Abstract
Rationale
Neonatal rat methamphetamine (MA) exposure has been shown to cause long-term behavioral impairments similar to some of those observed following neonatal stress. The mechanism by which MA induces impairments is unknown but may be related to early increases in corticosterone release. We previously developed a method to attenuate MA-induced corticosterone release using adrenal autotransplantation (ADXA) in neonatal rats. This exposure period corresponds to the second-half of human pregnancy.
Objective
To determine whether inhibition of neonatal MA-induced increases in corticosterone attenuates the long-term behavioral deficits associated with early MA treatment.
Results
ADXA successfully attenuated MA-induced plasma corticosterone increases by ~50% during treatment (P11-20) but did not attenuate the long-term behavioral effects of MA-treatment. MA-treated rats, regardless of surgery, showed increased errors and latencies in the Cincinnati water maze test of egocentric learning and increased latency, path length, and cumulative distance in three phases of Morris water maze spatial learning and reference memory. MA-treated offspring were hypoactive, had subtle reductions in anxiety in the elevated zero maze but not in the light-dark test. ADXA had no effect on MA-induced long-term 5-HT reductions in the neostriatum or entorhinal cortex or on 5-HIAA reductions in the hippocampus.
Conclusions
Fifty percent attenuation of neonatal MA-induced elevations in corticosterone does not alter the long-term egocentric or allocentric learning deficits or other behavioral effects of neonatal MA exposure. Because the ADXA effect was partial, the data cannot rule out the possibility that a more complete block of MA-induced corticosterone release might not prevent later cognitive deficits.
Keywords: methamphetamine, neonatal rat, spatial learning, allocentric learning, reference memory, egocentric learning, adrenalectomy, adrenal autotransplantation, serotonin, 5-HT, Morris water maze, Cincinnati water maze, corticosterone, brain development
Introduction
Methamphetamine (MA) abuse has become a worldwide problem (Johnston et al., 2008a; Johnston et al., 2008b; Srisurapanont et al., 2001). MA is abused primarily by adolescents and young adults (Johnston et al., 2008a; Johnston et al., 2008b). Some percentage of female users will become pregnant. Approximately 40% of pregnant MA users continue to use throughout pregnancy (Smith et al., 2003). One out of every 4 pregnant women seeking drug treatment in the U.S. reports MA as their primary drug of abuse (Terplan et al., 2009). Since MA readily crosses the placenta, (Burchfield et al., 1991; Garcia-Bournissen et al., 2007) fetal exposure in such cases is inevitable.
A limited number of human prenatal MA studies exist. Findings include reduced birth weight, length, and head circumference and higher rates of placental and intraventricular hemorrhage and anemia (Chomchai et al., 2004; Dixon, 1989; Dixon and Bejar, 1989; Little et al., 1988; Oro and Dixon, 1987; Smith et al., 2008). Additionally, increased agitation, vomiting, temperature instability, and rapid respiration have been noted (Chomchai et al., 2004). Magnetic resonance imaging studies report that exposed children have smaller volumes of the hippocampus, putamen, and globus pallidus, and reduced attention, visual motor integration, psychomotor speed, and spatial and verbal memory (Chang et al., 2004). Changes in white matter diffusivity have also been reported (Cloak et al., 2009) as have reduced object recognition scores on the Fagan Test of Infant Intelligence (Struthers and Hansen, 1992).
We have developed an animal model of MA exposure comparable to second-half of human pregnancy using the neonatal rat. This model is based on observations that cells in the dentate gyrus continue to proliferate until approximately postnatal day (P)19 in the rat which is approximately equivalent to post-conception day 240 in humans (Bayer et al., 1993). We also incorporate interspecies scaling algorithms and one such model shows that P11 rat brain is approximately equivalent to human gestation at 26 weeks for cortical structures and at 19 weeks for limbic structures (Clancy et al., 2006; Clancy et al., 2007). MA treatment from P11-20 produces reductions in neostriatal and hippocampal serotonin (5-HT) that are apparent within 24 h following the first MA treatment without affecting dopamine (DA) (Schaefer et al., 2008). P11-15 MA treatment also reduces hippocampal and neostriatal 5-HT 24 h after the P15 dose (Schaefer et al., 2008) whereas P11-only exposure does not reduce hippocampal or neostriatal 5-HT (Schaefer et al., 2006). Yet neither P11-15 nor P11-20 causes any 5-HT reductions when examined on P30 (Schaefer et al., 2008). This is in contrast to the profound effects of adult MA exposure. For example, adult rats treated with MA on a single day show large reductions in neostriatal DA and 5-HT that last for months (O’Callaghan and Miller, 2002). In a separate experiment DA reductions were apparent at P90 following P11-20 MA treatment (Crawford et al., 2003). P11, 11-15, or 11-20 MA treatment also induces large increases in plasma corticosterone (CORT) that last at least 24 h after the last dose (Schaefer et al., 2006; Schaefer et al., 2008; Williams et al., 2000). Since early stress induces changes in brain development and behavior, (Chapillon et al., 2002; Sanchez et al., 2001) this effect of the drug may be important.
Behaviorally, long-term deficits in spatial learning and memory in the Morris water maze (MWM) are seen after P11-20 MA treatment (Vorhees et al., 1994; Vorhees et al., 2000; Williams et al., 2002; Williams et al., 2003c). An experiment comparing P11-15 to P16-20 MA treatment on MWM performance showed disproportionately more effects after the P11-15 than after P16-20 exposure (Williams et al., 2003b). Follow-up experiments have revealed that 10-day exposures result in more severe MWM deficits than shorter intervals (Vorhees et al., 2008a) whereas the effects on a different maze, the Cincinnati water maze (CWM), result in reliable effects after P11-15 or P11-20 MA treatment (Vorhees et al., 2008a). By contrast, P1-10 MA treatment causes no deficits in MWM performance (Vorhees et al., 1994). Taken together, the data show that exposure intervals in which MA results in MWM and CWM deficits overlap. Intriguingly, the susceptible periods of MA-induced MWM and CWM deficits overlap with the stress hyporesponsive period (SHRP).
The SHRP is a period from approximately P4-14 when the adrenal gland is resistant to environmental stressor-induced CORT release (Sapolsky and Meaney, 1986). The SHRP is not a refractory period, but rather a phase of diminished responsiveness. For example, maternally separated neonatal rats show elevated CORT levels 30 min after exposure to novelty, saline injection, or adrenocorticotropic hormone (ACTH) exposure compared to controls but the levels are below those seen in adult stressed rats (Levine et al., 1991). Similarly, MA increases plasma ACTH and CORT in neonatal rats but to an even greater extent than do stressors such as forced swim or confinement (Grace et al., 2008; Williams et al., 2000; Williams et al., 2006).
In the following experiments, we tested whether the heightened CORT response caused by neonatal MA exposure during the SHRP contributes to the learning and behavioral deficits observed later in life. Adrenal autotransplantation (ADXA) has been previously shown in adults to inhibit the CORT response for a period of days with eventual recovery. Therefore, ADXA was performed on P9 followed by MA exposure from P11-20. We chose ADXA because we have previously demonstrated that it reduces CORT in neonates by ~50% from P11-20 (unpublished observation). Using alternative (unpublished observation) methods such as complete adrenalectomy (ADX) or pharmacological inhibition were not as successful and/or caused secondary effects (unpublished observation). Pharmacological inhibition of CORT synthesis using ketoconazole or metyrapone initially blocks the CORT response to MA, but CORT rebounds 24 h later even with continuing inhibitor treatment (unpublished observation). Complete bilateral ADX overcomes the problems with synthesis inhibitors and completely blocks MA-induced CORT release, but also causes depletions in hippocampal 5-HT greater than those seen in SHAM-MA treated animals (unpublished observation). This potential confounder is avoided using the ADXA method (unpublished observation) and was therefore used herein.
