17β-estradiol, myosin, estrogen receptor
INTRODUCTION
The loss of skeletal muscle strength occurs with age, but the reason why there are differences in rates and magnitude of losses between females and males is not clear. Sex hormones likely contribute to this difference. While lowered serum testosterone levels in aged men contribute to muscle weakness (4), the relationship between sex hormones and muscle strength in women is not so well understood. Furthermore, mechanisms by which testosterone improves strength in aged men are known (e.g., (6)), but mechanisms of how estrogens (e.g., estradiol, estrone, estriol) and/or progesterone affect skeletal muscle function have not been elucidated. Our working hypothesis is that estrogens do benefit muscle strength as demonstrated in both post-menopausal women and estrogen-deficient rodents. Furthermore, we hypothesize that the mechanism underlying estrogens’ effect on muscle strength results from actions of nuclear estrogen receptors that ultimately cause an improvement in the function of myosin. In the following sections, we provide mounting evidence supporting these suppositions.
Evidence that estrogens are beneficial to muscle strength in women
The loss of skeletal muscle strength is an undesirable consequence of aging. This decrease in a muscle’s ability to generate force is due in part to age-induced muscle atrophy. However, force generation normalized for muscle size, referred to as specific force or muscle quality, also declines with age. Hurley’s group, as part of the Baltimore Longitudinal Study on Aging, found that muscle quality was diminished in arm and leg muscles with age (12). Moreover, they showed that some of these declines were different between men and women. Pointing toward sex hormones as the underlying cause of these gender differences are studies showing that women have an accelerated decline in strength around the time of menopause. For example, whereas men had gradual decreases in knee extensor and handgrip strength between 20 and 80 years of age, women had a steep decline after the age of 55 (25). Similarly, Phillips and coworkers reported that strength of the adductor pollicis muscle was not different between men and women up to the age when menopause occurred but thereafter peri- and post-menopausal women had a striking decline in strength (Fig. 1, top panel) (22). The accelerated strength loss of the adductor pollicis muscle in the peri- and post-menopausal women was not due to enhanced muscle atrophy because the strength data were reported as specific force (maximum voluntary force relative to muscle cross-sectional area).
Figure 1.
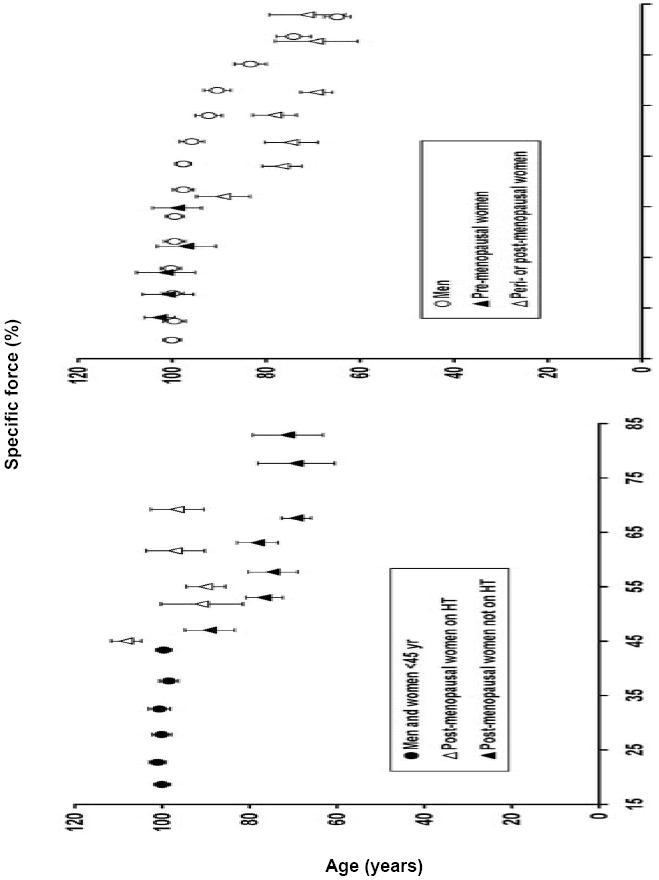
Relationship between specific muscle force of the adductor pollicis muscle and age for groups of subjects that did and did not experience loss of estrogens during the sixth decade of life. Within each graph, specific force is expressed as a percent of the mean for subjects aged 45 yr and younger. Top panel: Following menopause, women lose muscle strength with age at a greater rate than do men out until about the age of 70 yr. Bottom panel: Postmenopausal women who take an estrogen-based hormone therapy (HT) retain muscle strength to a greater extent than do women who do not take the therapy. Figure created from data originally published in (22).
