Abstract
Inflammation is associated with many neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, and multiple sclerosis. In this Review, we discuss inducers, sensors, transducers, and effectors of neuroinflammation that contribute to neuronal dysfunction and death. Although inducers of inflammation may be generated in a disease-specific manner, there is evidence for a remarkable convergence in the mechanisms responsible for the sensing, transduction, and amplification of inflammatory processes that result in the production of neurotoxic mediators. A major unanswered question is whether pharmacological inhibition of inflammation pathways will be able to safely reverse or slow the course of disease.
Introduction
Virchow's seminal descriptions of activated microglia anticipated by more than a century the current interest in roles of the innate and adaptive immune systems in diverse forms of neurodegenerative disease. Microglia, a type of glial cell, are macrophages that are resident in the brain and spinal cord and form the frontline defense of the innate immune system. Direct evidence for an innate inflammatory response in Alzheimer's disease (AD) was described nearly 20 years ago (reviewed in Akiyama, 1994), and subsequent studies have documented inflammatory components in Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and a growing number of other nervous system pathologies. Although inflammation may not typically represent an initiating factor in neurodegenerative disease, there is emerging evidence in animal models that sustained inflammatory responses involving microglia and astrocytes contribute to disease progression. A major unresolved question is whether inhibition of these responses will be a safe and effective means of reversing or slowing the course of disease. To effectively address this question, it will be necessary to learn more about how inflammatory responses are induced within the central nervous system and the mechanisms by which these responses ultimately contribute to pathology. Here, we review studies of the roles of inflammation mediated by the innate and adaptive immune systems in the pathogenesis of AD, PD, ALS, and MS and highlight some of the important areas for future investigation.
Inflammation, Immunity, and Repair
The immune system plays essential roles in the maintenance of tissue homeostasis and the response to infection and injury. Microglia are the major resident immune cells in the brain, where they constantly survey the microenvironment and produce factors that influence surrounding astrocytes (another type of glial cell with support functions) and neurons. Under physiological conditions, microglia exhibit a deactivated phenotype that is associated with the production of anti-inflammatory and neurotrophic factors (Streit, 2002). Microglia switch to an activated phenotype in response to pathogen invasion or tissue damage and thereby promote an inflammatory response that serves to further engage the immune system and initiate tissue repair. In most cases, this response is self-limiting, resolving once infection has been eradicated or the tissue damage has been repaired.
Sustained inflammation resulting in tissue pathology implies persistence of an inflammatory stimulus or a failure in normal resolution mechanisms. A persistent stimulus may result from environmental factors or the formation of endogenous factors (e.g., protein aggregates) that are perceived by the immune system as “stranger” or “danger” signals. Inflammatory responses that establish feed-forward loops may overwhelm normal resolution mechanisms. Although some inflammatory stimuli induce beneficial effects (e.g., phagocytosis of debris and apoptotic cells), and inflammation is linked to tissue repair processes, uncontrolled inflammation may result in production of neurotoxic factors that amplify underlying disease states. As a starting point for comparing and contrasting roles of inflammation in the pathogenesis of AD, PD, ALS, and MS, we will first briefly consider general features of inflammation in the context of innate and adaptive immunity.
Inducers and Sensors of Infection and Injury
The inflammatory response represents a highly regulated biological program that, on the one hand, must enable the innate and adaptive immune systems to effectively deal with rapidly dividing microbial pathogens but, on the other hand, involves the production of factors that are themselves capable of inducing significant tissue pathology. As a consequence, genes that play key roles in amplification or effector functions of inflammatory responses are actively repressed under normal conditions and are only induced when cells sense evidence of infection or injury. Inflammatory responses to infectious agents are typically initiated by pattern recognition receptors that bind to so-called pathogen-associated molecular patterns (stranger signals). One class of pattern recognition receptor is exemplified by the Toll-like receptors (TLRs), which recognize a diverse set of pathogen-associated molecules that are not present in the host (see Review by O. Takeuchi and S. Akira on page 805 of this issue). For example, TLR4 recognizes lipopolysaccharide (LPS) associated with gram-negative bacteria, whereas TLR3 recognizes viral double-stranded RNA. These receptors are expressed on many cell types but are highly expressed on cells that play central roles in innate immune responses, including macrophages and microglia. Pattern recognition receptors have more recently been found to also be capable of responding to endogenously derived molecules, such as components released from necrotic cells (danger signals) and by molecules that may be formed as a consequence of pathogenic mechanisms. Roles of TLR2 and TLR4 have recently been established in the pathogenesis of several chronic inflammatory diseases in animal models, and specific TLR4 polymorphisms are associated with several human age-related diseases, including atherosclerosis, type 2 diabetes, and rheumatoid arthritis, raising the question of whether these receptors also contribute to inflammatory programs associated with neurodegenerative disease (Balistreri et al., 2009). In addition to pattern recognition receptors, purinergic receptors are expressed on microglia and astrocytes and are capable of responding to ATP released from cells following cell death, traumatic injury, or ischemia (Di Virgilio et al., 2009). Microglia and astrocytes also express a number of so-called “scavenger receptors” that have been demonstrated to be involved in the uptake of a number of substrates, including oxidized proteins, lipids, and apoptotic cells, and that may also contribute to cell signaling (Husemann et al., 2002).
Transduction Systems
Ligation of pattern recognition receptors leads to the activation of signal transduction pathways that regulate diverse transcriptional and posttranscriptional processes. For example, the TLRs couple to signaling adaptor systems that are defined by the MyD88 and TRIF signal adaptor proteins, resulting in activation of downstream kinases including IkB kinases and MAP kinases. These in turn control the activities of multiple, signal-dependent transcription factors that include members of the NF-κB, AP-1, and interferon regulator factor (IRF) families (see Review by O. Takeuchi and S. Akira on page 805). These factors work in a combinatorial manner to regulate hundreds of genes, depending on the target cell that is activated.
Amplifiers and Effectors
To bring about an effective immune response, the initial detection of a microbial pathogen must be amplified to recruit additional cells to sites of infection, induce antimicrobial activities, and initiate the development of adaptive immunity. Important subsets of highly induced genes thus include cytokines (e.g., TNF-α, IL-1β) that are amplifiers of the program of inflammation and chemokines (e.g., MCP-1) that serve to recruit additional immune cells. In addition, genes that encode proteins with antimicrobial activities (e.g., iNOS) are induced, as are genes that influence substrate metabolism, protein synthesis, cell motility, phagocytosis, intracellular killing, and antigen presentation. The generation of reactive oxygen species (ROS), e.g., through the NADPH oxidase system, is an important antimicrobial mechanism but also exemplifies a system that can result in collateral damage to tissue, e.g., the parenchymal cells in the brain.
Cellular and Tissue Context
Inflammatory responses are typically localized and involve communication between immune, vascular, and parenchymal cells. Resident populations of tissue macrophages play key roles as sentinels of infection and injury but are also increasingly recognized as having an influence on normal tissue homeostasis. Macrophage phenotypes can be considered in the context of “activation” status, with M1 or “classical activation” describing the proinflammatory phenotypic response to IFN-γ produced by Th1 T lymphocytes or signaling through TLRs. M2 or “alternative activation” describes distinct phenotypic responses to cytokines, such as IL-4 and IL-13, produced by Th2 lymphocytes and other cell types (see Review by C. Nathan and A. Ding on page 871 of this issue). The term “deactivated” macrophage has been used to describe phenotypic responses to anti-inflammatory cytokines, such as IL-10. Transition of tissue-resident macrophages from the M2 to the M1 phenotype generally is associated with inflammation-induced pathologies, such as diet-induced insulin resistance (Lumeng et al., 2007). As noted above, microglia exhibit a deactivated phenotype in the healthy brain and may play important roles in maintenance of tissue homeostasis through communication with astrocytes and neurons, analogous to homeostatic roles of “alternatively activated” macrophages in other tissues. The central nervous system (CNS) is an immunologically privileged site and circulating immune cells normally do not have access to it in the absence of inflammation or injury. Dendritic cells with specialized antigen-presenting capabilities do not appear to be present under normal conditions. Microglia appear to be the major initial sensors of danger or stranger signals recognized by TLR4, and they secrete inflammatory mediators such as TNF-α and IL-1β that can act on astrocytes to induce secondary inflammatory responses (Saijo et al., 2009). Microglia are thus likely to play critical roles in establishing and maintaining inflammatory responses in the context of neurodegenerative diseases.
Counter-regulation
Numerous negative feedback mechanisms have been identified that serve to attenuate responses to inducers or amplifiers of inflammation. These include induction of proteins that inhibit signal transduction pathways (e.g., SOCS proteins), induction of transcriptional repressors and transrepressors (e.g., ATF3, Nurr1), and production of soluble or cell-surface mediators with anti-inflammatory activities (e.g., IL-10, TGF-β, resolvins, ligands for TAM receptors). How these negative feedback pathways are integrated with proinflammatory signaling pathways to bring about appropriate resolution of inflammation in any context remains poorly understood.
Alzheimer's Disease
Initially described almost 100 years ago by Alois Alzheimer, AD is one of the most common age-related neurodegenerative diseases, with approximately 7% of people older than 65 years and about 40% of people older than 80 years being affected in industrialized countries. The symptoms of AD are characterized by loss of memory, progressive impairment of cognition, and various behavioral and neuropsychiatric disturbances. The pathological hallmarks of AD in the brain include extracellular amyloid plaques comprising aggregated, cleaved products of the amyloid precursor protein (APP) and intracellular neurofibrillary tangles (NFTs) generated by hyperphosphorylated forms of the microtubule-binding protein tau.
Evidence of an inflammatory response in AD includes changes in microglia morphology—from ramified (resting) to amoeboid (active)—and astrogliosis (manifested by an increase in the number, size, and motility of astrocytes) surrounding the senile plaques. Moreover, microglia surrounding plaques stain positive for activation markers and proinflammatory mediators, including MHC class II, Cox-2, MCP-1, TNF-α, IL-1β, and IL-6 (Akiyama et al., 2000). MCP-1 is known to induce the chemotaxis of astrocytes and contributes to the recruitment of astrocytes around senile plaques (Wyss-Coray et al., 2003). In addition, elevated levels of chemokines and cytokines and their receptors, including IL-1α, CXCR2, CCR3, CCR5, and TGF-β, have been reported in post-mortem AD brains (Cartier et al., 2005).
Inducers and Sensors of Inflammation in AD
NFTs are composed of hyperphosphorylated forms of the micro-tubule-binding protein tau, which under normal physiological conditions regulates cytoskeletal changes. An inflammatory environment might activate the tau kinases to promote formation of NFTs (Ballatore et al., 2007), but whether hyperphosphorylated tau and NFTs affect inflammatory responses is not yet well understood.