Materials and Methods
Subjects and Conditions
Male (251-275 g) and nulliparous female (151-175 g) Sprague-Dawley CD IGS rats were obtained from Charles River Laboratories (Raleigh, NC) and acclimatized to the vivarium for at least one week prior to breeding. The offspring were the subjects of this experiment. Environmental stimuli (semicircular stainless steel enclosures) were placed in the cage upon receipt of the animals and remained in the home cage for the duration of the experiment (see (Vorhees et al., 2008b) for details). Food and water were provided ad libitum and the housing room was maintained on a 14 h light: 10 h dark cycle (lights on at 600 h EST). Litters were culled with preferential retention of males up to 10. Litters with < 8 males at birth had no more than 2 pups fostered from other litters born on the same day. For simplicity and because there are rarely MA-induced sex differences in later behavior, males were used (unpublished observation). Within each litter, 4 animals were assigned to the SHAM operation groups and 4-6 to the ADXA group. The extra 2 ADXA animals were assigned to the MA treatment condition in the event of mortality (unpublished observation). Hence, each litter contained 2 pups for each of 4 groups: 2 SHAM-SAL, 2 SHAM-MA, 2 ADXA-SAL, and 2 ADXA-MA. Extra ADXA-MA animals were removed from the experiment at weaning (P28) if not needed as replacements such that the final design contained 4 animals/treatment/litter. Animals were housed in pairs after being separated from the dam. Behavioral testing began on P60. Protocols were approved by the Institutional Animal Care and Use Committee. The vivarium is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Surgical Procedures
The ADXA procedure was derived from (Okamoto et al., 1992) in adult rats and adapted here for P9 rats. Our procedures differed in that specific placement of the adrenals under the cutaneous maximus was not performed and antibiotics were not given following surgery. Rats were transported in their home cage to a surgical suite and half of the litter was subjected to severing of the adrenal glands via a dorsal route and then the adrenals were placed into the peritoneum for spontaneous engraftment. The remaining animals in each litter were given the same operation except that the adrenals were untouched (SHAM). Animals in both surgery groups (ADXA or SHAM) were anesthetized with isoflurane and the dorsum was swabbed with 70% ethanol and betadine prior to surgery. After surgery the wound was sutured, the dermis stapled, and the dorsum swabbed with warm saline. Following surgery, subjects were returned to the home cage. Staples were removed on P21.
Treatments
(+)-Methamphetamine HCL (expressed as the freebase and > 95% pure, National Institute on Drug Abuse, Bethesda, MD) was injected at 10 mg/kg in a volume of 3 ml/kg s.c. or an equal volume of saline to controls. Animals were dosed four times per day at 2 h intervals from P11-20. Sites of injection were varied in order to prevent skin irritation.
Body Weights
Animals were weighed prior to each dose from P11-20, on P21, and weekly thereafter.
Behavioral Experiments
Animals were assigned to one of 2 different pathways for behavioral testing. One pup of each group/litter was assigned to receive the pathway A set of tests and one pup of each group/litter was assigned to receive the pathway B set of tests.
Pathway A tests
Light/Dark Exploration (P60)
Animals were placed in 41 × 41 cm locomotor test chambers (Accuscan Instruments, Columbus, OH) with a black acrylic enclosure placed inside. The enclosure filled half of the chamber with an opening of 10 cm × 6.5 cm (w × h) facing the open side of the chamber. Animals were placed in the middle of the open side. Rats were tested for 10 min and data were collected every minute. The number of transitions from light to dark (open to closed) and total time spent on each side were recorded. Chambers were cleaned with 70% ethanol between subjects.
Novel Object Recognition (P61-64)
On P61, subjects were placed in circular, polyethylene arenas (91 cm in diameter with 51 cm high walls) and allowed to habituate for 10 min/day to the test chambers for 3 days prior to testing; chambers were cleaned with 70% ethanol between trials. On the fourth day, testing was conducted in two phases. During familiarization, two identical objects were placed along a line bisecting the arena 41 cm apart and 25 cm from the sides. Rats were placed in the center and allowed to explore until 30 s of combined object exploration time was accumulated, after which the animals were removed. Exploration was scored using a video camera above the arena. If an animal did not accumulate 30 s of exploration time within 10 min, it was removed and not tested further. Object exploration was scored when the animal was oriented toward and within 1 cm of the object and when climbing on the object only if the rat was actively exploring it. Retention was tested 1 h after familiarization by being placed back in the test arena with a new object and an exact copy of the original object and allowed to accumulate 30 s of object investigation time.
Straight Channel (P65)
Each subject was placed in a 244 cm straight channel containing room temperature water. The animal was placed facing the wall at the opposite end of that containing an escape platform. Latency to reach the escape was recorded for 4 trials with a maximum of 2 min/trial.
Cincinnati Water Maze (P66-83)
On the day following straight channel, rats were tested in the CWM, a multiple-T water maze as previously described (Vorhees, 1987; Vorhees et al., 1991; Vorhees et al., 2009). The task was performed under infrared light and a CCD camera was mounted above the maze and attached to a monitor in an adjacent room. Rats were acclimated to the dark for 5 min before testing. Two trials were given per day for 18 consecutive days with a maximum of 5 min/trial and an intertrial interval (ITI) of 5 min. Latency to reach the escape and the number of errors were recorded. An error was committed when an animal left the direct path to the goal and entered a cul-de-sac. An error was scored when the head and shoulders crossed an imaginary boundary at the “stem” of the cul-de-sac and whenever it crossed into either arm of a “T”. Animals that did not complete the task within 5 min were given an error score equal to the maximum number of errors committed by the worst performing animal + 1 to correct for cases where an animal stopped searching before reaching the time limit.
Morris Water Maze Working Memory (P84-104)
A short-term working memory version of the MWM task was employed for 21 days (P84-104) adapted from (Morris et al., 1986). Animals were to locate a submerged platform (10 cm diameter) in a tank (210 cm diameter) of water (21 ± 1°C) based on the positions of distal cues in the room. The platform was submerged 1.5-2.0 cm below the water level. Each rat was given 2 trials/day with a time limit of 2 min/trial. The ITI was 15 s on the platform. If the platform was not located within 2 min, the animal was placed on the platform. Start and platform positions were pseudo-randomized each day but start positions were always a cardinal direction and platform positions always an ordinal direction. For example, on the first day of testing, animals were placed in the north position and the platform was situated at the southeast position. Both positions were fixed for both trials on a given day. Animals were tracked and data collected using AnyMaze software (Stoelting, Wood Dale, IL).
Pathway B tests
Elevated Zero Maze (P60)
The elevated zero maze (EZM) test began on P60 and was run in the morning to control for ultradian and circadian rhythm effects. The maze is 105 cm in diameter with a 10 cm wide circular runway divided into four equal quadrants with 2 quadrants being open and two being closed with black side walls. Both open and both closed quadrants were positioned across from each other. The runway was mounted 72 cm above the floor. The open quadrants had 1 cm clear acrylic curbs to prevent slipping and the closed quadrants had 28 cm high black acrylic walls. A dimmed halogen floor lamp was used as the lighting source. Each animal began the task in the middle of a closed quadrant and was tested for 5 min. Latency to enter the open, time spent in the open, and number of head dips were recorded from video recordings made using an overhead camera and DVD recorder. DVDR malfunction resulted in several trials being lost. The apparatus was cleaned with 70% ethanol between subjects.
Locomotor Activity (P61)
On the day following EZM, animals were placed in 41 × 41 cm locomotor chambers (Accuscan Instruments, Columbus, OH) and movements were recorded in 5 min intervals for 1 h. The number of vertical and horizontal movements as well as the amount of time spent in the center vs. periphery were extracted and analyzed. Chambers were cleaned with 70% ethanol between animals.