Phillips and coworkers linked the menopause-related strength loss to sex hormones by studying an additional group of women who were on an estrogen-based hormone therapy (HT) (22). They showed that the strength loss was prevented in peri- and post-menopausal women on HT (Fig. 1, bottom panel). Again, the strength differences between groups were not related to muscle size because strength was assessed as specific force. Despite these striking results, the finding that HT prevents muscle weakness in postmenopausal women has not been consistently reported, as was concluded in two narrative review papers on the topic of sex hormones and muscle strength and performance (13, 27).
To address the inconsistent findings in the literature regarding the effect of HT on muscle strength in post-menopausal women, we conducted a systematic review combined with a meta-analysis (8). Muscle strength data from 23 studies that included nearly 10,000 post-menopausal women who were or were not on HT were analyzed. The major finding was that postmenopausal women that had received HT had ~5% greater strength than those that did not receive treatment (effect size=0.23). Interestingly, when specific force was analyzed from the subset of studies that made that measurement, a trend for a larger effect equating to ~10% greater strength for postmenopausal women on HT was revealed (Fig. 2, top panel). Thus, by statistically combining all results from the published literature, it can be concluded that HT is indeed beneficial for muscle strength in postmenopausal women (8). Results of the meta-analysis also indicate that the effect of HT on muscle strength is by improving the function of the existing muscle, that is, by improving muscle quality not by muscle hypertrophy.
Figure 2.
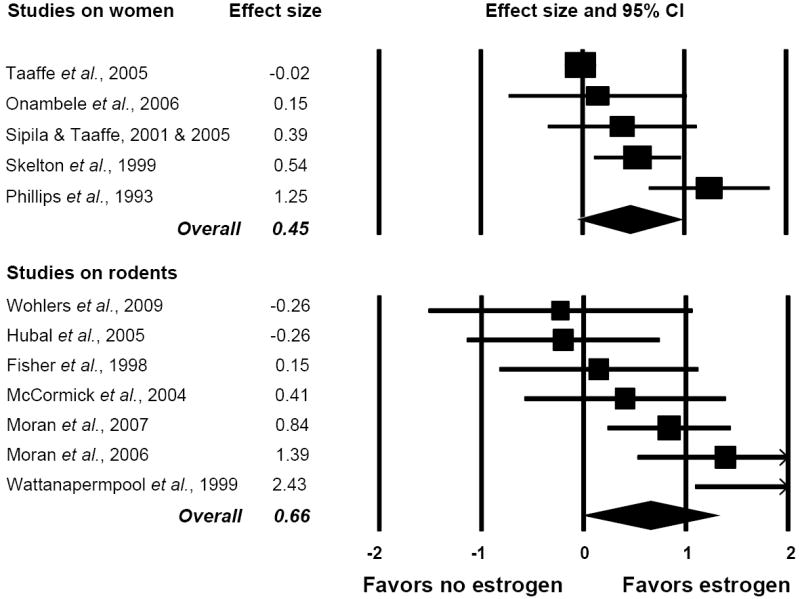
Forest plots of effect sizes from meta-analyses on studies that reported muscle strength normalized to muscle size (specific force) in subjects that were and were not estrogen deficient. Each square represents the effect size for that study with the size of the square equating to the weight of that study in the meta-analysis. The horizontal line through the square indicates the 95% confidence interval (CI) for that effect size. Within each plot, studies are arranged from lowest to highest effect sizes. Top panel: Results of five studies on postmenopausal women comparing muscle strength between women who were and were not on an estrogen-based hormone therapy. Bottom panel: Results of seven studies on rats or mice comparing specific force between those that were estradiol deficient via ovariectomy and those that were ovary intact or estradiol-replaced. Figure created from data originally published in (8); refer to this paper for references cited in the figure.
A recent study on postmenopausal monozygotic female twins who were discordant for HT also supports the contention that estrogens are good for muscle function (19, 24). The purpose of the twin analyses was to determine if long-term HT was associated with better lower limb muscle function in a cohort of women in which potential genetic and early environmental differences were minimized. Although they found that maximal isometric knee extension strength was not significantly different between sisters who did and did not take HT, other measures of muscle function were different. Specifically, HT was associated with significantly better maximal 10-m walking speed and lower-body muscle power. Interesting, it was also found that sisters on HT had greater relative muscle area and less relative fat area of the thigh than did the non-HT sisters, suggesting that in women estrogens may have some anabolic and/or metabolic influence. A commentary following the twin paper sums up the study and the past literature by stating that this study “incrementally advances the field and in fact tips the balance toward a positive and measureable (beneficial) impact of HRT” (19, 24).