Senile plaques containing the N-terminal APP cleavage products Aβ1–42 and/or Aβ1–40 (Aβ) are the hallmarks of AD pathology. Aβ1–42 and Ab1–40 are generated from APP by β and γ-secretases (Haass and Selkoe, 2007). Rare mutations in APP and the presenilin components of γ-secretase are causes of familial AD, providing one line of evidence for the hypothesis that Aβ contributes to the pathogenesis of AD (Bertram and Tanzi, 2008). Although a pathogenic role for Aβ is generally accepted, the mechanisms remain poorly understood. In addition to numerous cell-autonomous effects in neurons, aggregates of Aβ have also been shown to activate microglia and induce the production of factors, such as nitric oxide (NO), ROS, proinflammatory cytokines (e.g., TNF-α, IL-1β, IL-6), chemokines (e.g., IL-18), and prostaglandins (e.g., PGE2), that promote neuronal death (Akiyama et al., 2000; Kitazawa et al., 2004).
Microglia and astrocytes appear to be capable of detecting Ab through several sensors, including TLRs, that are expressed on glial cells (Landreth and Reed-Geaghan, 2009). In particular, Ab has been suggested to activate microglia and astrocytes through TLR4 (together with CD14 and MD2 in microglia), leading to the activation of signal-dependent transcription factors that drive expression of downstream inflammatory response genes (Reed-Geaghan et al., 2009; Walter et al., 2007). Consistent with this, mice carrying a nonfunctional TLR4 crossed with a mouse model of AD (APP/PS1 double transgenic mice) showed production of fewer inflammatory cytokines (Jin et al., 2008). It has been suggested that TLR4 may participate in the phagocytosis of Aβ plaques by microglia. Indeed, mice carrying mutant TLR4 crossed with AD transgenic mice exhibited more Aβ plaques (Tahara et al., 2006). A recent report suggests that Aβ fibrils trigger inflammatory responses through TLR4/TLR6 in the presence of CD36 (Stewart et al., 2009). Intriguingly, a polymorphism in the TLR4 extracellular domain has been reported to be associated with protection against late-onset AD in an Italian population (Minoretti et al., 2006), suggesting that sterile inflammation could influence AD pathology through TLR4 signaling. TLR9 stimulation by CpG-DNA (a mimic of bacterial DNA) showed neuroprotective roles both in vivo and in vitro (Doi et al., 2009). TLR2 also may be a sensor for fibrillar Aβ. Blocking TLR2 signaling with antibody or by knockdown of the receptor gene in vitro suggested that TLR2 stimulation by Aβ promotes neurotoxic inflammation. However, mice lacking TLR2 crossed with APP/PS1 transgenic AD mice were reported to show a delay in Aβ deposition and improved behavior on memory tests (Richard et al., 2008). These apparently divergent actions of TLR2, TLR4, and TLR9 in vivo and in vitro are currently difficult to reconcile but could reflect differences in the specific cell types that express these receptors as well as differences in the signaling/effector pathways that are engaged beyond the core NF-κB response. Sorting out the basis for these differences will be important.
A second sensing system for Aβ is provided by the receptor for advanced glycoxidation end-products (RAGE), a cell surface receptor belonging to the immunoglobulin superfamily (Neeper et al., 1992; Schmidt et al., 1992). RAGE was initially identified as a receptor for advanced glycoxidation end-products (AGEs). Pro-oxidant environments, such as an inflammatory milieu, can promote the production of AGEs that lead to the activation of RAGE on the surface of microglia, astrocytes, vascular endothelial cells, and neurons. Several reports suggest that Aβ peptide as well as Aβ oligomers bind to RAGE and activate glia cells, especially microglia (Yan et al., 1996). Blocking the interaction of Aβ with RAGE impaired the activation of microglia and reduced the production of proinflammatory mediators (Ramasamy et al., 2009). RAGE is also suggested to play an important role in the clearance of Aβ and to be involved in apoE-mediated cellular processing and signaling (Bu, 2009). In addition to AGEs and Aβ, RAGE recognizes other ligands including serum amyloid A (SAA), S100 protein, and high-mobility group box1 (HMGB1). The increased production of RAGE ligands is often observed in cellular and organ dysfunction, particularly where inflammation is involved (Schmidt et al., 2009; Srikanth et al., 2009). These molecules are often present in the altered tissue environments associated with type 2 diabetes, and the activation of RAGE might contribute to the increased risk of AD in patients with type 2 diabetes.
NOD-like receptors (NLRs) represent a third Aβ sensing system. NLRs are soluble, cytoplasmic pattern recognition receptors for pathogens and also act as sensors of cellular damage. (see Reviews by O. Takeuchi and S. Akira on page 805 and by K. Schroder and J. Tschopp on page 821 of this issue). In AD, Aβ oligomers and fibrils induce lysosomal damage and trigger NALP3, a member of the NLR family that is expressed in microglia (Halle et al., 2008). NALPs activate downstream signaling proteins, such as apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC). Caspase activation by an adaptor ASC induces apoptosis as well as the maturation of proinflammatory mediators like IL-1β and IL-18. Also, a lower cellular K+ concentration activates NALP1, another member of the NLR family that is expressed in neurons. Similar to NALP3 in glial cells, NALP1 activates ASC and caspases and induces the maturation of IL-1β and IL-18 (see Review by K. Schroder and J. Tschopp on page 821).
Additional Genetic Factors
As mutations in APP and β- and γ-secretases are rare causes of AD, additional genetic, environmental, and age-related factors must contribute to the formation of Aβ and other events that drive AD pathogenesis in most individuals. One of the strongest genetic associations with AD is the E4 allelic variant of apolipo-protein E (apoE). ApoE is a 34 kDa protein that acts, at least in part, as a transporter of lipids. In humans, there are three apoE isoforms: apoE2, apoE3, and apoE4. The apoE4 allele is associated with an increased risk of AD as well as other neurodegenerative and cardiovascular diseases, such as atherosclerosis (Bu, 2009). Numerous hypotheses have been advanced to account for these associations (reviewed in Kim et al., 2009), including differential effects of apoE alleles on inflammation and Aβ processing. In the brain, apoE is mainly produced by microglia and astrocytes and likely plays many important roles by binding to members of the low-density lipoprotein (LDL) receptor family, including the classical LDL receptor (LDLR) and LDLR-related protein 1 (LRP1) (Jaeger and Pietrzik, 2008). One recent study suggests that apoE has anti-inflammatory effects by activating LDLR-mediated signaling. When LDLRs and LRP1 are stimulated by peptide mimics of apoE, JNK kinase activity is suppressed and microglia activation is attenuated (Pocivavsek et al., 2009a, 2009b). This anti-inflammatory effect of apoE is isoform specific, with apoE4 exhibiting less activity (Licastro et al., 2007; Vitek et al., 2009). With respect to the roles of apoE and its receptors in the regulation of APP processing, LRP1 has been suggested to directly bind to Aβ or Aβ/apoE complexes, mediating their uptake by glial cells, which is neuroprotective (Marzolo and Bu, 2009). In this case, the apoE3/Aβ complex is preferentially taken up by LRP1 compared to the apoE4/Aβ complex. These studies suggest that expression of the apoE4 protein might result in defects in clearance of Aβ that would promote the deposition of Aβ aggregates in the brain, hence contributing to AD.
Environmental Factors
Many environmental factors may influence inflammatory responses that contribute to AD pathology, including traumatic injury, systemic infection, and diet (Migliore and Coppede, 2009). Traumatic injury activates both microglia and astrocytes and could potentially induce self-sustaining inflammatory responses in the brain (Van Den Heuvel et al., 2007). Activation of the systemic innate immune system by infection may be involved in the early stages of AD pathogenesis (Perry et al., 2007). Recently, a strong correlation between type 2 diabetes and AD has been recognized (Granic et al., 2009; Jones et al., 2009). Type 2 diabetes with hyperinsulinemia increases the risk of AD in elderly people (Luchsinger and Gustafson, 2009). Several different mechanisms have been proposed to explain this correlation. Prolonged consumption of excess calories induces obesity and a low-grade but chronic form of inflammation in adipose tissue, liver, and other organs that is associated with an insulin-resistant state (see Review by G.S. Hotamisligil on page 900 of this issue). Macrophages are now recognized to be major sources of proinflammatory mediators that act on various cell types to impair insulin signaling. It is possible that systemic inflammation contributing to insulin resistance might have a direct effect in the brain, or that the activation of tissue macrophages in type 2 diabetes might reflect an underlying defect shared by microglia that independently become activated within the CNS.
Inflammation-Dependent Pathology
Aβ formation and associated tauopathy appear to be sufficient to explain a cell-autonomous stress response in neurons, as recently supported by studies of the N-APP/DR6 mouse model of AD (Nikolaev et al., 2009). However, Aβ aggregates and products derived from dead cells can trigger microglia and astrocytes through the TLR and RAGE-dependent pathways, leading to local inflammation that may further amplify neuronal death (Figure 1). Although the relative roles of Aβ and other potential initiators of inflammation remain unclear, the activation of caspases and signal-dependent transcription factors such as NF-κB and AP-1 results in production of numerous amplifiers (e.g., IL-1β, TNF-α, IL-6) of inflammation. Proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, might act directly on neurons to induce apoptosis (McCoy and Tansey, 2008; Simi et al., 2007). Furthermore, factors such as TNF-α and IL-1β released by microglia can activate astrocytes, whereas factors released from astrocytes may lead to further activation of microglia (Saijo et al., 2009). In addition, APP, presenilin (a component of γ-secretase), and BACE1 (β-secretase) have NF-κB sites in their promoters, and proinflammatory cytokines are known to upregulate their expression in neurons (Sastre et al., 2008). Inflammatory mediators acting on neurons might contribute to more production of Aβ, further activating microglia-mediated inflammation. Thus, communication between neurons and glia may amplify the production of neurotoxic factors that contribute to AD pathology. Region-specific effects on neurons are likely to depend on the specific types of receptors expressed within different neuronal populations. For example, TNF-α binds to TNFR1, which activates cell survival pathways through NF-κB as well as apoptotic signaling pathways through activation of caspases. In contrast, TNFRII signaling only activates NF-κB. Although it is clear that symptoms of AD are caused by neuronal damage, it is not well understood which neurons are the primary targets of the neurotoxic process. It was reported that death of cholinergic neurons in the basal forebrain was an important component of AD pathology. However, recent evidence suggests that other neurons, such as glutaminergic and GABAnergic neurons, might also be important targets in AD pathology (Rissman et al., 2007; Yamin, 2009).
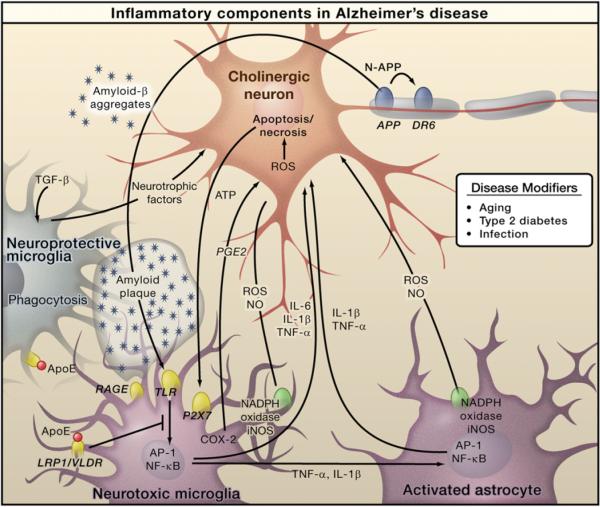
Figure 1. Inflammation in Alzheimer's Disease.