Straight Channel (P62)
Morris Water Maze Hidden Platform (P63-80)
Subjects were placed in a 210 cm diameter tank of water (21 ± 1°C) and required to find a submerged platform. The platform was submerged 1.5-2.0 cm below the water. There were three phases of the MWM hidden platform test each consisting of 6 days of platform trials with 4 trials/day, and on the sixth day a single probe trial with no platform present was performed and the animal started from a novel position. The three phases were: Acquisition (P63-68) with a 10 cm diameter platform in the southwest quadrant; reversal (P69-74) with a 7 cm platform in the northeast quadrant; and shift (P75-80) with a 5 cm platform in the northwest quadrant. Probe trials lasted for 30 s; learning trials were up to a maximum of 2 min with an ITI of 15 s on the platform. Curtains were opened during hidden platform trials so that distal room cues were revealed. Data were collected using video tracking software (AnyMaze, Stoelting, Wood Dale, IL).
Morris Water Maze Cued (P81-82)
MWM cued testing began the day following hidden platform trials and was conducted for 2 days. Subjects were placed in the tank and were tested for latency to reach a 10 cm diameter platform submerged 1.5-2.0 cm below the water with a yellow plastic ball mounted on a brass rod to mark its location. Curtains were drawn closed around the tank to minimize distal cues. On each day, subjects were given 4 trials with the locations of the platform and starting positions randomized ((2 min trial limit with ~30 s ITI) (15 s on the platform + 15-20 s to reposition the platform)).
Tissue Collection
On P109 after the end of behavioral testing, brains were removed and assayed for DA, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-HT, 5-hydroxyindoleacetic acid (5-HIAA) and norepinephrine (NE) in neostriatum, 5-HT, 5-HIAA, and NE in the hippocampus, and DA, DOPAC, 5-HT, 5-HIAA, and NE in entorhinal cortex for animals in pathway A only. Animals were taken into an adjacent suite, decapitated, and brains removed and dissected over ice with the aid of a brain block (Zivic-Miller, Pittsburgh, PA). The brain was sliced coronally at the optic chiasm and caudal to the mammillary body and the hippocampus dissected bilaterally. The neostriatum was dissected from the 2 mm section rostral to the optic chiasm and entorhinal cortex 2 mm caudal from the mammillary body. Tissues were stored at −80°C until assayed.
Corticosterone levels following an acute stressor were obtained in order to estimate the adrenal response. Litters assigned to pathway B were placed in 46 cm tall × 15 cm in diameter clear acrylic cylinders filled to a depth of 35 cm (22 ± 1 °C) for 10 min. Rats were removed, decapitated and blood collected in polyethylene tubes containing 2% EDTA (0.05 ml). Plasma was isolated from whole blood by centrifugation at 1300 RCF for 25 min and the supernatant collected and stored at −80°C.
Monoamine Assays
HPLC reagents were obtained from Sigma Chemical (Sigma-Aldrich Co., St. Louis, MO) unless otherwise specified. Frozen tissues were weighed, thawed, and sonicated in 0.1 N perchloric acid. Samples were centrifuged for 14 min at 13,000 × g at 4°C. The supernatant for each sample was transferred to a new vial for injection onto a Supelco Supelcosil™ LC-18 column (15 cm × 4.6 mm, 3 μm) (Sigma-Aldrich Co.). The HPLC system consisted of a Waters 717plus autosampler (Waters Corp., Milford, MA) and ESA 5840 binary pump and Coulochem III electrochemical detector. The potential settings were E1 at −150 mV and E2 at +250 mV, with a guard cell potential set at +350 mV. MD-TM mobile phase (ESA Inc., Chelmsford, MA) was used and consisted of 75 mM sodium dihydrogen phosphate (monohydrate), 1.7 mM 1-octanesulfonic acid sodium salt, 100 μl/l triethylamine, 25 μM EDTA, and 10% acetonitrile (pH = 3.0). The pump flow rate was 0.7 ml/min, and the samples were run at 28°C. Standards for DA, DOPAC, HVA, NE, 5-HT, and 5-HIAA were prepared in 0.1 N perchloric acid. All neurotransmitters were run on a single chromatogram.
Corticosterone Assay
Plasma samples were thawed and assayed with Octeia Corticosterone ELISA kits (IDS, Fountain Hills, AZ). Each sample was diluted 1:5 and assayed according to the manufacturer’s protocol. The ELISAs were measured and quantified on a SpectraMax Plus microtiter plate reader (Molecular Devices, Sunnyvale, CA).
Statistics
Data were analyzed using mixed linear analyses of variance (ANOVAs) (SAS Proc Mixed, SAS Institute, SAS v.9.1, Cary, NC). The covariance matrix for each data set was checked using best fit statistics and in each case the best fit was to the autoregressive-1 covariance structure. Proc Mixed uses Kenward-Roger adjusted degrees of freedom that do not match those obtained from general linear model ANOVAs and can be fractional. Measures taken repetitively on the same animal, such as day, were repeated measure factors. Significant interactions were analyzed using slice effect ANOVAs at each level of the repeated measure factor. Data are presented as least square (LS) mean ± LS SEM.
Results
Body Weight
During treatment, there were effects of surgery, F(1,138) = 28.4, p< 0.0001, drug, F (1,138) = 274.6, p< 0.0001, day, F(9,1333) = 502.9, p< 0.0001, surgery × day, F(9,1333) = 3.9, p<0.0001, and drug × day, F(9,1333) = 96.9, p< 0.0001. MA-treated and ADXA animals had decreased body weight gain but no drug × surgery interaction was observed. The surgery × day interaction revealed that ADXA animals had reduced weight gain compared to SHAM animals (P11-20). The drug × day interaction showed that MA animals had reduced weight gain beginning on P12 that lasted throughout the dosing period. For post weaning weights, there were main effects of surgery, F(1,138) = 4.2, p< 0.04, drug, F(1,138) = 21.2, p< 0.0001, and week, F(9,1112) = 2853.3, p< 0.0001, but no interactions. The ADXA animals weighed less than the SHAM animals and MA-treated animals weighed less than SAL-treated animals. Representative body weights are shown in Table 1.
Table 1.
Offspring body weight (g) as a function of age
| P11 | P15 | P20 | P56 | P90 | |
|---|---|---|---|---|---|
| SHAM-SAL | 26.7 ± 0.8 | 36.7 ± 0.8 | 50.7 ± 0.8 | 224.5 ± 5.2 | 511.5 ± 5.3 |
| ADXA-SAL | 25.0 ± 0.8# | 34.3 ± 0.8# | 46.4 ± 0.8# | 212.6 ± 5.5# | 508.0 ± 5.5# |
| SHAM-MA | 26.3 ± 0.8 | 29.0 ± 0.83* | 36.1 ± 0.8* | 203.7 ± 5.2* | 499.3 ± 5.3* |
| ADXA-MA | 25.1 ± 0.8# | 27.7 ± 0.8*# | 32.7 ± 0.8*# | 193.8 ± 5.2*# | 498.2 ± 5.3*# |
p< 0.01 combined ADXA groups vs. combined SHAM groups
p< 0.001 vs. SHAM
p< 0.01 combined MA groups vs. combined SAL groups.
Pathway A tests
Light/Dark
There were no significant effects of drug, surgery, or interactions for time in light, time in dark, latency to dark entry or number of dark entries. For example, time (s) in light ls means ± SEM were SHAM-SAL = 204.1 ± 18.7, SHAM-MA = 204.0 ± 19.1, ADXA-SAL = 177.0 ± 19.1 and ADXA-MA = 197.1 ± 18.7.
Novel Object Recognition
During familiarization there was no preference for one side over the other. During retention, there were no significant effects of surgery, drug, or interactions among these.