In summary, muscle weakness ensues with age and in women tends to become more pronounced when the production of estrogens and progesterone declines at menopause. This accelerated muscle weakness is linked to the loss of sex hormones because HT helps preserve strength. A caveat of the studies on postmenopausal women that precludes the determination of which specific hormone affects muscle function is that differing HT preparations were used across and even within studies (8). Beyond the hormone preparations used, between-study variations also include population characteristics and type of muscle strength measurement. To circumvent these issues that are inherent to studies on women, more controlled interventions utilizing rodents are reasonable and likely necessary to establish the specific sex hormone that improves strength in females and also to determine the mechanism(s) by which the hormone is working.
Evidence that estrogens are beneficial to muscle strength in rodents
In addition to studies showing that humans experience loss of strength with age, age-related muscle weakness has been substantiated in rodent models. This picture is heavily skewed, however, because the vast majority of these studies have been conducted on male rats and mice. Due to the void of information on aging female rodent muscle, we conducted a study specifically to determine if and when age-related decrements in strength occur in female mice (18). The major finding was that specific force generated by soleus muscle declined by ~25% with age and did so around the age at which ovaries fail in mice, that is, between 11 and 16 months of age (5). This prompted us to more critically look at the effect of estrogens on muscle function. We chose to begin our studies by investigating 17β-estradiol because it is the most biologically active form of estrogen. Moreover, we also chose a surgical approach of reducing ovarian hormones in adult mice, i.e., ovariectomy, in order to focus on ovarian hormones without the multiple, potentially confounding effects of aging.
In a series of studies, we consistently found that leg muscles from ovariectomized mice were 10%-20% weaker than corresponding muscles from ovary-intact and 17β-estradiol replaced mice (16, 17, 31). For example, soleus muscles of ovariectomized and sham-operated mice generated an average (± SD) 0.22 ± 0.04 and 0.27 ± 0.04 N·cm of force per milligram of contractile protein, respectively (17). Two important points are worth noting. First, muscle strength was reported as maximal force normalized to the sum of actin and myosin heavy chain contents because ovariectomy causes fluid accumulation in rodent muscle. If force was normalized by muscle cross-sectional area, as is normally done, then specific force deficits of muscles from the ovariectomized mice would appear to be even greater. Second, because several hormones are affected with ovariectomy, it was important to determine if estradiol was the key hormone affecting muscle. Indeed, we showed that 17β-estradiol replacement reversed the detrimental ovariectomy-induced effects on muscle function (16), confirming that estradiol is paramount. Additional analyses of fiber cross-sectional areas, fiber types, total and contractile protein contents, and dry muscle masses showed that the quantity of muscle was not affected by estradiol status but, instead, that the intrinsic quality of muscle was altered.
Previous studies that had been conducted on ovariectomized rodents reported that strength increased, did not change, or decreased with ovariectomy. Notably, most of those studies had been conducted on young, growing mice and rats, the ages being equivalent to adolescence or younger in humans illustrating the complex, critical roles of ovarian hormones during muscle development. In total, 10 rodent studies have been published addressing the question of how ovarian hormones affect muscle strength. We conducted a meta-analysis on data from these studies, similar to that done on post-menopausal women (8). The six studies that had been conducted on estrogen-deficient and -replete mice collectively provide strong evidence for the hormone imparting muscle strength (effect size = 0.88); however, the three studies that had been conducted on rats did not yield a significant effect. Irrespective of species and similar to the results from the meta-analysis on women, the hormonal effects were significant when the strength data were normalized to some measure of muscle size. In the rodents, muscles that were exposed to estrogens had ~7% greater normalized strength than those that were devoid of the hormone (Fig. 2, bottom panel). These data again indicate that the intrinsic quality of skeletal muscle and thus its capacity to generate force is better with estrogens. In addition, 5 of the 10 studies in the meta-analysis included a group of rodents that were ovariectomized and replaced specifically with 17-β estradiol and those results indicate that estradiol is the key ovarian hormone affecting muscle strength (8).
Evidence that myosin function is affected by age and by estradiol in females
At the molecular level, force is produced by the interaction of the contractile proteins, myosin and actin. Myosin is referred to as the molecular motor in muscle because during contraction, in concert with adenosine triphosphate (ATP) hydrolysis, the protein changes conformation to cycle between strong- and weak-binding structural states. Notably, force is only generated when the myosin head is in its strong-binding state, that is, when it is strongly bound to actin. Electron paramagnetic resonance (EPR) spectroscopy paired with site-specific spin labeling has been key in determining myosin structural states because it is the only technique that is capable of detecting and quantifying weak- and strong-binding states in intact muscle fibers during a muscle contraction (29). We originally used EPR spectroscopy to test the hypothesis that low specific force in fibers from aged rats is a result of structural changes in the myosin head (11). We showed that the fraction of myosin heads in the strong-binding structural state during contraction averaged 0.316 ± 0.021 in fibers from young rats but only 0.221 ± 0.013 in fibers from aged rats (11). This was a seminal result showing that the 30% decrement in strong-binding myosin in fibers from aged rats corresponded to the 30% decrement in specific force in those fibers. Thus, we concluded that myosin structural changes provide a molecular explanation for age-related declines in specific force. Others had suggested that age-related muscle weakness is a result of decreases in strong-binding myosin (2, 21). However, our EPR study provided the first direct evidence supporting this hypothesis.