Amyloid-β peptide, produced by cleavage of amyloid precursor protein (APP), forms aggregates that activate microglia, in part by signaling through Toll-like receptors (TLRs) and RAGE. These receptors activate the transcription factors NF-κB and AP-1, which in turn induce the production of reactive oxygen species (ROS) and drive the expression of inflammatory mediators such as cytokines. These inflammatory factors act directly on cholinergic neurons and also stimulate astrocytes, which amplify proinflammatory signals to induce neurotoxic effects. Apoptosis and necrosis of neurons result in release of ATP, which further activates microglia through the purinergic P2X7 receptor. Microglia can also play protective roles by mediating clearance of Aβ through ApoE-dependent and ApoE-independent mechanisms. Cholinergic neurons in the basal forebrain, the neurons that are primarily affected in AD, are presumed to be important targets of inflammation-induced toxicity, but other types of neurons, such as glutaminergic and GABAergic neurons, may also be affected.
Divergent results have been obtained in attempts to assess the overall impact of microglia on AD pathology in mice. In one approach, APP/PS1 transgenic AD mice were crossed to mice in which microglia, but not macrophages, could be conditionally depleted. Three weeks after conditional depletion of microglia, amyloid plaque formation and neuronal damage had not changed compared to control mice (Grathwohl et al., 2009). Although these results could be interpreted to indicate that microglia are essentially passive bystanders in AD pathology at least in this model, the lack of effect of microglia depletion could also reflect the relatively short timeframe of the experiment or a balanced reduction in both beneficial and deleterious activities. A contrasting result was provided by recent experiments in which the growth factor M-CSF was systemically administered to APP/PS1 transgenic mice for 4 months. This procedure resulted in a significant increase in the number of parenchymal microglia, decreased Aβ deposits, and decreased cognitive loss (Boissonneault et al., 2009), thereby supporting a neuroprotective function. The authors suggested that M-CSF injection primarily resulted in the expansion of bone marrow-derived microglia. This might provide another explanation for the apparent discrepancy between these results and the data obtained by selectively depleting microglia resident in the CNS.
Parkinson's Disease
Parkinson's disease (PD) is the second most common neurode-generative disease after AD and is the most common movement disorder. Currently, about 2% of the population over the age of 60 is affected. Prominent clinical features are motor symptoms (bradykinesia, tremor, rigidity, and postural instability) and non-motor-related symptoms (olfactory deficits, autonomic dysfunction, depression, cognitive deficits, and sleep disorders). Like AD, PD is a proteinopathy; it is characterized by the accumulation and aggregation of misfolded α-synuclein. Neuropathological hallmarks are intracellular inclusions containing α-synuclein called Lewy bodies and Lewy neurites and the loss of dopaminergic neurons in the substantia nigra of the midbrain and in other brain regions as well (Braak et al., 2003). Loss of dopaminergic neurons is not the only neuropathological alteration in PD, as microglial activation and an increase in astroglia and lymphocyte infiltration also occur. An increase in astroglial cells in postmortem tissue from the brains of PD patients (Damier et al., 1993) and an increased number of dystrophic astrocytes (Braak et al., 2007) have also been reported. Positron emission tomography of PD patients has shown a marked increase in the peripheral benzodiazepine receptor expressed by glial cells. Additional studies are needed to validate the specificity of this imaging approach, but these findings are suggestive of increased glial activation in PD patients (Gerhard et al., 2006).
The etiologies of most common forms of PD remain poorly understood. Originally thought to be a disease characterized by loss of one neuronal type, PD is now recognized to have an inflammatory component (Block and Hong, 2007; McGeer and McGeer, 2008; Nagatsu and Sawada, 2005). Reactive microglia expressing human leukocyte antigen (HLA)-DR and CD11b, along with Lewy bodies, are found in the substantia nigra of PD patients (McGeer et al., 1988). In addition, increased levels of cytokines in the colony-stimulating factor (CSF) (Nagatsu and Sawada, 2005) and in the blood have been reported. Although these inflammatory components are not specific for PD, they might provide useful biomarkers for monitoring progression of the disease.
Inducers and Sensors of Inflammation in PD
As is the case for AD, rare mutations in a number of genes cause familial forms of PD and provide insights into general pathogenic mechanisms (Gasser, 2009). Among these are mutations in α-synuclein (PARK1 and PARK4) and DJ-1 (PARK7). α-synuclein is a 140 amino acid protein that is found physiologically in the presynaptic terminals of neurons. It plays a major role in PD neuropathology: α-synuclein aggregates in PD and becomes the major fibrillar protein in Lewy bodies in both sporadic and inherited forms of PD. Moreover, point mutations (A53T, A30P, E46K) and gene multiplications of human wild-type α-synuclein are related to rare familial autosomal-dominant forms of early-onset PD. In PD, the aggregation of α-synuclein from monomers, via oligomeric intermediates, into fibrils is considered the disease-causing toxic mechanism. Recent reports indicate that the accumulation of α-synuclein can result in the formation of intermediate state oligomers, which lead to neuronal cell death (Danzer et al., 2007). One line of research proposes that neuronal death itself, including release of protein aggregates, induces activation of microglia. Additional activation of microglia may be due to the release of aggregated proteins from neurons into the extracellular space (Roodveldt et al., 2008). This finding is interesting, as the α-synuclein-related neuropathological alterations in sporadic PD and in most forms of familial PD were initially thought to be intracellular. Recent data challenge this model and indicate the importance of extracellular α-synuclein aggregates in PD (Lee, 2008). Extracellular α-synuclein is phagocytosed by microglia (Zhang et al., 2005), and aggregated, nitrated, and oxidized forms of α-synuclein have been found to induce microglial activation (Reynolds et al., 2008; Zhang et al., 2005). Sensing mechanisms for α-synuclein aggregates are similar to those for viruses and toxins. Extracellular α-synuclein is suggested to be sensed and internalized by cell surface gangliosides in BV-2 cells (a microglia cell line) in vitro. An important role has been reported for GM1 gangliosides in endocytosing α-synuclein, most likely via lipid rafts (Park et al., 2009). α-synuclein-mediated neurotoxicity is enhanced by microglial activation and release of proinflammatory cytokines. The internalization of α-synuclein by microglia is followed by activation of NADPH oxidase and production of ROS (Zhang et al., 2005). Recently, microglial immunity stimulated by nitrated α-synuclein was reported to be regulated by CD4+ T regulatory (Treg) cells. Treg cells may alter the proteome of microglia in response to nitrated α-synuclein, thereby protecting dopaminergic neurons (Benner et al., 2008; Reynolds et al., 2008).
A characteristic of dopaminergic neurons in the substantia nigra is an increase in intracellular oxidative processes related to the synthesis of dopamine (Kuhn et al., 2006), making them particularly vulnerable to oxidative stress. In addition, high rates of catecholamine metabolism drive the production of neuromelanin, and high amounts of neuromelanin are suspected to increase the vulnerability of dopaminergic midbrain neurons to oxidative stress (Kastner et al., 1992). A direct effect of neuromelanin on activation of microglia through activation of NF-κB has been shown in cultures of rodent microglial cells (Wilms et al., 2003).
Environmental Factors
As the vast majority of PD cases are sporadic, environmental factors that interact with common but less penetrant susceptibility genes are likely to influence the onset of most cases of sporadic PD (Tansey et al., 2007). Although not directly sensed by microglia, PD-causing toxins are known to induce neuronal degeneration that is associated with reactive microgliosis, which exacerbates neurotoxicity. MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is a neurotoxin that causes permanent symptoms of PD. Metabolized to 1-methyl-4-phenylpyridinium (MPP+) by glial cells, MPP+ is taken up by dopaminergic neurons via the dopamine transporter and induces oxidative stress, leading to mitochondrial damage and neuronal cell death. Despite a lack of Lewy bodies in MPTP parkinsonism, activated microglia were found in patients even 16 years after MPTP exposure. Although these patients may have had further access to other drugs in the meantime, 18-year-old nonhuman primates given MPTP years earlier also exhibited activated microglia and dopaminergic neuronal loss in the absence of Lewy bodies, suggesting a long-lasting and self-driven reactive microgliosis (McGeer and McGeer, 2008). The role of bacterial or viral infection as an initiating factor in human PD is unclear, but intracranial infusion of bacterial LPS is used as an alternative model for microglia-induced loss of tyrosine hydroxylase-positive dopaminergic neurons in rodents (Castano et al., 1998). LPS-induced inflammation can also synergize with mutations in α-synuclein and Parkin that are associated with familial PD to potentiate the loss of tyrosine hydroxylase-positive neurons in animal models (Gao et al., 2008). Whether other neuronal populations are affected remains to be established.
Inflammation-Dependent Pathology
Several lines of evidence suggest that inflammatory mediators such as ROS, NO, TNF-α, and interleukin (IL)-1β derived from non-neuronal cells including microglia modulate the progression of neuronal cell death in PD (Hirsch and Hunot, 2009)(Figure 2). Evidence that inflammatory responses originating from nonneuronal cells are sufficient to cause loss of dopaminergic neurons is provided by studies of LPS-mediated neurotoxity (Castano et al., 1998). Injection of LPS into the rodent brain results in increased levels of inflammatory mediators, including COX-2 and iNOS, prior to loss of dopaminergic neurons (Hunter et al., 2007). TLR4, the main receptor for LPS, is preferentially expressed on microglia compared to astrocytes (Kim et al., 2000), but it is present at very low or undetectable levels on neurons. Consistent with this finding, microglia are much more responsive than astrocytes to LPS when assayed in a tissue culture environment, whereas neurons are virtually unresponsive (Saijo et al., 2009). Direct application of LPS to neurons has little effect on gene expression or survival. In contrast, conditioned media from LPS-treated microglia are neurotoxic, and this effect is enhanced when conditioned media are added to mixed cultures of astrocytes and neurons. Separation of the different cell type components of the mixed culture system is most consistent with the interpretation that microglia are the primary initial responders to LPS and produce mediators such as TNF-α and IL-1β that activate astrocytes (Saijo et al., 2009). The combination of factors that are produced by activated microglia and astrocytes in turn may promote neurotoxicity. Intriguingly, these factors are preferentially toxic to dopaminergic neurons, raising the question of whether differential sensitivity of neurons in PD is due to factors that are relatively specific to dopaminergic neurons or whether dopaminergic neurons are more sensitive to generic neurotoxic factors.