Straight Channel Swimming
There was a main effect of trial, F(3,359) = 96.8, p< 0.0001, but no effect of drug, surgery, or interaction of these on latency to swim the straight channel.
Cincinnati Water Maze
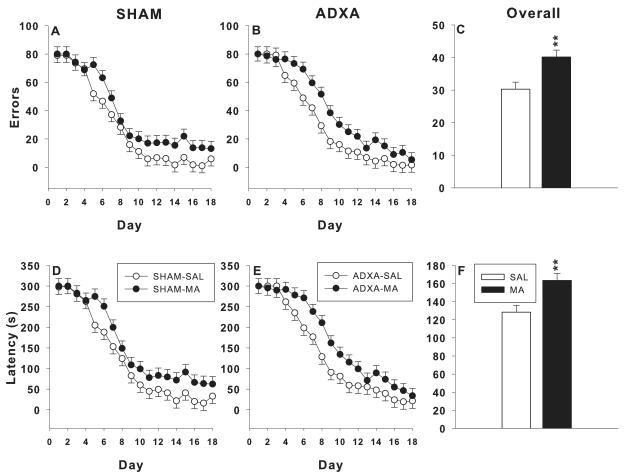
For errors (Figure 1A-C), there were main effects of drug, F(1,78.4) = 10.8, p< 0.002, and day, F(17,1125) = 50.2, p< 0.0001. Both MA-treated groups committed more errors than SAL-treated groups regardless of surgery condition (Figure 1C). For latency (Figure 1D-F) to the platform, there were main effects of drug, F(1,79.1) = 10.7, p< 0.002, and day, F(17, 1122) = 48.05, p< 0.0001; a similar pattern as errors was seen. MA-treated groups had longer latencies to the platform (Figure 1F) than SAL-treated groups regardless of surgical condition. No main effect of surgery or interactions between surgery and drug were obtained for errors or latency.
Figure 1.
Cincinnati water maze: The MA-treated groups show significant deficits in CWM performance regardless of surgery. A-C: Errors: D-F: latency to reach the escape platform. Learning curves for the SHAM and ADXA groups are shown separately in the left panels for each dependent measure and the main effect of drug treatment with surgical conditions combined are shown in the right-hand panel; **p< 0.01 or ***p < 0.001 combined MA-treated groups vs. combined SAL-treated groups.
Morris Water Maze Working Memory
For the trial-dependent, matching-to-sample (working memory) version of the MWM, there was a surgery × drug × day interaction, F(20,1345) = 1.9, p< 0.01 for latency. The ADXA-MA group showed an increased percent change in latency between trial-1 and trial-2 only on day 6 and the SHAM-MA group showed decreased percent change compared to SHAM-SAL on days 15 and 17. Analysis of swim speed showed a main effect of day, F(20,1352) = 2.7, p<0.0001, and interactions of surgery × day, F(20,1352) = 1.6, p< 0.05, and drug × day, F(20,1352) = 2.0, p< 0.01. The ADXA groups had decreased percent change in speed on days 10, 16, and 18 and increased percent change on day 20 compared to the SHAM groups. In addition, the MA groups had decreased percent change on day 5 and 10 and increased percent change on day 20. All these effects were sporadic and small and showed no consistent pattern of effects (not shown).
Pathway B tests
Elevated Zero Maze
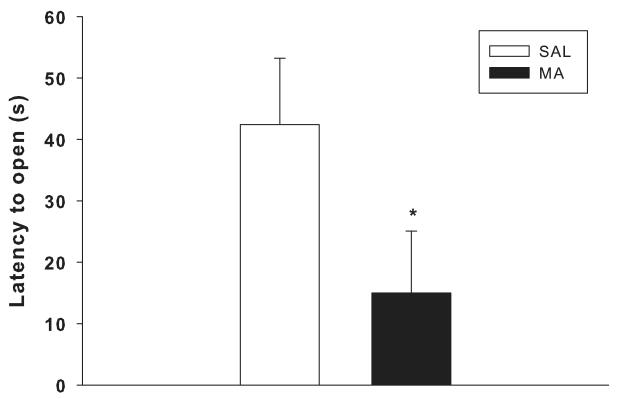
Latency to first open quadrant entry, time in open, and number of head dips were analyzed. For latency, there was a main effect of drug, F(1,49) = 4.5, p< 0.04; the MA-treated groups had decreased latency to first open entry compared to the SAL-treated groups (Figure 2). There were no main effects or interactions on time in open or head dips.
Figure 2.
Elevated zero-maze: The combined MA-treated groups had reduced time to first entry to an open quadrant compared to the combined SAL-treated groups *p < 0.05.
Locomotor Activity
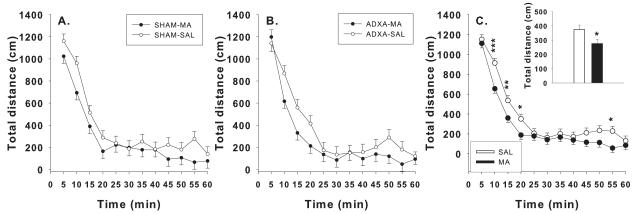
Total, central, and peripheral distances, horizontal activity, and vertical activity were analyzed. For total distance, there were no surgery × treatment × day interactions, (Figure 3 A and B), but there were main effects of drug, F(1,72.8) = 5.1, p< 0.03, time, F(11,674) = 92.5, p< 0.0001, and interactions of surgery × time, F(11,674) = 1.9, p< 0.04, and drug × time, F(11,674) = 2.3, p< 0.01. MA-treated groups were hypoactive compared to SAL-treated groups (Figure 3C inset). Further analysis of the drug × time interaction revealed that the MA-treated groups had reduced locomotion from 10-20 min and at 55 min compared to SAL-treated groups (Figure 3C). Similar results were obtained for horizontal activity. For peripheral distance, there were main effects of drug, F(1,72.8) = 10.4, p< 0.002, time, F(11,679) = 65.2, p< 0.0001, and interactions of surgery × time, F(11,679) = 2.1, p< 0.02, and drug × time, F(11,679) = 4.2, p< 0.0001. MA-treated groups moved less in the periphery than did SAL-treated groups (not shown). The drug × time interaction showed decreased peripheral distance in MA-treated groups from 10-20 and 50-55 min compared to SAL-treated groups (not shown). For center distance, an overall effect of time was observed, F(11,658) = 83.5, p< 0.001, but no treatment-related effects were seen. For vertical activity, there were main effects of drug, F(1,55.9) = 11.24, p< 0.001, and time, F(11,679) = 3.5, p< 0.0001. MA-treated groups had increased vertical movements compared to SAL-treated groups (not shown).
Figure 3.
Locomotor activity: Ls mean ± SEM total distance moved (cm) during a 60 min test session. A: Activity for SHAM and B: ADXA per treatment group. MA groups had reduced total distance (C inset) compared to SAL groups, and specifically MA groups had reduced total distance from 10-20 min and at 55 min (C). *p< 0.05, **p< 0.01, ***p< 0.001, all compared to SAL controls.