As with the overwhelming majority of studies addressing age-induced muscle weakness, we originally investigated myosin as a molecular mechanism of muscle dysfunction in male rodents. The picture becomes a bit more complicated in female rodents because, like in women, aging is accompanied by hormonal changes, particularly the decline of ovarian hormones as the reproductive system fails. Phillips and coworkers, in their 1993 paper that was highlighted in a previous section, hypothesized that ovarian hormones influence muscle strength by directly affecting contractile proteins (22). This hypothesis was addressed in one study by using permeabilized muscle fibers. Permeabilized fibers do not have intact nerve or membranes so excitation-coupling mechanisms are bypassed and contraction is initiated by exposure to exogenous calcium. Thus, force generation directly reflects function of the contractile proteins. In support of the hypothesis that hormones affect contractile proteins, permeabilized fibers from rat soleus muscles generated 20% lower specific force 10-14 weeks following ovariectomy compared with fibers from control, ovary-intact rats (Fig. 3, far left set of bars) (32). Once again these data are suggestive of a qualitative difference between fibers that are devoid of and exposed to sex hormones. In other words, fibers that came from ovariectomized rats were weaker not because they were smaller but rather because there was an intrinsic deficit that resulted in a decreased ability to generate force. Authors of that paper speculated that deprivation of ovarian hormones caused the reduced “tension” by either a decrease in the number of force-generating crossbridges or a decrease in the force per crossbridge (32).
Figure 3.
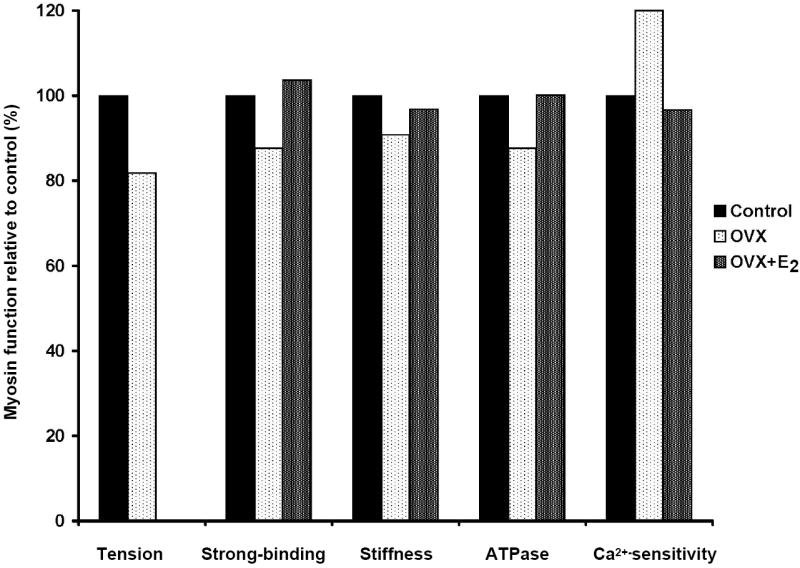
Decrements in the functions of myosin occur when muscle fibers or myofibrils are analyzed from rodents deficient in estrogens via ovariectomy (OVX). Conversely, when ovariectomized rodents are treated with estradiol (OVX+E2), myosin functions return to control levels. Tension is specific force generated by permeabilized fibers and is reflective of the overall ability of acto-myosin filaments to make force (data from (32)). The fraction of myosin in the strong-binding structural state during contraction directly reflects the force-generating capacity of myosin and active stiffness of intact muscle indirectly reflects myosin strongly bound to actin during contraction; both are affected by estrogen status (data from reference #16). Both myofibrillar adenosine triphosphate hydrolysis (ATPase) and control of calcium (Ca2+) are required for myosin to cycle between strong- and weak-binding states to actin and both are influenced by estrogens (data from (33)).