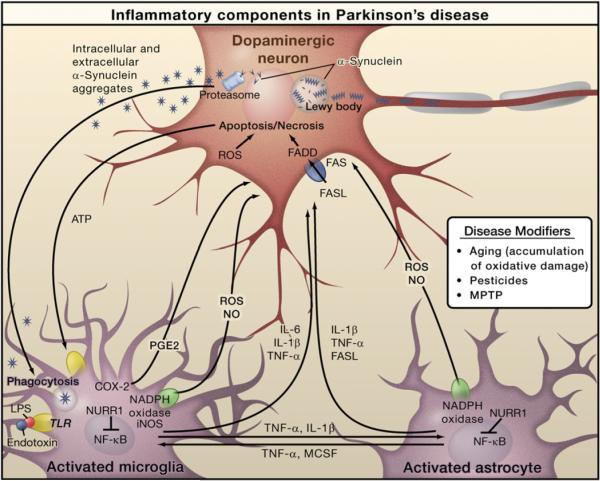
Figure 2. Inflammation in Parkinson's Disease.
Prominent neuropathological hallmarks of Parkinson's disease (PD) are the loss of dopaminergic neurons in the substantia nigra of the midbrain and the presence of intracellular inclusions containing aggregates of the α-synuclein protein, called Lewy bodies. Besides forming Lewy bodies, aggregates of α-synuclein form intermediate-state oligomers that when released from neurons activate microglia through Toll-like receptor (TLR)-independent mechanisms. This leads to activation of NF-κB and production of reactive oxygen species (ROS) and proinflammatory mediators. These factors act directly on dopaminergic neurons of the substantia nigra, which are the principal (although not the only) neurons that die in PD. These factors also activate microglia, which amplify the inflammatory response in a positive feedback loop, leading to further activation of microglia. Products derived from microglia and astrocytes act in a combinatorial manner to promote neurotoxicity. Bacterial lipopolysaccharide (LPS), acting primarily through TLR4 expressed by microglia, is sufficient to induce an inflammatory response in the substantia nigra that results in loss of dopaminergic neurons. The transcription factor NURR1 acts to suppress inflammatory responses in microglia and astrocytes by inhibiting NF-κB target genes.
PD-associated, activated microglial cells release NO produced by iNOS as well as ROS. NADPH oxidase is the major source of ROS production in activated microglia in PD (Hunot et al., 1996). A direct effect of α-synuclein on microglia (as opposed to a TLR-dependent pathway as observed following LPS treatment) has been demonstrated. Extracellular α-synuclein is phagocytosed by microglia, resulting in activation of NADPH oxidase and ROS production (Zhang et al., 2005). NADPH oxidase activation and ROS production are a crucial mechanism for microglia activation after exposure to α-synuclein as the toxic effect was less strong in mice lacking NADPH oxidase (Zhang et al., 2005). The oxidative stress-induced nitration of α-synuclein is a potent inducer of microglial activation in vitro and in vivo and is associated with activation of NF-κB-related genes and increased expression of neurotrophins (NFKB1, TNF, TNFRSF1A, BDNF, GDNF) (Reynolds et al., 2008). In addition to microglia activation, a recent study using the MPTP mouse model of PD suggested that infiltration of CD4+ T lymphocytes may be involved in PD. Dopaminergic toxicity is mediated by CD4+ T cells and requires the expression of FasL but not IFN-γ (Benner et al., 2008; Brochard et al., 2009).
Counter-regulation
Mechanisms that act to counter-regulate or resolve inflammatory responses have recently been identified that may be relevant to PD pathology. The chemokine receptor CX3CR1 is present on microglia, and CX3CR1 knockout mice show increased toxicity in response to systemic LPS treatment and augmented neurodegeneration in the substantia nigra following MPTP administration (Cardona et al., 2006). A negative feedback mechanism operating at the level of NF-κB target genes was recently described for the orphan nuclear receptor Nurr1. Nurr1 was originally described as being required for the generation and maintenance of dopaminergic neurons, with rare mutations associated with familial PD. Nurr1 unexpectedly also inhibits expression of proinflammatory neurotoxic mediators in microglia and astrocytes. Reduced Nurr1 expression results in exaggerated inflammatory responses in microglia that are further amplified by astrocytes, leading to the production of factors that cause the death of tyrosine hydroxylase-positive neurons. Nurr1 exerts anti-inflammatory effects by docking to NF-κB-p65 on target inflammatory gene promoters in a signal-dependent manner. Subsequently, Nurr1 recruits the CoREST corepressor complex, resulting in clearance of NF-κB-p65 and transcriptional repression. These studies suggest that Nurr1 protects against loss of dopaminergic neurons in PD in part by limiting the production of neurotoxic mediators by microglia and astrocytes (Saijo et al., 2009).
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS), or Lou Gehrig's disease, is a progressive fatal neurodegenerative disease that affects motor neurons in the brainstem, spinal cord, and motor cortex. The most common clinical features of ALS were described more than 150 years ago by the French neurologist, Jean Martin Char-cot. Clinical features involve degeneration of motor neurons producing fasciculation, muscle wasting and weakness, increased spasticity, and hyper-reflexia. Respiratory complications usually develop in patients with advanced disease, and the cause of death is generally paralysis of the respiratory muscles and diaphragm. With a projected lifetime risk of 1/2000, ALS is considered one of the most common motor neuron diseases (Eisen, 2009). ALS is universally fatal, with a median age of onset of 55 years and a survival of 2–5 years after the onset of symptoms. Although the exact pathophysiological mechanisms underlying neurodegeneration in ALS remain uncertain, a common pathological hallmark is the presence of ubiquitin-immunoreactive cytoplasmic inclusions in degenerating neurons, followed by a strong inflammatory reaction (McGeer and McGeer, 2002).
Prominent neuroinflammation can be readily observed in pathologically affected areas of the CNS and in spinal cords from both human ALS patients and mouse models of the disease (McGeer and McGeer, 2002). Typically, inflammation in ALS is characterized by gliosis and the accumulation of large numbers of activated microglia and astrocytes. Activation of glia in ALS has been extensively characterized and is marked by elevated production of potentially cytotoxic molecules such as ROS, inflammatory mediators such as COX-2, and proinflammatory cytokines such as IL-1β, TNF-α, and IL-6 (McGeer and McGeer, 2002). Major histocompatibility complex molecules and complement receptors are highly expressed by reactive microglia in the primary motor cortex and in the anterior horn of the spinal cords of ALS patients (McGeer and McGeer, 2002).
Genetically Determined Factors
The majority of ALS cases are sporadic, likely resulting from a complex gene-gene and gene-environment interplay. Only 10% of the cases are familial. Studies in families with adult-onset ALS have identified genes responsible for genetic heritability, such as superoxide dismutase 1 (SOD1) (Rosen et al., 1993), transactive response (TAR) DNA-binding protein (TARDBP) (Neumann et al., 2006), and FUS/TLS (fused in sarcoma or translocation in liposarcoma) (Kwiatkowski et al., 2009; Vance et al., 2009). Genetically engineered transgenic mouse models expressing the human SOD1 protein carrying familial ALS mutations recapitulate the disease (Clement et al., 2003). ALS patients with SOD1 mutations have neuronal inclusions in their motor neurons that are ubiquitinated. The observation that SOD1 knockout mice have a very mild phenotype and that several strains with SOD1 mutations still have superoxide dismutase activity strongly suggests a gain-of-function form of toxicity in ALS (Turner and Talbot, 2008). The identification of the TAR DNA-binding protein 43 (TDP-43) as a major component of ubiquitinated inclusions in sporadic ALS patients and in patients with another neurodegenerative disease called frontotemporal lobar degeneration has focused attention on mutations in TARDBP, the gene that encodes TDP-43 (Neumann et al., 2006). Recently, dominant mutations in the FUS/TLS gene were also identified in several ALS families (Kwiatkowski et al., 2009; Vance et al., 2009). The wild-type FUS/TLS protein contains RNA-binding motifs and is believed to be involved in transcriptional regulation (Uranishi et al., 2001; Wang et al., 2008). Like TDP-43, wild-type FUS/TLS is frequently localized in the nucleus, but in its mutant form it may exist as aggregates in the cytoplasm of motor neurons in ALS patients (Kwiatkowski et al., 2009; Vance et al., 2009). FUS/TLS is a coactivator of NF-κB, and recent data suggest that it is involved in the inflammatory response (Amit et al., 2009; Uranishi et al., 2001).
Sensors and Transduction Systems
Increasing evidence points to receptors of the innate immune response as potential sensors of molecules that induce or amplify inflammation in ALS (Letiembre et al., 2009). CD14, a protein that facilitates TLR4 responses to LPS, and TLR2 are upregulated in the spinal cords of mice with ALS (Nadeau and Rivest, 2000; Nguyen et al., 2001) and ALS patients (Letiembre et al., 2009; Liu et al., 2009). Chronic infusion of a presymptomatic ALS mouse with LPS enhanced the innate immune response and also exacerbated disease progression (Nguyen et al., 2004). Microglia expressing mutant SOD1 exhibited an increase in NADPH oxidase-dependent production of ROS (Liu et al., 2009). Furthermore, the oxidation boost was followed by an increase in secretion of TNF-α and the metalloproteinases ADAM10–17, reinforcing a potential link between oxidative stress and inflammatory responses in ALS. Extracellular mutant SOD1 can also induce microglial activation via the MyD88-dependent pathway. This was shown by injecting mutant SOD1 protein into the brains of normal or MyD88−/− mice and observing that a proinflammatory response was only induced (measured by expression of TLR2 and IL-1β) in the brains of wild-type animals (Kang and Rivest, 2007). Interestingly, this inflammatory response was associated with infiltration of brain tissue by bone marrow-derived microglia (or macrophages); reconstitution of the hematopoietic system of SOD1G37R mutant mice with MyD88-deficient cells resulted in earlier disease onset and a shortened life span. These findings point to potential neuroprotective mechanisms in which bone marrow-derived myeloid cells eliminate secreted mutant SOD1 protein, perhaps analogous to the role of bone marrow-derived microglia in the clearance of Aβ (Boissonneault et al., 2009). An increase in cell death-associated extracellular ATP in the spinal cords of ALS patients may induce the purinergic receptor (P2X7) expressed by microglia to release IL-1β (Yiangou et al., 2006). It is possible that dying neurons in ALS patients may release ATP and, in turn, promote activation of glial cells. The principal transcription factors involved in regulating the expression of genes responsible for production of potential neurotoxic molecules remain to be established. Based on the suggested roles of TLRs and purinergic receptors as sensing systems, candidate transcription factors include AP-1 and NF-κB, but further investigation is required.