Morris Water Maze Hidden Platform
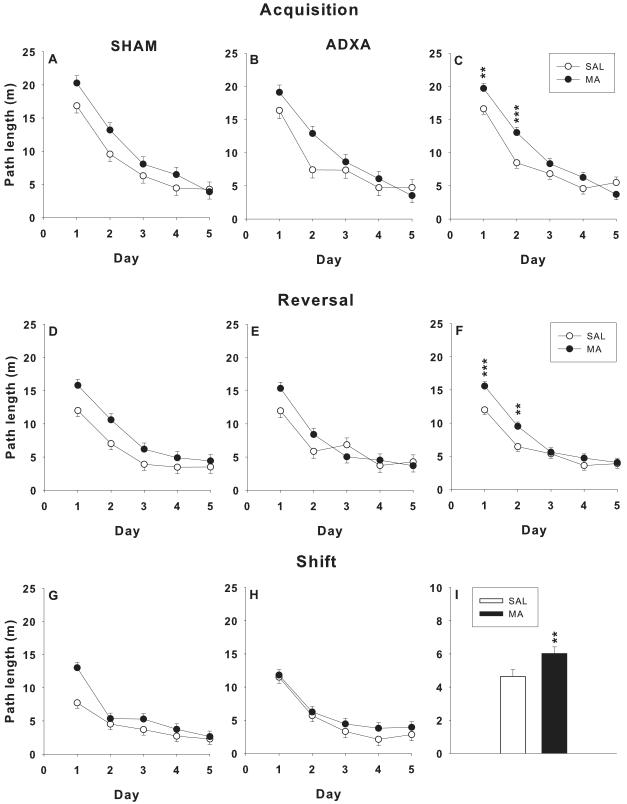
Spatial learning in the MWM was analyzed for path length, latency to the target platform, cumulative distance, and mean speed. For acquisition path length (Figure 4A-C), there were main effects of drug, F(1,54.7) = 8.6, p< 0.005, and day, F(4,201) = 97.2, p< 0.0001, and the interaction of drug × day, F(4,201) = 3.5, p< 0.01. Further analysis of the interaction showed that on days 1 and 2, MA-treated groups had increased path length compared to SAL-treated groups, regardless of surgery (Figure 4C). Similar findings were observed for latency and cumulative distance (not shown). For swim speed, there were main effects of drug, F(1,55.7) = 4.5, p< 0.04, and day, F(4,212) = 4.4, p< 0.002. MA-treated groups swam slower compared to SAL-treated groups (not shown). There were no other significant effects.
Figure 4.
Morris water maze learning trials: Ls mean ± SEM path length (cm) averaged by day (4 trials/day) in neonatally MA vs. SAL-treated rats having ADXA or SHAM surgery. Left and middle panels show each treatment group as a function of surgical condition. Right panels show the main effect of drug treatment with the two MA-treated and two SAL-treated groups merged. Top row: acquisition; middle row: reversal; bottom row: shift. Panels C and F are shown by day to illustrate a significant drug × day interaction. Panel I is shown averaged across days to illustrate a drug main effect with no drug × day interaction. *p < 0.05, **p < 0.01, ***p < 0.001 combined MA-treated groups vs. combined SAL-treated groups.
For reversal path length (Figure 4D-F), there were main effects of drug, F(1,74) = 8.6, p< 0.005, and day, F(4,220) = 66.9, p< 0.0001, and a drug × day interaction, F(4,220) = 2.8, p< 0.03. The interaction was attributable to increased path length in MA-treated groups on days 1 and 2 (Figure 4F). Analyses of latency and cumulative distance were similar. For swim speed, the only main effect was day, F(4,210) = 13.0, p< 0.0001.
For shift path length (Figure 4G-I), there were main effects of drug, F(1,53.3) = 9.5, p< 0.003 (Figure 4I), and day, F(4,204) = 66.7, p<0.0001, but no interactions. The MA-treated animals had longer path lengths than the SAL-treated animals, regardless of surgery. Similar findings were observed for latency and cumulative distance. For speed the only main effect was for day, F(4,214) = 12.3, p< 0.0001.
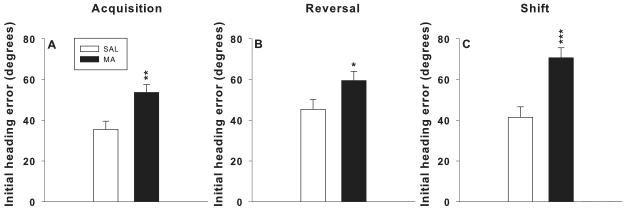
For probe trials, platform site crossovers, initial heading error, and average distance from the target were analyzed. For acquisition probe initial heading error, there was a main effect of drug, F(1,53) = 10.7, p< 0.002, which was attributable to increased heading error in MA-treated groups compared to SAL-treated groups (Figure 5A). There were no effects for crossovers or average distance.
Figure 5.
Morris water maze probe trials: Ls mean ± SEM average heading error to the former location of the platform 24 h after the last platform trial for each phase of MWM testing. There were no significant main effects of surgical condition and no interactions between drug and surgical condition. There were significant main effects of drug. For clarity the main effect of drug (with surgical conditions averaged together) are shown. *p < 0.05, **p < 0.01, ***p < 0.001 combined MA-treated groups vs. combined SAL-treated groups.
For reversal probe crossovers, there was a main effect of surgery, F(1,53) = 6.0, p< 0.02. For initial heading error, there was a main effect of drug, F(1,53) = 4.6, p< 0.04. SHAM groups had fewer crossovers than the ADXA groups (not shown) and MA-treated groups had increased initial heading errors than SAL-treated groups (Figure 5B).
For shift probe initial heading error, there was a main effect of drug, F(1,53) = 18.6, p< 0.0001, in which MA-treated groups had greater heading errors than SAL-treated groups (Figure 5C). There were no effects for average distance from the target on acquisition, reversal, or shift probe trials.
Morris Water Maze Cued
There was a latency main effect of day, F(1,71) = 29.6, p< 0.0001, but no effects of drug, surgery, or interactions.
Corticosterone
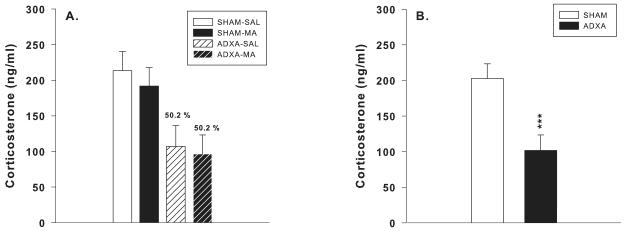
Corticosterone levels were measured following 10 min forced swim in adulthood to determine the extent of which ADXA attenuated CORT release. There was an overall effect of surgery, F(1,45) = 17.7, p< 0.001, in which the ADXA groups had plasma CORT levels that were significantly lower than SHAM groups (Figure 6B). CORT levels were 50.2% of their respective controls (Figure 6A). There were no effects of drug or interactions.
Figure 6.
Corticosterone levels following forced swim: Ls mean ± SEM (ng/ml) plasma CORT levels in rats following completion of behavioral testing (P106). Rats were euthanized 10 min following 30 min of forced swim. ADXA reduced CORT levels by 50.2% in both ADXA-SAL and ADXA-MA animals compared to their respective SHAM controls (A). The main effect of decreased CORT in ADXA animals is shown, regardless of surgery (B). ***p< 0.001 compared to SHAM controls.