Recently we completed a series of studies using EPR spectroscopy to directly investigate the effects of estradiol on myosin in muscles from female mice. Ovariectomy caused reductions in strong-binding myosin that was equivalent percentagewise to the reduction in specific force, and 17β-estradiol treatment completely reversed those reductions (Fig. 3) (16). These results extended previous findings in three ways. First, the ovarian hormone 17β-estradiol was pinpointed as an influential hormone affecting force generation at the molecular level. Second, myosin was identified as a distinct contractile protein detrimentally affected by lack of the hormone. Third, because the percent decrement in strong-binding myosin was the same extent as that in specific force, it can be deduced that there was a decrease in the number of force-generating crossbridges and not in force per crossbridge. Again, to reiterate, total and actin and myosin protein contents of muscle fibers were not different between these estradiol-deficient and -replete mice, substantiating that the quantity of contractile machinery was not affected by estradiol status, only the quality was affected.
Three other pieces of evidence support the contention that myosin is detrimentally affected by the loss of estradiol. In intact, isolated soleus and extensor digitorum longus (EDL) muscles from ovariectomized mice, active stiffness was reduced, agreeing with the EPR data that the fraction of myosin heads strongly bound to actin during contraction is depressed (17). Furthermore, estradiol replacement reversed the effect on active stiffness (Fig. 3) (16). The cycling of myosin between strong- and weak-binding states is dependent on hydrolysis of ATP by the myosin head. As such, myofibrillar ATPase activity reflects myosin function. In cardiac myofibrils, maximum myofibrillar ATPase activity was reduced by ovariectomy and restored by estradiol replacement (Fig. 3) (33). Lastly, although more indicative of myofilament function as opposed to myosin function per se, the loss of estrogens induces calcium hypersensitivity in cardiac myofibrils and this effect was also reversed by estradiol replacement (Fig. 3) (33). Collectively, these results strongly support the supposition that myosin function is affected by estradiol and is a molecular mechanism underlying the qualitative defect in muscle that is not exposed to this specific sex hormone. Myosin heavy chain has multiple isoforms but there are no indications that estradiol status results in changes in isoform expression (16, 32).
The challenge now becomes to determine how estradiol causes alterations in myosin functions. Because oxidative stress is postulated to be a leading mechanism underlying overall biological aging and because we know that myosin is susceptible to oxidation, this is one avenue to consider. In relation to gender, there are suggestions that oxidative stress is countered better in premenopausal women than in similar aged men resulting in lower incidences of many diseases such as cardiovascular disease in this group of women (3). However, older women, specifically those who are postmenopausal, lose this advantage over men of the same age. Observations, such as these, point toward sex hormones playing a role in oxidative stress during aging and we speculate that this may occur in skeletal muscle as well. There is plenty of evidence showing that oxidation occurs in skeletal muscle of aged male rodents (e.g., (7)) but studies in females are lacking. Also, the connection between biochemical oxidative modifications and functional decrements in skeletal muscle were lacking until a recent report where we showed that myosin is susceptible to oxidation and that the oxidation significantly impairs contractile function (23). The approach we used in those studies was an in vitro oxidation of muscle fibers by hydrogen peroxide that yielded irreversible, physiologically-relevant modifications of muscle proteins. Specifically, we found oxidation of methionine residues within the myosin heavy chain and essential light chains of hydrogen peroxide-treated muscle fibers. These fibers also had decrements in myosin function, as determined by measurements of force generation and the fraction of myosin heads in the strong-binding structural state during contraction. Our current working hypothesis is that similar oxidative stress occurs in vivo in female muscle as a result of estradiol deficiency, and an antioxidant effect may be one mechanism by which estrogen treatment is protective. Admittedly, much work lies ahead to substantiate this hypothesis.
Evidence that estradiol works through estrogen receptors in skeletal muscle
An important step in determining how estradiol is able to alter the function of myosin in a fiber during a muscle contraction is to consider how estradiol typically functions in cells. The most well-described actions of estradiol are its genomic effects. These genomic effects are mediated through nuclear α- and β-estrogen receptors (ER) which function as transcription factors once bound with their ligand. For this type of estrogenic mechanism to be a reasonable one in skeletal muscle, it would be expected that ERs in muscle fibers would be responsive to circulating estradiol levels. In other words, it is essential that ERs in skeletal muscle have a typical steroid response. To this end, we hypothesize that ovariectomy- and age-induced estradiol deficiency should result in changes in ER content in skeletal muscle and conversely that estradiol replacement reverses the effects. It has been shown in nine tissues other than skeletal muscle that ovariectomy in rats caused α-ER messenger RNA (mRNA) expression to increase as much as three-fold or to not change, while estradiol treatment reversed any effects (14). The results of that study are important because it shows that regulation of ER mRNA expression by plasma estradiol is tissue specific. There are also reports of age-related changes in ERs in different tissues, but again effects in skeletal muscle are unknown (10, 26, 34).