Inflammation in ALS Pathogenesis
The major determinants of motor neuron death in ALS remain to be established. Although IL-1β and TNF-α are neurotoxic in vitro, deletion of either gene alone did not alter disease progression in SOD1 mutant mice (Gowing et al., 2006; Nguyen et al., 2001). Although it is possible that IL-1 and TNF-α are not important contributors to in vivo pathology, an alternative interpretation is that motor neuron death in ALS is the consequence of multiple factors acting in a redundant manner. In this scenario, loss of a single effector molecule is not sufficient to alter the disease phenotype. Consistent with this possibility, administration of the anti-inflammatory drug lenalidomide extended survival of SOD1 mutant mice and improved motor behavior even after the onset of symptoms; these improvements correlated with the reduced expression of TNF-α, IL-1β, and FasL (Neymotin et al., 2009). A motor neuron-specific death pathway has been suggested for ALS based on the finding that motor neurons isolated from transgenic SOD1 mutant mice were more sensitive to Fas- or NO-triggered cell death than wild-type motor neurons (Raoul et al., 2002). Upon binding of FasL to the Fas receptor, the intracellular portion of Fas recruits the adaptor molecule FADD, which then activates a caspase cascade that culminates in the death of motor neurons. This pathway was also activated in presymptomatic ALS mice (Raoul et al., 2006). Given that astrocytes and microglia produce NO and that astrocytes from SOD1 mutant mice produce FasL (Barbeito et al., 2004), glial cells could be the executioners that directly kill motor neurons. Another member of the same receptor family, the p75 neurotrophin receptor, has also been implicated in ALS-dependent motor neuron death (Pehar et al., 2004). Specifically, nerve growth factor secreted by SOD1 mutant astrocytes induced the death of motor neurons expressing p75 by a mechanism involving the formation of NO and peroxide (Pehar et al., 2004).
Even though motor neurons are the main cells affected in ALS, increasing evidence points to the involvement of neighboring glia cells during pathogenesis (Figure 3) (Clement et al., 2003; Yamanaka et al., 2008b). Cell-specific deletion of mutant SOD1 in astrocytes and microglia using GFAP-Cre or CD11b-Cre transgenes, respectively, reduced disease severity and boosted the survival of ALS mice (Boillee et al., 2006; Yamanaka et al., 2008b). Although there are some concerns related to the timing and efficiency of Cre-mediated excision in these experiments, evidence for a role for non-neuronal cells has been obtained using chimeric mice selectively expressing SOD1 in neurons or in non-neuronal cells (Yamanaka et al., 2008a). In chimeric mice expressing high levels of mutant SOD1 in 100% of motor neurons and oligodendrocytes, the presence of wild-type neighboring support cells substantially delayed the onset of motor neuron degeneration. We and others have shown that glial cells expressing various mutant forms of SOD1 can exert toxic effects on healthy (nonmutated) human motor neurons when cocultured with them in vitro (Di Giorgio et al., 2008; Marchetto et al., 2008). Gene expression profiling implies that inflammatory cascades are activated before the initiation of motor neuron degeneration, suggesting that inflammation could be involved in the presymptomatic phase of the disease (Vargas et al., 2008). Mutant SOD1, but not the wild-type protein, has been proposed to be secreted into the extracellular space via chromogranin vesicles, causing activation of microglia and resulting in motor neuron death in culture (Urushitani et al., 2006). Consistently, mutant SOD1 is present in the cerebrospinal fluid of ALS patients and it is toxic to rodent spinal cord cultures (Tikka et al., 2002). Moreover, intracerebral infusion of mutant SOD1 into wild-type mice induced microglial activation and cytokine production (Kang and Rivest, 2007). Although there is no evidence for direct binding, mutant SOD1 in the extracellular space could be a ligand that sensor molecules detect resulting in activation of the inflammatory response. In the case of mutations in TARDBP and FUS/TLS and in sporadic forms of ALS, it is still not clear which molecules are responsible for triggering of the immune response. One could speculate that misfolded, ubiquitinated proteins may play a role. The initial inflammatory reaction could also come from extracellular ATP released by injured neurons, which is sensed by purinergic receptors on glia (Yiangou et al., 2006).
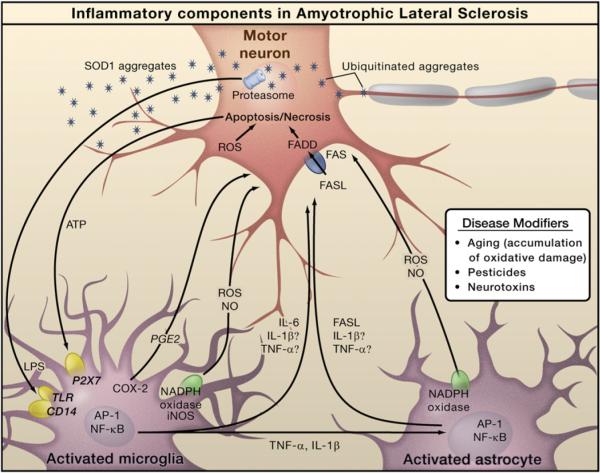
Figure 3. Inflammation in Amyotrophic Lateral Sclerosis.
The pathology of amyotrophic lateral sclerosis (ALS) is characterized by degeneration of motor neurons. Familial ALS is caused by mutations in the SOD1 gene, but the genes mutated in sporadic ALS are not yet defined. Progressive neurodegeneration of motor neurons in ALS may result from a combination of intrinsic motor neuron vulnerability to aggregates of mutant SOD1 protein and non-cell-autonomous toxicity exerted by neighboring cells. Toxic aggregates can induce inflammatory responses by microglia via Toll-like receptor 2 (TLR2) and CD14. Microglia can induce astrocyte activation by producing cytokines. Activated microglia and astrocytes amplify the initial damage to the motor neurons by activating AP-1 and NF-κB through production of proinflammatory cytokines and apoptosis-triggering molecules such as TNF-α and FASL. TNF-α and IL-1β exert neurotoxic effects in vitro, but deletion of the individual genes does not affect the course of the disease in an animal model. Dying motor neurons release ATP that can further activate microglia through the purinergic receptor P2X7 expressed by microglia.
Emerging evidence points to an involvement of the adaptive immune response in ALS disease progression. An increase in IL-12 has been found in the brains of SOD1 mutant mice that have been chronically treated with LPS (Nguyen et al., 2004). IL-12 is a cytokine involved in the transition from the innate to the adaptive immune response that promotes the differentiation of CD4+ lymphocytes into IFN-γ-producing Th1 helper cells (see Review by O. Takeuchi and S. Akira on page 805). Indeed, increased levels of CD4+ and CD8+ T lymphocytes and dendritic cells were detected in close proximity to dying motor neurons in the spinal cords of SOD1 mutant mice and in the brain parenchyma of ALS patients (Mantovani et al., 2009). The role of infiltrating T lymphocytes in ALS pathology is not yet clear, but recent reports suggest that they may have a neuroprotective function (Banerjee et al., 2008; Beers et al., 2008; Chiu et al., 2008). In fact, it has recently been proposed that infiltrating T cells (Th2) can be neuroprotective after secreting IL-4, which signals reactive microglia to produce neurotrophic factors such as insulin growth factor (IGF1) (Chiu et al., 2008).
Multiple Sclerosis
Multiple sclerosis (MS) is a heterogeneous and complex autoimmune disease that is characterized by inflammation, demyelination, and axon degeneration in the CNS. This pathology results from a primary defect in the immune system that targets components of the myelin sheath, resulting in secondary effects on neurons. Thus, in contrast to AD, PD, and ALS, protein aggregates are not pathogenic factors. The manifestations of MS include defects in sensation and in the motor, autonomic, visual, and cognitive systems. MS predominantly affects young adults and 2–3 times more females than males. In the early stage of the disease, approximately 85% of MS patients show the relapse-remission type of disease. However, with time, the recovery of these relapsing-remitting patients is impaired and eventually leads to irreversible progression, that is, secondary progressive MS. The majority of relapsing-remitting MS patients progress to secondary progressive MS. In contrast, about 10% of MS patients do not show any remission, and the primary neurological symptoms exhibit continuous so-called primary progression (Goverman, 2009; Sospedra and Martin, 2005).
MS lesions are characterized by infiltration of lymphocytes and antibody-producing plasma cells into the perivascular region of the brain and spinal cord white matter, an increase in microglia and astrocytes, and demyelination (Lassmann et al., 2001). The deposition of antibodies and complement around demyelinated lesions (Frohman et al., 2006) and axonal degeneration in the progression phase of MS have also been observed (Trapp and Nave, 2008). When damage and the ensuing inflammatory response are transient, remyelination of nerves can take place as part of normal repair. However, in the presence of chronic inflammation, such as in MS, remyelination is severely impaired and leads to axon degeneration and the eventual demise of the neuron.
Initiators and Sensors of MS
In contrast to AD, PD, and ALS, there is no clear familial form of MS that could help to identify endogenous initiators of disease. The development of MS is generally thought to require a combination of environmental factors acting on genetic traits that ultimately lead to an autoimmune response that targets the myelin sheath surrounding nerves. Experimental autoimmune encephalomyelitis (EAE), in which rodents are immunized with a myelin-derived antigen and adjuvant, is the most common animal model of MS. By varying the genetic background and immunization protocol, EAE can reproduce the symptoms of the major forms of human MS.
Viral and bacterial infections are strong candidates for factors that could initiate MS because regions of pathogen-associated proteins resemble myelin proteins, such as myelin basic protein (MBP), and are antigenic. For example, a peptide from hepatitis B virus (HBV), which is known to be associated with MS, is structurally very similar to a peptide derived from MBP when presented in the context of MHC molecules by antigen-presenting immune cells such as dendritic cells (Sospedra and Martin, 2005). In combination with other factors, HBV infection provokes MS by activating T cells that respond to the HBV peptide antigen and the MBP peptide (Fujinami and Oldstone, 1985), suggesting the possibility that molecular mimicry may underlie the targeting of the adaptive immune system to specific myelin components. Other common pathogens, such as Epstein-Barr virus and some enterobacteria, have also been associated with the onset of MS (Lang et al., 2002), although the critical antigens have not been defined.
Both the innate and acquired immune systems are involved in MS pathology. Several lines of evidence demonstrate that immune cells outside of the CNS such as dendritic cells are key players in MS pathogenesis (Bailey et al., 2007; Figure 4 shows a simplified view of MS pathogenesis within the CNS). Although autoreactive T and B cells play major roles in MS pathology, it is the innate immune system that initiates the disease. For example, naive T cells recognize the myelin-specific antigen MBP when this autoantigen is presented in the context of MHC by antigen-presenting cells such as dendritic cells, macrophages, and microglia. Antigen-presenting cells not only present the antigen to T lymphocytes but also provide costimulation and produce the cytokines required for T cells to differentiate into effector cells.
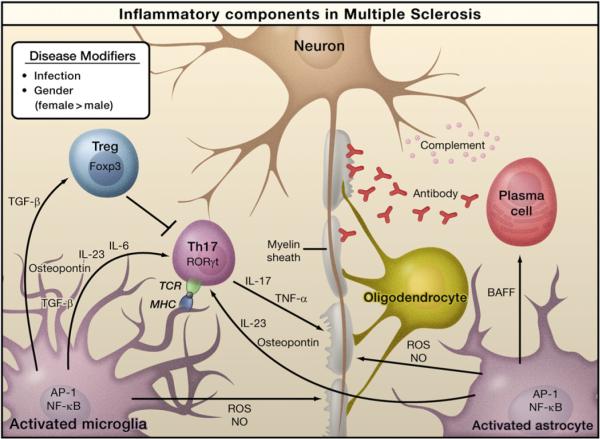
Figure 4. Inflammation in Multiple Sclerosis.