Monoamines
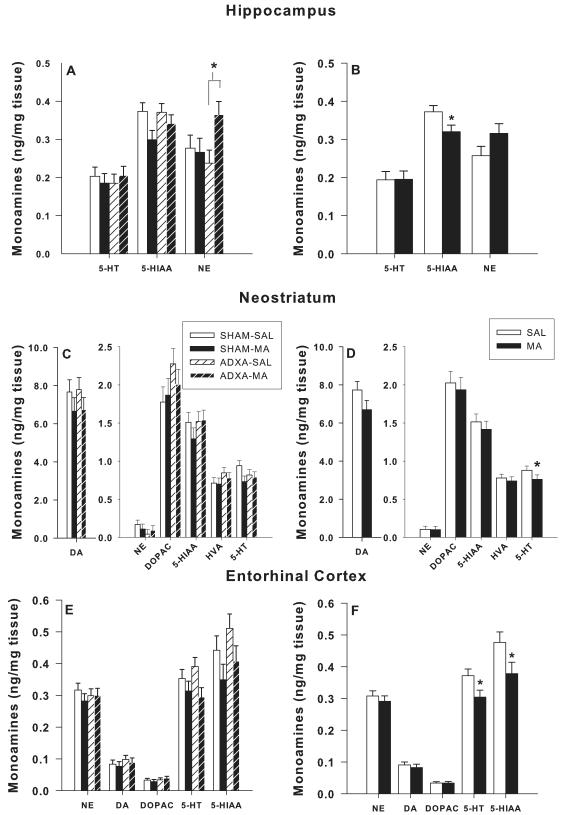
Monoamines were analyzed in the hippocampus, neostriatum, and entorhinal cortex (Figure 7). In the hippocampus (Figure 7A-B), there was a main effect of drug for 5-HIAA, F(1,40) = 5.1, p< 0.03, and a surgery × drug interaction for NE, F(1,40) = 4.0, p< 0.05. 5-HIAA was decreased in the MA-treated groups compared to SAL-treated groups (Figure 7B) and NE was increased in the ADXA-MA group (Figure 7A) compared to the ADXA-SAL group. For neostriatal 5-HT (Figure 7C-D), there was a main effect of drug, F(1,39) = 2.6, p< 0.05, in which the MA-treated groups had reduced 5-HT compared to the SAL-treated groups (Figure 7D). No neonatal surgery × drug interactions were observed. In the entorhinal cortex (Figure 7E-F), there were main effects of drug for both 5-HT, F(1,39) = 5.7, p< 0.02, and 5-HIAA levels, F(1,38) = 4.6, p< 0.04. 5-HT and 5-HIAA levels were reduced in the MA-treated groups compared to the SAL-treated groups (Figure 7F).
Figure 7.
Monoamine concentrations: DA and 5-HT in neonatally MA vs. SAL treated rats as a function of ADXA vs. SHAM surgery following the completion of behavioral testing (P106). A, C, E: monoamines in each region analyzed by group. B, D, F: main effect of drug with surgical conditions combined. *p < 0.05 combined MA-treated groups vs. combined SAL-treated groups.
Discussion
In the present experiment, it was determined that attenuation of the CORT response to MA was produced using ADXA, but did not reduce the long-term cognitive deficits seen in MWM or CWM learning/memory. These data suggest that large increases in plasma CORT during neonatal MA exposure may not be responsible for the later cognitive deficits. Despite a substantial literature demonstrating long-term alterations in HPA-axis reactivity (Aisa et al., 2007; Biagini et al., 1998; Felszeghy et al., 2000; Hodgson et al., 2001; Kalinichev et al., 2002; Shanks et al., 1995; Wigger and Neumann, 1999), deficits in spatial learning, deficits in novel object recognition (Aisa et al., 2007), and alterations in anxiety (Huot et al., 2001; Kalinichev et al., 2002; Knuth and Etgen, 2007; Romeo et al., 2003; Wigger and Neumann, 1999) following neonatal exposure to physical or psychological stressors, the present data did not demonstrate a beneficial effect on later behavior by reducing the magnitude of the MA-induced release of CORT during an early and sensitive period of brain development. Another possible explanation for the lack of attenuation of the cognitive deficits by ADXA is that there may be a threshold at which CORT elicits its adverse effects. It is conceivable that the CORT reductions by ADXA are not sufficient to be below such a threshold and therefore do not provide neuroprotection. In this regard, we have shown that MA exposure during the neonatal period produces a larger increase in CORT than physical or psychological stressors (Grace et al., 2008). Therefore the ~50% reduction in CORT increase caused by MA may still be within the range of that produced by stressors that have been shown to elicit long-term changes in behavior. Experiments to determine if a further reduction in CORT release following MA may alleviate the learning and memory changes will require different approaches or modification of the ADXA method to make it more complete. Alternatively, the type of stressor involved may be important at least for causing alterations in long-term HPA-axis reactivity. Here we examined CORT levels following an acute forced swim stressor in adulthood and observed no effects of MA treatment, suggesting normal HPA axis reactivity to stress. We have observed a similar absence of long-term MA effects on CORT release in adult offspring following forced swim, confinement to a small space, or acute MA treatment (unpublished observation). These data, combined with those in the present experiment, suggest that a pharmacological neonatal stressor such as MA may be different from physical or psychological stressors at least in terms of altering the developing HPA axis. Alternatively, ADXA may have induced secondary/compensatory changes that offset any beneficial effect of reducing MA-related CORT increases. For example, it is known that ADX causes compensatory increases in the release of ACTH and CRF (Dallman et al., 1987) which may have deleterious consequences. With the removal of CORT, changes in both the mineralocorticoid receptors (MR) and glucocorticoid receptors (GR) are likely to occur. It is conceivable that alterations in MR and GR by ADXA could affect later cognition and future experiments will be designed to test this hypothesis. Another important issue in future studies testing the possible involvement of early drug-induced increases in plasma CORT levels will be to assess these potential compensatory systems to determine if they change in ways that effectively cancel the beneficial effect of preventing large increases in CORT release.
The positive control group (SHAM-MA) replicated our previous findings and since the ADXA treatment (ADXA-MA) failed to prevent this effect, this group too effectively replicated our previous findings. We have previously demonstrated that deficits in hippocampal-dependent spatial learning and reference memory in the MWM emerge in adulthood following neonatal exposure to MA (Vorhees et al., 2008b; Vorhees et al., 2009; Williams et al., 2002; Williams et al., 2003a; Williams et al., 2003b), an effect seen here in both the SHAM-MA and ADXA-MA treated groups. In the acquisition phase, MA-treated animals had reductions in mean speed compared to controls, which we do not typically see. However, these changes cannot account for the navigational impairment for several reasons. First, MA animals had increased path lengths to the target platform regardless of the time it took them to get there. Second, the straight channel showed no differences in maximal swimming speed across groups. Third, there were no differences observed between groups in the cued (visible platform) version of the MWM showing that speed differences were not instrumental in determining the outcome on hidden platform trials. Taken together, these data represent consistent evidence of spatial learning deficits in MA-treated groups not confounded by unrelated performance differences.
We have previously demonstrated that neonates exposed to MA have deficits in egocentric learning in the CWM (Vorhees et al., 2009) and this effect was also seen here. Egocentric learning is the ability to navigate to a destination using self-motion cues and routes rather than distant landmarks and is thought to involve the hippocampus (Etienne et al., 1996; Etienne and Jeffery, 2004), grid and border cells in the entorhinal cortex, head direction cells in the presubiculum, and cells in other regions. MA-treated animals showed long-term alterations in monoamines in both the hippocampus (5-HIAA) and entorhinal cortex (5-HT and 5-HIAA), which suggest that the serotonergic system may correlate to changes in egocentric learning; however, further analysis showed that such correlations were not significant (not shown). However, the changes in the serotonergic system seen after testing are smaller than those induced during the period of drug administration, limiting the likelihood of finding high correlations between these variables.
We found that rats developmentally exposed to MA have reduced locomotor activity prior to maze testing. Several experiments using early stress exposure find no differences in spontaneous locomotion in adulthood (Aisa et al., 2007; Knuth and Etgen, 2007), demonstrating that effects may depend upon the stressor involved. However, as discussed, these effects do not appear to affect MWM or CWM learning or memory since no changes in swimming speed or straight channel swimming times were found and therefore represent a separate effect of the drug independent of its effects on learning and memory.
MA-treated animals also showed a subtle reduction in the elevated zero maze test of anxiety; but no effect in light/dark exploration. Taken with our previous observations (Skelton et al., 2007), long-term effects on anxiety by neonatal MA exposure appear to be minor. A number of studies have demonstrated that neonatal maternal separation increases anxiety in the elevated plus maze in adulthood (Huot et al., 2001; Kalinichev et al., 2002; Knuth and Etgen, 2007; Romeo et al., 2003; Wigger and Neumann, 1999) again suggesting that neonatal MA exposure works through different mechanisms than maternal separation. A possible explanation for this is that the increases in CORT may not be associated with a particular event as with maternal separation. For example, rats that exhibit CORT increases following maternal separation also incur a psychological event of prolonged maternal deprivation of care in the form of nursing, grooming, and physical contact. During drug treatment, the animals were only briefly separated from the dam and remained with their littermates and hence did not experience the same type of deprivation as in maternal separation experiments.