This lack of information on ERs in skeletal muscle was the underlying rationale for determining these receptor levels in a recent study (1). We theorize that this information in turn could provide insight into the mechanism of estradiol action in muscle. Hindlimb muscles from estradiol-deficient and -replete mice were analyzed for α-ER, β-ER, and G protein-coupled receptor (Gper) mRNA expression by real-time polymerase chain reaction (PCR) and for α-ER protein levels. The most important and novel finding from the study is that α-ER mRNA and protein levels were responsive to circulating estradiol levels. Specifically, α-ER mRNA and protein levels were ~two-fold greater in soleus, EDL, and tibialis anterior muscles of ovariectomized mice relative to muscles of estradiol-replaced mice (1). These results indicate that skeletal muscle is an estrogen-responsive tissue and presents a plausible mechanism of estrogenic action in skeletal muscle through ERs. How this ultimately affects myosin and strength remains to be determined. We speculate that improving the reduction-oxidation state in fibers and thus keeping muscle proteins like myosin free from posttranslational oxidative modifications could contribute to the maintenance of protein structure-function and ultimately strength. This speculation is based on findings that antioxidant proteins such as superoxide dismutases and glutathione peroxidase are regulated through ERs by 17β-estradiol (28, 30). This idea is illustrated in Figure 4.
Figure 4.
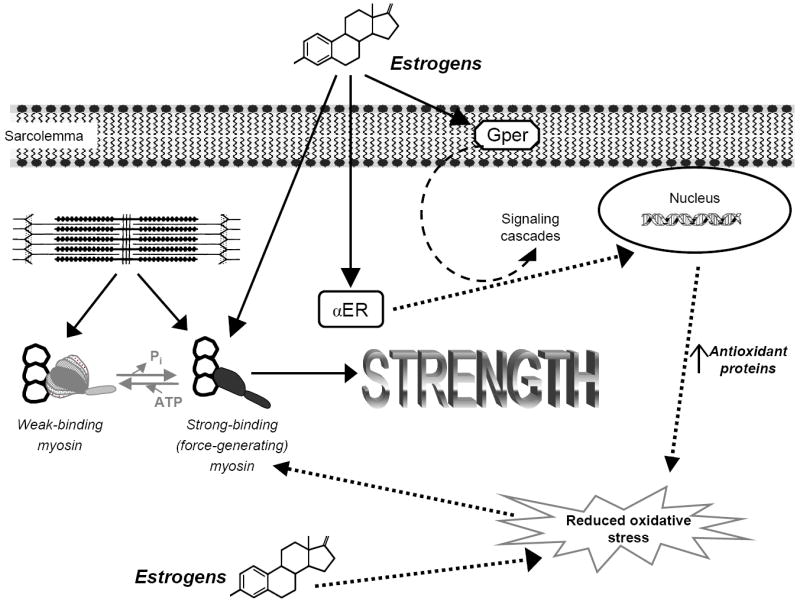
Schematic of how estrogens may benefit muscle strength. Ovariectomized mice display a reduction in muscle strength and this is attributed to a decrement in the fraction of strong-binding myosin during contraction. Conversely, treatment with estrogens increases strong-binding myosin and ultimately strength. Estrogen receptor (ER) content in muscle is responsive to circulating estrogens, and we hypothesize that ERs may initiate signaling cascades and/or regulate genes that result in an overall reduction in oxidative stress in fibers. Alternatively, estrogens may have some direct antioxidant affect. We speculate that reducing oxidative stress would preserve myosin structure-function, conferring a beneficial effect on strength. Solid arrows represent experimental evidence from our lab and others. Dashed arrows represent hypothesized mechanisms of estrogens’ actions in skeletal muscle. Abbreviations: adenosine triphosphate (ATP), inorganic phosphate (Pi), G protein-coupled receptor (Gper).
In addition to estradiol’s genomic effects via α- and β-ERs, estradiol has been shown to regulate many other physiological responses including cell signaling, which may or may not involve ERs. For example, Gper when bound with estrogen initiates signaling cascades that mediate rapid cellular responses (9). These cellular responses have not been investigated in skeletal muscle. It is likely that decoding estrogen-sensitive signaling pathways will be complex as was shown recently by the indirect ER-regulation of MyoD that occurs by preventing repression of AP-1 binding (20). Finally, many steroids including estrogens are weak antioxidants (15) and as such it is possible that estradiol itself has some direct affect on the oxidative status of muscle fibers. These concepts are also illustrated in Figure 4. In sum, we know that estrogen replacement improves myosin function and strength in muscle that is devoid of sex hormones and that the content of ERs in muscle also increases. The mechanism by which these events occur is speculative and may include genomic effects via ERs, cell signaling events (e.g., via Gper), and direct antioxidant consequences.