Infection by bacteria or viruses or other environmental stimuli trigger the activation of microglia and astrocytes in multiple sclerosis (MS), leading to the production of proinflammatory cytokines through activation of the transcription factors NF-κB and AP-1. Naive T cells recognize myelin-derived antigen presented in the context of MHC molecules by antigen-presenting cells. In the presence of IL-6 and TGF-β, the naïve T cells are induced to express retinoic acid receptor-related orphan receptor γt (RORγt) and differentiate into Th17 cells. Activated microglia and astrocytes secrete IL-23 and osteopontin, which induce Th17 cells to secrete IL-17 and TNF-α resulting in damage to the myelin sheath that protects nerve axons. Activated astrocytes produce BAFF, a survival factor for autoreactive B cells, which differentiate into plasma cells and produce anti-myelin antibodies. Activated microglia and astrocytes are also sources of reactive oxygen species (ROS) and nitric oxide (NO), which contribute to the destruction of the myelin sheath and of the neurons themselves. Regulatory T cells (Treg) that express Foxp3 suppress the activity of Th17 cells and thus help to suppress inflammation.
TLRs are expressed by antigen-presenting cells and astrocytes. Triggering such receptors will affect the differentiation and activation of T cells. Somewhat surprisingly, the Asp299Gly polymorphism in TLR4, which is associated with susceptibility to type 2 diabetes, is not associated with susceptibility to MS (Kroner et al., 2005). The functions of TLRs in mouse models of MS are complex, with both stimulatory and protective roles identified. These observations can be explained by the responses of innate immune cells and their influence on the differentiation of distinct subsets of T helper cells (Marta et al., 2009). Recently, phagocytosis of infected apoptotic cells (which trigger TLR signaling) was reported to be a physiological signal that induces activation of effector T cells in vivo (Torchinsky et al., 2009).
Autoreactive T and B lymphocytes play roles as amplifiers and effectors in MS. Th1 helper T cells were initially thought to play a crucial role in MS pathogenesis. However, characterization of specific functions of IL-12, IL-23, and other IL-12 family members has uncovered essential roles for a subset of T helper cells called Th17 cells in the pathogenesis of MS (Cua et al., 2003). These Th17 cells secrete members of the IL-17 proinflammatory cytokine family, especially IL-17A and IL-17F (Korn et al., 2009), and play a key role in infection by pathogens and in gut immunity (see Review by D.R. Littman and A.Y. Rudensky on page 845 of this issue). The differentiation and activation of Th17 cells requires signaling though the T cell receptor (TCR) as well as a mixture of cytokines produced by antigen-presenting cells. These include IL-1β for human and IL-6 for mice, and TGF-β as well as IL-23 and other cytokines. Th17 cells also secrete IL-21, which induces activation of Th17 cells in an autocrine manner (Korn et al., 2009). The retinoic acid receptor-related orphan receptor γt (RORγt) plays a key role in Th17 cell differentiation. Treg cells, which are anti-inflammatory helper T cells, play an opposing role by inhibiting the activity of Th17 cells. Differentiation of this cell type requires the Foxp3 transcription factor (see Review by D.R. Littman and A.Y. Rudensky on page 845).
Although MS is recognized as a noninherited disease, there are many polymorphisms reported to influence susceptibility to MS. In particular, MHC haplotype is an important determinant of susceptibility to MS. In the human, the HLA-DR/DQ haplotype and the HLA-A3 and HLA-B7 haplotype are known to influence the susceptibility to or protection from MS among certain populations (for reviews, see Fugger et al., 2009). Additional factors that influence risk include polymorphisms in the T cell receptor β chain (TCR-β), CTLA4, TNF-α, ICAM1, CCR5, IL-10, IL-4R α chain, IL-2R β chain, IL-7R α chain, IFN-γ, CD6, IRF8, TNFSF1A (TNFRI), vitamin D receptor, and the estrogen receptor (Fugger et al., 2009). Genome-wide association studies have identified additional loci on chromosomes 17, 5, and 19, and a recent large genetic study identified more MS-susceptible loci on chromosomes 12 and 20, for which the responsible genes need to be determined (ANZgene, 2009; see Essay by Zenewicz et al. in this issue). Many of the candidate genes residing within these loci are expressed in T and B lymphocytes and regulate the differentiation, activation, and migration of effector T and B cells, consistent with their potential influence on MS pathogenesis.
Relapse and Remission
Relapse and remission are among the characteristic features of MS. What triggers the relapse of MS is not fully understood, but activation of the innate immune system, for example by infection, is one reported cause (Sospedra and Martin, 2005). Deregulation of immunity by memory T and B cells might be another cause of relapse. α4β1-integrin (VLA4) is an adhesion molecule expressed by T cells that allows autoreactive T cells to break through the blood brain barrier and migrate into the brain parenchyma; thus, one therapeutic strategy is to block VLA4 signaling using monoclonal antibodies (Yednock et al., 1992). Osteopontin is a cytokine secreted by activated microglia, astrocytes, and neurons that binds to VLA4, stimulating the production of proinflammatory cytokines and blocking apoptosis of autoreactive T cells. These two molecules may cooperate to induce relapse in the CNS in MS (Hur et al., 2007). With respect to remission, αβ-crystallin mRNA has been identified as the most abundant mRNA in MS lesions. Mice lacking αβ-crystallin exhibit exacerbated symptoms of MS, and administration of recombinant αβ-crystallin improves the symptoms of relapsing-remitting MS and secondary progressive MS in animal models (Ousman et al., 2007).
Neuroinflammation: Common and Disease-Specific Features
Viewed from the perspective of inducers, sensors, transducers, and effectors, each of the neurodegenerative diseases considered here is distinguished by a disease-specific mechanism for induction of inflammatory responses. The distinct pathways for production of inducers of inflammation—such as Aβ, α-synuclein, mutant SOD1, and myelin peptide mimetic—and the specific anatomical locations at which these processes occur are likely determinants of the specific pathologicial features of each disease. Remarkably, however, once inducers are generated, there appears to be considerable convergence in the sensor, transducer, and effector mechanisms that lead to amplification of inflammatory responses, neurotoxicity, and neuronal death. Activation of innate immune cells in the CNS, such as microglia and astrocytes, is one of the universal components of neuroinflammation (Figures 1–4). In particular, TLRs and other pattern recognition receptors expressed on microglia are likely to play significant roles in initiating inflammatory responses that are further amplified by astrocytes. Similarly, signal transduction pathways downstream of these receptors that regulate the activities of the transcription factors NF-κB and AP-1 appear to play general roles in mediating the production of amplifiers and effector molecules, such as cytokines (e.g., TNF-α, IL-1β, and IL-6), ROS, and NO. Several of these factors could be general neurotoxic factors for all of the neurodegenerative diseases discussed above. However, the marked involvement of the adaptive immune system clearly distinguishes MS from AD, PD, and ALS, although T cells do seem to play a neuroprotective role in ALS.
It is likely that sustained inflammatory responses that contribute to neurodegeneration are driven, at least in part, by positive feedback loops. Crosstalk between microglia and astrocytes is predicted to lead to amplification of inflammation and release of ATP by necrotic neurons that would be expected to activate microglia in each of the disease contexts considered here. Such feedback loops could, in principle, become independent of the original inducing molecules that are required to initiate inflammatory responses. In addition, inflammation may itself influence the production of disease-specific inducers, such as Aβ.
It will be interesting to determine how the output of activated, innate immune cells affects specific types of neurons. For example, many of the same cytokines are suggested to play pathological roles in AD, PD, and ALS, but the patterns of neuronal loss are distinct. It will therefore be important to determine whether this difference reflects different sensitivities of specific neurons to generic neurotoxic factors or the production of neurotoxic factors with neuron-specific activities. Finally, much more effort will be required to understand the gene networks that underlie the neuroprotective roles for microglia and astrocytes, and how these networks are perturbed in chronic disease states.
Therapeutic Implications
A major unanswered question is whether it will be possible to safely and effectively target inflammatory mechanisms that contribute to the pathogenesis of AD, PD, ALS, and MS. Table 1 lists examples of therapeutic approaches that have been tested, are in clinical trials, or are currently being developed that directly or indirectly influence inflammatory responses. Notable progress has been made in the case of MS, where the function of the adaptive immune system can be altered with therapeutic antibodies against key cell surface antigens. For example, based on the contribution of B cells to MS pathology, a monoclonal antibody (rituximab) that depletes memory B cells has been investigated as a potential therapeutic strategy (Dalakas, 2008). Although clinically effective, this treatment carries a risk of progressive multifocal leukoencephalopathy, a rare but highly adverse side effect. Indeed, most of the biological agents currently being evaluated in MS carry some risk of eliciting autoimmunity, and effective and safe therapies remain elusive.
Table 1.
Therapeutic Targets for Treating Neuroinflammation
| Target | Disease | Agent | Study | Status | |
|---|---|---|---|---|---|
| Inducers | Aβ | AD | Tarenflurbil | Phase III | No benefit |
| Sensors | RAGE | AD | PF-04494700 | Phase II | Ongoing |
| Transducers | PPARγ | AD | Rosiglitazone | Phase II | Improved cognition |
| MLK | PD | CEP-1347 | Phase III | No benefit | |
| Effectors | Cox-2 | AD; ALS | Celecoxib | Phase III; phase II/III | No benefit; no benefit |
| Cox-2 | ALS | Nimesulide | Phase I | Ongoing | |
| ROS | AD; ALS | Vitamin E+selenium; Vitamin E | Phase III | Ongoing; no benefit | |
| ROS | AD; ALS | CoenzymeQ | Phase III; phase II | Ongoing; no benefit | |
| ROS | ALS | Celastrol | Phase I | Ongoing | |
| Cells | Microglia | PD; ALS | Minocycline | Phase II; phase III | Ongoing; no Benefit |
| Leukocytes | MS | Alemtuzumab | Phase III | Improvement | |
| Astrocytes | ALS | ONO-2506 | Phase III | Ongoing |
In the cases of AD, PD, ALS, and other neurodegenerative diseases in which microglia and astrocytes are the primary contributors to inflammation, therapeutic approaches logically would aim to modulate the sensor/transducer/effector functions of the innate immune system, that is, TLRs, NF-κB, and TNF-α, respectively. These approaches also face numerous challenges with respect to timing, efficacy, and safety. Epidemiological studies suggest that cyclooxygenase inhibitors reduce the risk of PD and AD (reviewed by Choi et al., 2009), but clinical trials of these agents in patients with manifest disease have failed in AD (Aisen et al., 2003; Reines et al., 2004) and ALS (Cudkowicz et al., 2006). In concert with studies in animal models, these findings suggest that targeting inflammatory pathways might be more effective in preventing disease progression than in reversing existing pathology. If so, the application of these strategies might require identification and treatment of high-risk patients prior to the development of overt or severe symptoms. Biomarkers of subclinical pathology would thus be of great utility for identifying at risk patients and for monitoring the efficacy of treatments.