Several studies have found that following adrenal autotransplantation in adult rats, plasma CORT levels are detectable 1 to 5 weeks post-transplantation (Nabishah et al., 1998; Okamoto et al., 1992; Srougi et al., 1978; Srougi and Gittes, 1978; Vendeira et al., 1992) and are similar to controls by 6 to 9 weeks (Nabishah et al., 1998; Srougi and Gittes, 1978). The regenerative properties of the adrenal gland have been examined and grafts containing adrenal cortical tissue, regardless of size, have the ability to regenerate (Belloni et al., 1982; Nabishah et al., 1998; Okamoto et al., 1992; Srougi et al., 1978; Vendeira et al., 1992). Histological evidence shows that following ADXA, early necrosis of cells in the adrenal occurs, followed by cortical cell proliferation, differentiation into zones, formation of an adrenal capsule, and return of function (Belloni et al., 1982; Nabishah et al., 1998; Okamoto et al., 1992; Srougi et al., 1978; Vendeira et al., 1992) which resembles normal morphology by 180 days (Vendeira et al., 1992). The timeline by which these processes occur differ slightly between experiments. Necrosis occurs and regeneration begins within one week (Srougi et al., 1978; Srougi and Gittes, 1978; Vendeira et al., 1992). Organization into zones occurs by 15 days and encapsulation by 30 days (Vendeira et al., 1992). There is also evidence that adrenal autografts are reinnervated (Ulrich-Lai and Engeland, 2000). The adrenal medulla does not regenerate (Srougi and Gittes, 1978), possibly due to its increased sensitivity to anoxia; CORT levels remain low in animals in which only adrenal medulla was engrafted (Nabishah et al., 1998). Despite the evidence concerning ADXA in adult rats, nothing is known about this approach in neonatal rats. We recently demonstrated that in P9 rats, ADXA causes rapid re-engraftment and restoration of partial function in as little as 2-3 days (unpublished observation).
The mechanisms by which MA causes long-term learning and memory deficits are unknown. A candidate other than increased CORT release is reduction of brain 5-HT. Hippocampal serotonin has been shown to be reduced throughout the period of neonatal MA exposure (Schaefer et al., 2006; Schaefer et al., 2008). The current experiment demonstrates that depletions in 5-HT in the neostriatum and entorhinal cortex and 5-HIAA in the hippocampus and entorhinal cortex persist into adulthood. Even with alterations in the serotonergic system in brain regions that are known to be involved in allocentric and egocentric learning, how serotonin mediates such effects remains to be determined. However, a test of the role of 5-HT in contributing to the long-term effects of MA is a logical step in future experiments in order to understand how MA induces cognitive deficits.
Acknowledgment
The authors express their appreciation to Mary Moran for assistance with the statistical analyses of the data. This research was supported by NIH project grant RO1 DA006733 and training grant T32 ES007031 (CEG; TLS).
Abbreviations
- MA
methamphetamine
- CORT
corticosterone
- ADXA
adrenal autotransplantation
- DA
dopamine
- 5-HT
serotonin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aisa B, Tordera R, Lasheras B, Del RJ, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Belloni AS, Vassanelli P, Robba C, Rebuffat P, Mazzocchi G, Nussdorfer GG. Ultrastructural observations on the regeneration of adrenocortical autotransplants in the rat spleen. J.Anat. 1982;135:245–253. [PMC free article] [PubMed] [Google Scholar]
- Biagini G, Pich EM, Carani C, Marrama P, Agnati LF. Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. Int.J.Dev.Neurosci. 1998;16:187–197. doi: 10.1016/s0736-5748(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Burchfield DJ, Lucas VW, Abrams RM, Miller RL, DeVane CL. Disposition and pharmacodynamics of methamphetamine in pregnant sheep. JAMA. 1991 Apr 17;265:1968–1973. [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004 Dec 15;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chapillon P, Patin V, Roy V, Vincent A, Caston J. Effects of pre- and postnatal stimulation on developmental, emotional, and cognitive aspects in rodents: a review. Dev.Psychobiol. 2002;41:373–387. doi: 10.1002/dev.10066. [DOI] [PubMed] [Google Scholar]
- Chomchai C, Na MN, Watanarungsan P, Yossuck P, Chomchai S. Methamphetamine abuse during pregnancy and its health impact on neonates born at Siriraj Hospital, Bangkok, Thailand. Southeast Asian J.Trop.Med.Public Health. 2004;35:228–231. [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007 Feb 15;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Darlington RB, Anand KJ, Finlay BL. Web-Based Method For Translating Neurodevelopment From Laboratory Species To Humans. Neuroinformatics. 2006;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Cloak CC, Ernst T, Fujii L, Hedemark B, Chang L. Lower diffusion in white matter of children with prenatal methamphetamine exposure. Neurology. 2009 Jun 16;72:2068–2075. doi: 10.1212/01.wnl.0000346516.49126.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, Williams MT, Newman ER, McDougall SA, Vorhees CV. Methamphetamine exposure during the preweanling period causes prolonged changes in dorsal striatal protein kinase A activity, dopamine D2-like binding sites, and dopamine content. Synapse. 2003 Jun 1;48:131–137. doi: 10.1002/syn.10197. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Jacobson L, Levin N, Cascio CS, Shinsako J. Characterization of corticosterone feedback regulation of ACTH secretion. Ann.N.Y.Acad.Sci. 1987;512:402–414. doi: 10.1111/j.1749-6632.1987.tb24976.x. [DOI] [PubMed] [Google Scholar]
- Dixon SD. Effects of transplacental exposure to cocaine and methamphetamine on the neonate. West J.Med. 1989;150:436–442. [PMC free article] [PubMed] [Google Scholar]
- Dixon SD, Bejar R. Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: incidence and clinical correlates. J.Pediatr. 1989;115:770–778. doi: 10.1016/s0022-3476(89)80661-4. [DOI] [PubMed] [Google Scholar]
- Etienne AS, Jeffery KJ. Path integration in mammals. Hippocampus. 2004;14:180–192. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- Etienne AS, Maurer R, Seguinot V. Path integration in mammals and its interaction with visual landmarks. J.Exp.Biol. 1996;199:201–209. doi: 10.1242/jeb.199.1.201. [DOI] [PubMed] [Google Scholar]
- Felszeghy K, Bagdy G, Nyakas C. Blunted pituitary-adrenocortical stress response in adult rats following neonatal dexamethasone treatment. J.Neuroendocrinol. 2000;12:1014–1021. doi: 10.1046/j.1365-2826.2000.00551.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Bournissen F, Rokach B, Karaskov T, Koren G. Methamphetamine detection in maternal and neonatal hair: implications for fetal safety. Arch.Dis.Child Fetal Neonatal Ed. 2007;92:F351–F355. doi: 10.1136/adc.2006.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace CE, Schaefer TL, Herring NR, Skelton MR, McCrea AE, Vorhees CV, Williams MT. (+)-Methamphetamine increases corticosterone in plasma and BDNF in brain more than forced swim or isolation in neonatal rats. Synapse. 2008;62:110–121. doi: 10.1002/syn.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson DM, Knott B, Walker FR. Neonatal endotoxin exposure influences HPA responsivity and impairs tumor immunity in Fischer 344 rats in adulthood. Pediatr.Res. 2001;50:750–755. doi: 10.1203/00006450-200112000-00020. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2007. Volume I: Secondary school students. National Institute on Drug Abuse; Bethesda, MD: 2008a. pp. 1–738. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2007. Volume II: College students and adults ages 19-45. National Institute on Drug Abuse; Bethesda, MD: 2008b. pp. 1–342. [Google Scholar]
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol.Biochem.Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Knuth ED, Etgen AM. Long-term behavioral consequences of brief, repeated neonatal isolation. Brain Res. 2007 Jan 12;1128:139–147. doi: 10.1016/j.brainres.2006.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Huchton DM, Wiener SG, Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Dev.Psychobiol. 1991;24:547–558. doi: 10.1002/dev.420240803. [DOI] [PubMed] [Google Scholar]
- Little BB, Snell LM, Gilstrap LC., III Methamphetamine abuse during pregnancy: outcome and fetal effects. Obstet.Gynecol. 1988;72:541–544. [PubMed] [Google Scholar]
- Morris RG, Hagan JJ, Rawlins JN. Allocentric spatial learning by hippocampectomised rats: a further test of the “spatial mapping” and “working memory” theories of hippocampal function. Q.J.Exp.Psychol.B. 1986;38:365–395. [PubMed] [Google Scholar]
- Nabishah BM, Khalid BA, Morat PB, Zanariyah A. Regeneration of adrenal cortical tissue after adrenal autotransplantation. Exp.Clin.Endocrinol.Diabetes. 1998;106:419–424. doi: 10.1055/s-0029-1212009. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB. Neurotoxic Effects of Substituted Amphetamines in Rats and Mice: Challenges to the Current Dogma. 2002. pp. 269–301.