CONCLUSIONS
The collective results of studies on post-menopausal women and ovariectomized rodents support the contention that estradiol is beneficial to muscle strength. The evidence indicates that estradiol does not accomplish this by affecting muscle size, rather by improving the intrinsic quality of skeletal muscle whereby fibers are enabled to generate force. Our EPR studies show that force generation at the molecular level, that is, myosin strongly binding to actin during contraction, is implicated in this qualitative effect of estradiol on skeletal muscle. Finally, evidence is mounting that estradiol may be affecting muscle through ERs, as these receptors are responsive to estradiol status in a typical steroid receptor manner. Why might these findings be important? Additional information for women approaching menopause to base decisions about HT would be worthwhile. While HT alone is not likely to be the best way to prevent muscle weakness in the elderly, in combination with other avenues it may prove to be very beneficial. For example, it may be that HT in combination with resistance exercise could even better improve muscle strength in post-menopausal women. It may also be possible to strategize alternative estrogen-like approaches to thwart off skeletal muscle weakness in other populations including men. Finally, perhaps estrogens’ effects on bone would be enhanced if in addition to the direct effects of the hormone on the skeleton, muscle contractions were more forceful and/or numerous creating additional osteogenic stimuli.
Acknowledgments
The authors apologize to the researchers whose scientific contributions were not noted in this article because of the constraint on citations. Our research has been supported by grants from the University of Minnesota Graduate School and the National Institutes of Health (AG031743).
Footnotes
Disclosure of funding: This research has been supported by grants from the University of Minnesota Graduate School and the National Institutes of Health (AG031743).
References
- 1.Baltgalvis KA, Greising SM, Lowe DA. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLoS ONE. doi: 10.1371/journal.pone.0010164. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brzezinski A, Danenberg HD. Estrogen, progesterone, and cardiovascular health: when shall we complete the puzzle? Menopause. 2005;12:488–91. doi: 10.1097/01.gme.0000177320.31629.df. [DOI] [PubMed] [Google Scholar]
- 4.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95:1717–27. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 5.Felicio LS, Nelson JF, Finch CE. Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol Reprod. 1984;31:446–53. doi: 10.1095/biolreprod31.3.446. [DOI] [PubMed] [Google Scholar]
- 6.Ferrando AA, Sheffield-Moore M, Yeckel CW, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–7. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 7.Fugere NA, Ferrington DA, Thompson LV. Protein nitration with aging in the rat semimembranosus and soleus muscles. J Gerontol A Biol Sci Med Sci. 2006;61:806–12. doi: 10.1093/gerona/61.8.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greising SM, Baltgalvis KA, Lowe DA, Warren GL. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071–81. doi: 10.1093/gerona/glp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hewitt SC, Deroo BJ, Korach KS. Signal transduction. A new mediator for an old hormone? Science. 2005;307:1572–3. doi: 10.1126/science.1110345. [DOI] [PubMed] [Google Scholar]
- 10.Kaur J, Thakur MK. Effect of age on physico-chemical properties of the uterine nuclear estrogen receptors of albino rats. Mech Ageing Dev. 1991;57:111–23. doi: 10.1016/0047-6374(91)90028-x. [DOI] [PubMed] [Google Scholar]
- 11.Lowe DA, Surek JT, Thomas DD, Thompson LV. Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2001;280:C540–7. doi: 10.1152/ajpcell.2001.280.3.C540. [DOI] [PubMed] [Google Scholar]
- 12.Lynch NA, Metter EJ, Lindle RS, et al. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol. 1999;86:188–94. doi: 10.1152/jappl.1999.86.1.188. [DOI] [PubMed] [Google Scholar]
- 13.Meeuwsen IB, Samson MM, Verhaar HJ. Evaluation of the applicability of HRT as a preservative of muscle strength in women. Maturitas. 2000;36:49–61. doi: 10.1016/s0378-5122(00)00132-8. [DOI] [PubMed] [Google Scholar]
- 14.Mohamed MK, Abdel-Rahman AA. Effect of long-term ovariectomy and estrogen replacement on the expression of estrogen receptor gene in female rats. Eur J Endocrinol. 2000;142:307–14. doi: 10.1530/eje.0.1420307. [DOI] [PubMed] [Google Scholar]
