Abstract
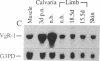
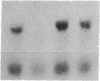
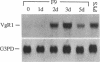
The transforming growth factor beta (TGF-beta)-related products of the Xenopus Vg-1 and Drosophila decapentaplegic (DPP) genes have been implicated in the control of growth and differentiation during embryogenesis. We have isolated a mouse cDNA, Vgr-1, that encodes a polypeptide structurally related to Xenopus Vg-1. Sequence comparisons indicate that the Vgr-1 protein belongs to a family of DPP-like gene products within the TGF-beta superfamily. The levels of Vgr-1 RNA were determined in embryos and tissues isolated at various stages of development. A 3.5-kilobase mRNA increases throughout development and into adulthood in many tissues and in F9 teratocarcinoma cells differentiating into endoderm in response to retinoic acid and cAMP. The amino acid homologies and patterns of expression suggest that, like the DPP gene product, Vgr-1 plays a role at various stages of development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cate R. L., Mattaliano R. J., Hession C., Tizard R., Farber N. M., Cheung A., Ninfa E. G., Frey A. Z., Gash D. J., Chow E. P. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986 Jun 6;45(5):685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Derynck R., Lindquist P. B., Lee A., Wen D., Tamm J., Graycar J. L., Rhee L., Mason A. J., Miller D. A., Coffey R. J. A new type of transforming growth factor-beta, TGF-beta 3. EMBO J. 1988 Dec 1;7(12):3737–3743. doi: 10.1002/j.1460-2075.1988.tb03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsave S. F., Isaacs H. V., Slack J. M. Mesoderm-inducing factors: a small class of molecules. Development. 1988 Mar;102(3):555–566. doi: 10.1242/dev.102.3.555. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Embryonic induction--molecular prospects. Development. 1987 Mar;99(3):285–306. doi: 10.1242/dev.99.3.285. [DOI] [PubMed] [Google Scholar]
- Hughes S. M., Lillien L. E., Raff M. C., Rohrer H., Sendtner M. Ciliary neurotrophic factor induces type-2 astrocyte differentiation in culture. Nature. 1988 Sep 1;335(6185):70–73. doi: 10.1038/335070a0. [DOI] [PubMed] [Google Scholar]
- Irish V. F., Gelbart W. M. The decapentaplegic gene is required for dorsal-ventral patterning of the Drosophila embryo. Genes Dev. 1987 Oct;1(8):868–879. doi: 10.1101/gad.1.8.868. [DOI] [PubMed] [Google Scholar]
- Kashima N., Nishi-Takaoka C., Fujita T., Taki S., Yamada G., Hamuro J., Taniguchi T. Unique structure of murine interleukin-2 as deduced from cloned cDNAs. 1985 Jan 31-Feb 6Nature. 313(6001):402–404. doi: 10.1038/313402a0. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Krumlauf R., Holland P. W., McVey J. H., Hogan B. L. Developmental and spatial patterns of expression of the mouse homeobox gene, Hox 2.1. Development. 1987 Apr;99(4):603–617. doi: 10.1242/dev.99.4.603. [DOI] [PubMed] [Google Scholar]
- Laughon A., Carroll S. B., Storfer F. A., Riley P. D., Scott M. P. Common properties of proteins encoded by the Antennapedia complex genes of Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1985;50:253–262. doi: 10.1101/sqb.1985.050.01.032. [DOI] [PubMed] [Google Scholar]
- Mason A. J., Hayflick J. S., Ling N., Esch F., Ueno N., Ying S. Y., Guillemin R., Niall H., Seeburg P. H. Complementary DNA sequences of ovarian follicular fluid inhibin show precursor structure and homology with transforming growth factor-beta. Nature. 1985 Dec 19;318(6047):659–663. doi: 10.1038/318659a0. [DOI] [PubMed] [Google Scholar]
- Mason A. J., Niall H. D., Seeburg P. H. Structure of two human ovarian inhibins. Biochem Biophys Res Commun. 1986 Mar 28;135(3):957–964. doi: 10.1016/0006-291x(86)91021-1. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Translocation of a localized maternal mRNA to the vegetal pole of Xenopus oocytes. Nature. 1987 Jul 2;328(6125):80–82. doi: 10.1038/328080a0. [DOI] [PubMed] [Google Scholar]
- Mercola M., Melton D. A., Stiles C. D. Platelet-derived growth factor A chain is maternally encoded in Xenopus embryos. Science. 1988 Sep 2;241(4870):1223–1225. doi: 10.1126/science.3413486. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. W., St Johnston R. D., Gelbart W. M. A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-beta family. Nature. 1987 Jan 1;325(6099):81–84. doi: 10.1038/325081a0. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Lillien L. E., Richardson W. D., Burne J. F., Noble M. D. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988 Jun 9;333(6173):562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- Rebagliati M. R., Weeks D. L., Harvey R. P., Melton D. A. Identification and cloning of localized maternal RNAs from Xenopus eggs. Cell. 1985 Oct;42(3):769–777. doi: 10.1016/0092-8674(85)90273-9. [DOI] [PubMed] [Google Scholar]
- Rosa F., Roberts A. B., Danielpour D., Dart L. L., Sporn M. B., Dawid I. B. Mesoderm induction in amphibians: the role of TGF-beta 2-like factors. Science. 1988 Feb 12;239(4841 Pt 1):783–785. doi: 10.1126/science.3422517. [DOI] [PubMed] [Google Scholar]
- Sharpe C. R., Fritz A., De Robertis E. M., Gurdon J. B. A homeobox-containing marker of posterior neural differentiation shows the importance of predetermination in neural induction. Cell. 1987 Aug 28;50(5):749–758. doi: 10.1016/0092-8674(87)90333-3. [DOI] [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- St Johnston R. D., Gelbart W. M. Decapentaplegic transcripts are localized along the dorsal-ventral axis of the Drosophila embryo. EMBO J. 1987 Sep;6(9):2785–2791. doi: 10.1002/j.1460-2075.1987.tb02574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks D. L., Melton D. A. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell. 1987 Dec 4;51(5):861–867. doi: 10.1016/0092-8674(87)90109-7. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Yedvobnick B., Finnerty V. G., Artavanis-Tsakonas S. opa: a novel family of transcribed repeats shared by the Notch locus and other developmentally regulated loci in D. melanogaster. Cell. 1985 Jan;40(1):55–62. doi: 10.1016/0092-8674(85)90308-3. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Peters G., Dickson C., McMahon A. P. Expression of the FGF-related proto-oncogene int-2 during gastrulation and neurulation in the mouse. EMBO J. 1988 Mar;7(3):691–695. doi: 10.1002/j.1460-2075.1988.tb02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. Novel regulators of bone formation: molecular clones and activities. Science. 1988 Dec 16;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Hansen P., Iwata K. K., Pieler C., Foulkes J. G. Identification of another member of the transforming growth factor type beta gene family. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4715–4719. doi: 10.1073/pnas.85.13.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]