- Okamoto T, Fujimoto Y, Obara T, Ito Y, Kodama T. Experimental study on adrenal autografts in rats to preserve normal adrenocortical function after bilateral adrenalectomy. Eur.Surg.Res. 1992;24:112–118. doi: 10.1159/000129196. [DOI] [PubMed] [Google Scholar]
- Oro AS, Dixon SD. Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. J.Pediatr. 1987;111:571–578. doi: 10.1016/s0022-3476(87)80125-7. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm.Behav. 2003;43:561–567. doi: 10.1016/s0018-506x(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev.Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Schaefer TL, Ehrman LA, Gudelsky GA, Vorhees CV, Williams MT. Comparison of monoamine and corticosterone levels 24 h following (+)methamphetamine, (+/−)3,4-methylenedioxymethamphetamine, cocaine, (+)fenfluramine or (+/−)methylphenidate administration in the neonatal rat. J.Neurochem. 2006;98:1369–1378. doi: 10.1111/j.1471-4159.2006.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Skelton MR, Herring NR, Gudelsky GA, Vorhees CV, Williams MT. Short- and long-term effects of (+)-methamphetamine and (+/−)-3,4-methylenedioxymethamphetamine on monoamine and corticosterone levels in the neonatal rat following multiple days of treatment. J.Neurochem. 2008;104:1674–1685. doi: 10.1111/j.1471-4159.2007.05112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J.Neurosci. 1995;15:376–384. doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Schaefer TL, Vorhees CV. Neonatal (+)-methamphetamine increases brain derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits. Psychoneuroendocrinology. 2007;32:734–745. doi: 10.1016/j.psyneuen.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Yonekura ML, Wallace T, Berman N, Kuo J, Berkowitz C. Effects of prenatal methamphetamine exposure on fetal growth and drug withdrawal symptoms in infants born at term. J.Dev.Behav.Pediatr. 2003;24:17–23. doi: 10.1097/00004703-200302000-00006. [DOI] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Grotta SD, Fallone M, Liu J, Lester BM. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol.Teratol. 2008;30:20–28. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisurapanont M, Jarusuraisin N, Kittarattanapaiboon P. World Health Organization; Geneva, Switzerland: Systematic review of treatment for amphetamine-related disorders. 2001:1–32.
- Srougi M, Gittes RF. Adrenal autotransplantation. Urol.Surv. 1978;28:41–48. [PubMed] [Google Scholar]
- Srougi M, Gittes RF, Underwood R. Influence of exogenous glucocorticoids and adrenocorticotropic hormone on experimental adrenal autografts. Surg.Forum. 1978;29:109–111. [PubMed] [Google Scholar]
- Struthers JM, Hansen RL. Visual recognition memory in drug-exposed infants. J.Dev.Behav.Pediatr. 1992;13:108–111. doi: 10.1097/00004703-199204000-00005. [DOI] [PubMed] [Google Scholar]
- Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstet.Gynecol. 2009;113:1285–1291. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Engeland WC. Rat adrenal transplants are reinnervated: an invalid model of denervated adrenal cortical tissue. J.Neuroendocrinol. 2000;12:881–893. doi: 10.1046/j.1365-2826.2000.00534.x. [DOI] [PubMed] [Google Scholar]
- Vendeira P, Magalhaes MM, Magalhaes MC. Autotransplantation of the adrenal cortex: a morphological and autoradiographic study. Anat.Rec. 1992;232:262–272. doi: 10.1002/ar.1092320211. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Maze learning in rats: a comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol.Teratol. 1987;9:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Ahrens KG, Acuff-Smith KD, Schilling MA, Fisher JE. Methamphetamine exposure during early postnatal development in rats: I. Acoustic startle augmentation and spatial learning deficits. Psychopharmacology (Berl) 1994;114:392–401. doi: 10.1007/BF02249328. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, Williams MT. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int.J.Dev.Neurosci. 2008a;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, Williams MT. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int.J.Dev.Neurosci. 2008b;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS. Adult learning deficits after neonatal exposure to D-methamphetamine: selective effects on spatial navigation and memory. J.Neurosci. 2000 Jun 15;20:4732–4739. doi: 10.1523/JNEUROSCI.20-12-04732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Skelton MR, Grace CE, Schaefer TL, Graham DL, Braun AA, Williams MT. Effects of (+)-methamphetamine on path integration and spatial learning, but not locomotor activity or acoustic startle, align with the stress hyporesponsive period in rats. Int.J.Dev.Neurosci. 2009;27:289–298. doi: 10.1016/j.ijdevneu.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Weisenburger WP, Acuff-Smith KD, Minck DR. An analysis of factors influencing complex water maze learning in rats: effects of task complexity, path order and escape assistance on performance following prenatal exposure to phenytoin. Neurotoxicol.Teratol. 1991;13:213–222. doi: 10.1016/0892-0362(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Blankenmeyer TL, Schaefer TL, Brown CA, Gudelsky GA, Vorhees CV. Long-term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Brain Res.Dev.Brain Res. 2003a Dec 30;147:163–175. doi: 10.1016/j.devbrainres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Williams MT, Inman-Wood SL, Morford LL, McCrea AE, Ruttle AM, Moran MS, Rock SL, Vorhees CV. Preweaning treatment with methamphetamine induces increases in both corticosterone and ACTH in rats. Neurotoxicol.Teratol. 2000;22:751–759. doi: 10.1016/s0892-0362(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology (Berl) 2003b;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Wallace TL, Fukumura M, Broening HW, Vorhees CV. Developmental D-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse. 2003c Jun 1;48:138–148. doi: 10.1002/syn.10159. [DOI] [PubMed] [Google Scholar]
- Williams MT, Schaefer TL, Furay AR, Ehrman LA, Vorhees CV. Ontogeny of the adrenal response to (+)-methamphetamine in neonatal rats: The effect of prior drug exposure. Stress. 2006;9:153–163. doi: 10.1080/10253890600902842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Vorhees CV, Boon F, Saber AJ, Cain DP. Methamphetamine exposure from postnatal day 11 to 20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Res. 2002 Dec 27;958:312–321. doi: 10.1016/s0006-8993(02)03620-x. [DOI] [PubMed] [Google Scholar]