- 15.Mooradian AD. Antioxidant properties of steroids. J Steroid Biochem Mol Biol. 1993;45:509–11. doi: 10.1016/0960-0760(93)90166-t. [DOI] [PubMed] [Google Scholar]
- 16.Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA. Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol. 2007;102:1387–93. doi: 10.1152/japplphysiol.01305.2006. [DOI] [PubMed] [Google Scholar]
- 17.Moran AL, Warren GL, Lowe DA. Removal of ovarian hormones from mature mice detrimentally affects muscle contractile function and myosin structural distribution. J Appl Physiol. 2006;100:548–59. doi: 10.1152/japplphysiol.01029.2005. [DOI] [PubMed] [Google Scholar]
- 18.Moran AL, Warren GL, Lowe DA. Soleus and EDL muscle contractility across the lifespan of female C57BL/6 mice. Exp Gerontol. 40:2005. 966–75. doi: 10.1016/j.exger.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Onambele-Pearson GL. HRT affects skeletal muscle contractile characteristics: a definitive answer? J Appl Physiol. 2009;107:4–5. doi: 10.1152/japplphysiol.00448.2009. [DOI] [PubMed] [Google Scholar]
- 20.Pedraza-Alva G, Zingg JM, Donda A, Perez-Martinez L. Estrogen receptor regulates MyoD gene expression by preventing AP-1-mediated repression. Biochem Biophys Res Commun. 2009;389:360–5. doi: 10.1016/j.bbrc.2009.08.153. [DOI] [PubMed] [Google Scholar]
- 21.Phillips SK, Bruce SA, Woledge RC. In mice, the muscle weakness due to age is absent during stretching. J Physiol. 1991;437:63–70. doi: 10.1113/jphysiol.1991.sp018583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond) 1993;84:95–8. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- 23.Prochniewicz E, Lowe DA, Spakowicz DJ, et al. Functional, structural, and chemical changes in myosin associated with hydrogen peroxide treatment of skeletal muscle fibers. Am J Physiol Cell Physiol. 2008;294:C613–26. doi: 10.1152/ajpcell.00232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ronkainen PH, Kovanen V, Alen M, et al. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: a study with monozygotic twin pairs. J Appl Physiol. 2009;107:25–33. doi: 10.1152/japplphysiol.91518.2008. [DOI] [PubMed] [Google Scholar]
- 25.Samson MM, Meeuwsen IB, Crowe A, Dessens JA, Duursma SA, Verhaar HJ. Relationships between physical performance measures, age, height and body weight in healthy adults. Age Ageing. 2000;29:235–42. doi: 10.1093/ageing/29.3.235. [DOI] [PubMed] [Google Scholar]
- 26.Sharma PK, Thakur MK. Estrogen receptor alpha expression in mice kidney shows sex differences during aging. Biogerontology. 2004;5:375–81. doi: 10.1007/s10522-004-3191-6. [DOI] [PubMed] [Google Scholar]
- 27.Sipila S, Poutamo J. Muscle performance, sex hormones and training in peri-menopausal and post-menopausal women. Scand J Med Sci Sports. 2003;13:19–25. doi: 10.1034/j.1600-0838.2003.20210.x. [DOI] [PubMed] [Google Scholar]
- 28.Strehlow K, Rotter S, Wassmann S, Adam O, Grohe C, Laufs K, Bohm M, Nickenig G. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003;93:170–7. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- 29.Thomas DD, Ramachandran S, Roopnarine O, Hayden DW, Ostap EM. The mechanism of force generation in myosin: a disorder-to-order transition, coupled to internal structural changes. Biophys J. 1995;68:135S–41S. [PMC free article] [PubMed] [Google Scholar]
- 30.Vina J, Sastre J, Pallardo FV, Gambini J, Borras C. Modulation of longevity-associated genes by estrogens or phytoestrogens. Biol Chem. 2008;389:273–7. doi: 10.1515/BC.2008.027. [DOI] [PubMed] [Google Scholar]
- 31.Warren GL, Moran AL, Hogan HA, Lin AS, Guldberg RE, Lowe DA. Voluntary run training but not estradiol deficiency alters the tibial bone-soleus muscle functional relationship in mice. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2015–26. doi: 10.1152/ajpregu.00569.2007. [DOI] [PubMed] [Google Scholar]
- 32.Wattanapermpool J, Reiser PJ. Differential effects of ovariectomy on calcium activation of cardiac and soleus myofilaments. Am J Physiol. 1999;277:H467–73. doi: 10.1152/ajpheart.1999.277.2.H467. [DOI] [PubMed] [Google Scholar]
- 33.Wattanapermpool J, Riabroy T, Preawnim S. Estrogen supplement prevents the calcium hypersensitivity of cardiac myofilaments in ovariectomized rats. Life Sci. 2000;66:533–43. doi: 10.1016/s0024-3205(99)00623-2. [DOI] [PubMed] [Google Scholar]
- 34.Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mech Ageing Dev. 2002;123:593–601. doi: 10.1016/s0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]