To be clinically effective, anti-inflammatory therapies will have to gain access to the CNS and target specific cells and pathways that are quantitatively important in disease pathogenesis in humans. Animal models and cellular assays are useful for defining key pathways and neurotoxic factors for specific types of neurons but should be tuned as much as possible to contexts that are representative of human disease. An important case in point is provided by the anti-inflammatory drug minocycline, which delayed disease progression in an ALS transgenic mouse model but worsened disease symptoms in phase III clinical trials in ALS patients (Gordon et al., 2007; Kriz et al., 2002), suggesting that the transgenic model is a weak representation of sporadic ALS. The recent demonstration that induced pluripotent stem (iPS) cells derived from ALS patients can be differentiated into astrocytes and specific types of neurons represents an important advance for developing in vitro humanized systems for mechanistic studies and evaluation of therapeutic agents (Dimos et al., 2008; Ebert et al., 2009). In addition to looking at neurons as endpoints, these systems may also facilitate efforts to define the genetic programs that underlie the neuroprotective as well as the neurotoxic functions of microglia and astrocytes and to therapeutically modulate these genetic programs.
As AD, PD, and ALS are chronic degenerative diseases, it is likely that their prevention and treatment will require long-term therapy, imposing a corresponding requirement for a high level of safety. The major obvious risks are in suppressing innate immune function to the point of enabling opportunistic infections. Based on the broad range of anti-inflammatory therapies that are in clinical use for systemic inflammation that do not compromise innate immune function, selectivity should be possible. An interesting case in point is provided by the thiazoli-dinedione class of drugs that are ligands for PPARγ and are used in the treatment of type 2 diabetes. These drugs regulate lipid and glucose metabolism and suppress inflammatory responses by macrophages and microglia (Tontonoz and Spiegelman, 2008). Recent studies suggest that these drugs have preventive activity in AD (Risner et al., 2006; Watson et al., 2005). Although currently available PPARγ agonists have a number of unwanted side effects, they are not associated with suppression of immunity. Furthermore, due to the complex nature of these diseases and the number of cell types involved, it may take more than one drug to confer a therapeutic benefit. This approach will require a new clinical trial design, exemplified by a recent phase II randomized trial of a celecoxib and creatine (a COX-2 inhibitor and antioxidant) combination therapy in ALS patients (Gordon et al., 2008).
Finally, recent developments in embryonic stem cell and iPS cell technologies are facilitating efforts to replace damaged neurons in a variety of neurodegenerative diseases. However, if cultured neurons are engrafted into regions of the brain in which there is persistent inflammation, then they will be exposed to a neurotoxic environment and it may be difficult to achieve a beneficial clinical outcome. It is thus possible that inhibition of inflammation may also be a prerequisite for successful cell replacement therapy.
Conclusion
Neurodegenerative diseases, exemplified by AD, PD, ALS, and MS, represent major unmet challenges for therapeutic interventions. Characterization and targeting of the processes that initiate specific disease pathologies and act primarily at the level of neurons are clearly important areas for continued investigation. The emerging evidence for both protective and pathogenic roles of microglia and astrocytes and the activation of common inflammation pathways in these cells in several neurodegenerative diseases supports the concept that glia-induced inflammation is an amplifier of pathology. Although inhibition of neuroinflammation may not alter the underlying cause of disease, it may reduce the production of factors that contribute to neurotoxicity, thereby resulting in clinical benefit. Further knowledge of the inducers, sensors, transducers, and effectors of neuroinflammation may make attainment of this goal possible.
ACKNOWLEDGMENTS
We thank Mel Simon for helpful discussions and Jamie Simon for figure preparation. K.S., F.H.G., and C.K.G. are supported by the NIH. F.H.G., B.W., and M.C.M. are supported by CIRM. C.K.G. is supported by a Leducq Transatlantic Network Foundation Grant. B.W. is a Feodor-Lynen fellow of the Alexander von Humbolt Foundation.
REFERENCES
- ANZgene Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat. Genet. 2009;41:824–828. doi: 10.1038/ng.396. [DOI] [PubMed] [Google Scholar]
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- Akiyama H. Inflammatory response in Alzheimer's disease. Tohoku J. Exp. Med. 1994;174:295–303. doi: 10.1620/tjem.174.295. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. Inflammation and Alzheimer's disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit I, Garber M, Chevrier N, Leite AP, Donner Y, Eisenhaure T, Guttman M, Grenier JK, Li W, Zuk O, et al. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science. 2009;326:257–263. doi: 10.1126/science.1179050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides `preferentially' polarize CD4+ T(H)-17 cells in relapsing EAE. Nat. Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- Balistreri CR, Colonna-Romano G, Lio D, Candore G, Caruso C. TLR4 polymorphisms and ageing: implications for the pathophysiology of age-related diseases. J. Clin. Immunol. 2009;29:406–415. doi: 10.1007/s10875-009-9297-5. [DOI] [PubMed] [Google Scholar]
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat. Rev. Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Mosley RL, Reynolds AD, Dhar A, Jackson-Lewis V, Gordon PH, Przedborski S, Gendelman HE. Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PLoS ONE. 2008;3:e2740. doi: 10.1371/journal.pone.0002740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeito LH, Pehar M, Cassina P, Vargas MR, Peluffo H, Viera L, Estevez AG, Beckman JS. A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res. Brain Res. Rev. 2004;47:263–274. doi: 10.1016/j.brainresrev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc. Natl. Acad. Sci. USA. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner EJ, Banerjee R, Reynolds AD, Sherman S, Pisarev VM, Tsiperson V, Nemachek C, Ciborowski P, Przedborski S, Mosley RL, et al. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS ONE. 2008;3:e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nat. Rev. Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem. Soc. Trans. 2007;35:1127–1132. doi: 10.1042/BST0351127. [DOI] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Boissonneault V, Filali M, Lessard M, Relton J, Wong G, Rivest S. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer's disease. Brain. 2009;132:1078–1092. doi: 10.1093/brain/awn331. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Sastre M, Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson's disease. Acta Neuropathol. 2007;114:231–241. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat. Rev. Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res. Brain Res. Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Castano A, Herrera AJ, Cano J, Machado A. Lipopolysaccha-ride intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J. Neurochem. 1998;70:1584–1592. doi: 10.1046/j.1471-4159.1998.70041584.x. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Chen A, Zheng Y, Kosaras B, Tsiftsoglou SA, Vartanian TK, Brown RH, Jr., Carroll MC. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc. Natl. Acad. Sci. USA. 2008;105:17913–17918. doi: 10.1073/pnas.0804610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase -1 and –2 in neuroinflammation: implications for translational research. Trends Pharmacol. Sci. 2009;30:174–181. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Cudkowicz ME, Shefner JM, Schoenfeld DA, Zhang H, Andreasson KI, Rothstein JD, Drachman DB. Trial of celecoxib in amyotrophic lateral sclerosis. Ann. Neurol. 2006;60:22–31. doi: 10.1002/ana.20903. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. B cells as therapeutic targets in autoimmune neurological disorders. Nat. Clin. Pract. Neurol. 2008;4:557–567. doi: 10.1038/ncpneuro0901. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson's disease. Neuroscience. 1993;52:1–6. doi: 10.1016/0306-4522(93)90175-f. [DOI] [PubMed] [Google Scholar]
- Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 2007;27:9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009;32:79–87. doi: 10.1016/j.tins.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Doi Y, Mizuno T, Maki Y, Jin S, Mizoguchi H, Ikeyama M, Doi M, Michikawa M, Takeuchi H, Suzumura A. Microglia activated with the toll-like receptor 9 ligand CpG attenuate oligomeric amyloid {beta} neurotoxicity in in vitro and in vivo models of Alzheimer's disease. Am. J. Pathol. 2009;175:2121–2132. doi: 10.2353/ajpath.2009.090418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose FF, Jr., Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen A. Amyotrophic lateral sclerosis: A 40-year personal perspective. J. Clin. Neurosci. 2009;16:505–512. doi: 10.1016/j.jocn.2008.07.072. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis–the plaque and its pathogenesis. N. Engl. J. Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Fugger L, Friese MA, Bell JI. From genes to function: the next challenge to understanding multiple sclerosis. Nat. Rev. Immunol. 2009;9:408–417. doi: 10.1038/nri2554. [DOI] [PubMed] [Google Scholar]
- Fujinami RS, Oldstone MB. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985;230:1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J. Neurosci. 2008;28:7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser T. Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert Rev. Mol. Med. 2009;11:e22. doi: 10.1017/S1462399409001148. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol. Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, Hilton JF, Spitalny GM, MacArthur RB, Mitsumoto H, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- Gordon PH, Cheung YK, Levin B, Andrews H, Doorish C, Macarthur RB, Montes J, Bednarz K, Florence J, Rowin J, et al. A novel, efficient, randomized selection trial comparing combinations of drug therapy for ALS. Amyotroph. Lateral Scler. 2008;9:212–222. doi: 10.1080/17482960802195632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverman J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing G, Dequen F, Soucy G, Julien JP. Absence of tumor necrosis factor-alpha does not affect motor neuron disease caused by superoxide dismutase 1 mutations. J. Neurosci. 2006;26:11397–11402. doi: 10.1523/JNEUROSCI.0602-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granic I, Dolga AM, Nijholt IM, van Dijk G, Eisel UL. Inflammation and NF-kappaB in Alzheimer's disease and diabetes. J. Alzheimers Dis. 2009;16:809–821. doi: 10.3233/JAD-2009-0976. [DOI] [PubMed] [Google Scholar]
- Grathwohl SA, Kalin RE, Bolmont T, Prokop S, Winkelmann G, Kaeser SA, Odenthal J, Radde R, Eldh T, Gandy S, et al. Formation and maintenance of Alzheimer's disease beta-amyloid plaques in the absence of microglia. Nat. Neurosci. 2009;12:1361–1363. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Hunot S, Boissiere F, Faucheux B, Brugg B, Mouatt-Prigent A, Agid Y, Hirsch EC. Nitric oxide synthase and neuronal vulnerability in Parkinson's disease. Neuroscience. 1996;72:355–363. doi: 10.1016/0306-4522(95)00578-1. [DOI] [PubMed] [Google Scholar]
- Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG, Bing G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J. Neurochem. 2007;100:1375–1386. doi: 10.1111/j.1471-4159.2006.04327.x. [DOI] [PubMed] [Google Scholar]
- Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat. Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40:195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- Jaeger S, Pietrzik CU. Functional role of lipoprotein receptors in Alzheimer's disease. Curr. Alzheimer Res. 2008;5:15–25. doi: 10.2174/156720508783884675. [DOI] [PubMed] [Google Scholar]
- Jin JJ, Kim HD, Maxwell JA, Li L, Fukuchi K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer's disease. J. Neuroinflammation. 2008;5:23. doi: 10.1186/1742-2094-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Kulozik P, Ostertag A, Herzig S. Common pathological processes and transcriptional pathways in Alzheimer's disease and type 2 diabetes. J. Alzheimers Dis. 2009;16:787–808. doi: 10.3233/JAD-2009-0973. [DOI] [PubMed] [Google Scholar]
- Kang J, Rivest S. MyD88-deficient bone marrow cells accelerate onset and reduce survival in a mouse model of amyotrophic lateral sclerosis. J. Cell Biol. 2007;179:1219–1230. doi: 10.1083/jcb.200705046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner A, Hirsch EC, Lejeune O, Javoy-Agid F, Rascol O, Agid Y. Is the vulnerability of neurons in the substantia nigra of patients with Parkinson's disease related to their neuromelanin content? J. Neurochem. 1992;59:1080–1089. doi: 10.1111/j.1471-4159.1992.tb08350.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J. Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M, Yamasaki TR, LaFerla FM. Microglia as a potential bridge between the amyloid beta-peptide and tau. Ann. N Y Acad. Sci. 2004;1035:85–103. doi: 10.1196/annals.1332.006. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kriz J, Nguyen MD, Julien JP. Minocycline slows disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2002;10:268–278. doi: 10.1006/nbdi.2002.0487. [DOI] [PubMed] [Google Scholar]
- Kroner A, Vogel F, Kolb-Maurer A, Kruse N, Toyka KV, Hemmer B, Rieckmann P, Maurer M. J. Neuroimmunol. 2005;Impact of the Asp299Gly polymorphism in the toll-like receptor 4 (tlr-4) gene on disease course of multiple sclerosis;165:161–165. doi: 10.1016/j.jneuroim.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Francescutti-Verbeem DM, Thomas DM. Dopamine quinones activate microglia and induce a neurotoxic gene expression profile: relationship to methamphetamine-induced nerve ending damage. Ann. N Y Acad. Sci. 2006;1074:31–41. doi: 10.1196/annals.1369.003. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Jr., Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- Landreth GE, Reed-Geaghan EG. Toll-like receptors in Alzheimer's disease. Curr. Top. Microbiol. Immunol. 2009;336:137–153. doi: 10.1007/978-3-642-00549-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang HL, Jacobsen H, Ikemizu S, Andersson C, Harlos K, Madsen L, Hjorth P, Sondergaard L, Svejgaard A, Wucherpfennig K, et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat. Immunol. 2002;3:940–943. doi: 10.1038/ni835. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Bruck W, Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol. Med. 2001;7:115–121. doi: 10.1016/s1471-4914(00)01909-2. [DOI] [PubMed] [Google Scholar]
- Lee SJ. Origins and effects of extracellular alpha-synuclein: implications in Parkinson's disease. J. Mol. Neurosci. 2008;34:17–22. doi: 10.1007/s12031-007-0012-9. [DOI] [PubMed] [Google Scholar]
- Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, Schulz-Schaeffer W, Fassbender K. Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol. Aging. 2009;30:759–768. doi: 10.1016/j.neurobiolaging.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Licastro F, Porcellini E, Caruso C, Lio D, Corder EH. Genetic risk profiles for Alzheimer's disease: integration of APOE genotype and variants that up-regulate inflammation. Neurobiol. Aging. 2007;28:1637–1643. doi: 10.1016/j.neurobiolaging.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hao W, Dawson A, Liu S, Fassbender K. Expression of amyotrophic lateral sclerosis-linked SOD1 mutant increases the neurotoxic potential of microglia via TLR2. J. Biol. Chem. 2009;284:3691–3699. doi: 10.1074/jbc.M804446200. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Gustafson DR. Adiposity, type 2 diabetes, and Alzheimer's disease. J. Alzheimers Dis. 2009;16:693–704. doi: 10.3233/JAD-2009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani S, Garbelli S, Pasini A, Alimonti D, Perotti C, Melazzini M, Bendotti C, Mora G. Immune system alterations in sporadic amyotrophic lateral sclerosis patients suggest an ongoing neuroinflammatory process. J. Neuroimmunol. 2009;210:73–79. doi: 10.1016/j.jneuroim.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Marta M, Meier UC, Lobell A. Regulation of autoimmune encephalomyelitis by toll-like receptors. Autoimmun. Rev. 2009;8:506–509. doi: 10.1016/j.autrev.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Marzolo MP, Bu G. Lipoprotein receptors and cholesterol in APP trafficking and proteolytic processing, implications for Alzheimer's disease. Semin. Cell Dev. Biol. 2009;20:191–200. doi: 10.1016/j.semcdb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J. Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:459–470. doi: 10.1002/mus.10191. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Glial reactions in Parkinson's disease. Mov. Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- Migliore L, Coppede F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat. Res. 2009;667:82–97. doi: 10.1016/j.mrfmmm.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Minoretti P, Gazzaruso C, Vito CD, Emanuele E, Bianchi M, Coen E, Reino M, Geroldi D. Effect of the functional toll-like receptor 4 Asp299Gly polymorphism on susceptibility to late-onset Alzheimer's disease. Neurosci. Lett. 2006;391:147–149. doi: 10.1016/j.neulet.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and nuclear factor kappa B activity in the brain during endotoxemia. J. Neurosci. 2000;20:3456–3468. doi: 10.1523/JNEUROSCI.20-09-03456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T, Sawada M. Inflammatory process in Parkinson's disease: role for cytokines. Curr. Pharm. Des. 2005;11:999–1016. doi: 10.2174/1381612053381620. [DOI] [PubMed] [Google Scholar]
- Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J. Biol. Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Neymotin A, Petri S, Calingasan NY, Wille E, Schafer P, Stewart C, Hensley K, Beal MF, Kiaei M. Lenalidomide (Revlimid) administration at symptom onset is neuroprotective in a mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2009;220:191–197. doi: 10.1016/j.expneurol.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S. Induction of proinflammatory molecules in mice with amyotrophic lateral sclerosis: no requirement for proapoptotic interleukin-1beta in neurodegeneration. Ann. Neurol. 2001;50:630–639. doi: 10.1002/ana.1256. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, D'Aigle T, Gowing G, Julien JP, Rivest S. Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2004;24:1340–1349. doi: 10.1523/JNEUROSCI.4786-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O'Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- Park JY, Kim KS, Lee SB, Ryu JS, Chung KC, Choo YK, Jou I, Kim J, Park SM. On the mechanism of internalization of alpha-synuclein into microglia: roles of ganglioside GM1 and lipid raft. J. Neurochem. 2009;110:400–411. doi: 10.1111/j.1471-4159.2009.06150.x. [DOI] [PubMed] [Google Scholar]
- Pehar M, Cassina P, Vargas MR, Castellanos R, Viera L, Beckman JS, Estevez AG, Barbeito L. Astrocytic production of nerve growth factor in motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J. Neurochem. 2004;89:464–473. doi: 10.1111/j.1471-4159.2004.02357.x. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Pocivavsek A, Burns MP, Rebeck GW. Low-density lipoprotein receptors regulate microglial inflammation through c-Jun N-terminal kinase. Glia. 2009a;57:444–453. doi: 10.1002/glia.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Mikhailenko I, Strickland DK, Rebeck GW. Microglial low-density lipoprotein receptor-related protein 1 modulates c-Jun N-terminal kinase activation. J. Neuroimmunol. 2009b;214:25–32. doi: 10.1016/j.jneuroim.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy R, Yan SF, Schmidt AM. RAGE: therapeutic target and biomarker of the inflammatory response-the evidence mounts. J. Leukoc. Biol. 2009;86:505–512. doi: 10.1189/jlb.0409230. [DOI] [PubMed] [Google Scholar]
- Raoul C, Estevez AG, Nishimune H, Cleveland DW, deLapeyriere O, Henderson CE, Haase G, Pettmann B. Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35:1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- Raoul C, Buhler E, Sadeghi C, Jacquier A, Aebischer P, Pettmann B, Henderson CE, Haase G. Chronic activation in presymptomatic amyotrophic lateral sclerosis (ALS) mice of a feedback loop involving Fas, Daxx, and FasL. Proc. Natl. Acad. Sci. USA. 2006;103:6007–6012. doi: 10.1073/pnas.0508774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J. Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reines SA, Block GA, Morris JC, Liu G, Nessly ML, Lines CR, Norman BA, Baranak CC. Rofecoxib: no effect on Alzheimer's disease in a 1-year, randomized, blinded, controlled study. Neurology. 2004;62:66–71. doi: 10.1212/wnl.62.1.66. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Kadiu I, Garg SK, Glanzer JG, Nordgren T, Ciborowski P, Banerjee R, Gendelman HE. Nitrated alpha-synuclein and microglial neuroregulatory activities. J. Neuroimmune Pharmacol. 2008;3:59–74. doi: 10.1007/s11481-008-9100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard KL, Filali M, Prefontaine P, Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1–42 and delay the cognitive decline in a mouse model of Alzheimer's disease. J. Neurosci. 2008;28:5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- Rissman RA, De Blas AL, Armstrong DM. GABA(A) receptors in aging and Alzheimer's disease. J. Neurochem. 2007;103:1285–1292. doi: 10.1111/j.1471-4159.2007.04832.x. [DOI] [PubMed] [Google Scholar]
- Roodveldt C, Christodoulou J, Dobson CM. Immunological features of alpha-synuclein in Parkinson's disease. J. Cell. Mol. Med. 2008;12:1820–1829. doi: 10.1111/j.1582-4934.2008.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M, Walter J, Gentleman SM. Interactions between APP secretases and inflammatory mediators. J. Neuroinflammation. 2008;5:25. doi: 10.1186/1742-2094-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, Esposito C, Hegarty H, Hurley W, Clauss M, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J. Biol. Chem. 1992;267:14987–14997. [PubMed] [Google Scholar]
- Schmidt AM, Sahagan B, Nelson RB, Selmer J, Rothlein R, Bell JM. The role of RAGE in amyloid-beta peptide-mediated pathology in Alzheimer's disease. Curr. Opin. Investig. Drugs. 2009;10:672–680. [PubMed] [Google Scholar]
- Simi A, Tsakiri N, Wang P, Rothwell NJ. Interleukin-1 and inflammatory neurodegeneration. Biochem. Soc. Trans. 2007;35:1122–1126. doi: 10.1042/BST0351122. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, Munch G. Advanced glycation endproducts and their receptor RAGE in Alzheimer's disease. Neurobiol. Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.04.016. Published online May 21, 2009. 10. 1016/j.neurobiolaging.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2009;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey MG, McCoy MK, Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson's disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp. Neurol. 2007;208:1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikka TM, Vartiainen NE, Goldsteins G, Oja SS, Andersen PM, Marklund SL, Koistinaho J. Minocycline prevents neurotoxicity induced by cerebrospinal fluid from patients with motor neurone disease. Brain. 2002;125:722–731. doi: 10.1093/brain/awf068. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu. Rev. Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature. 2009;458:78–82. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Talbot K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog. Neurobiol. 2008;85:94–134. doi: 10.1016/j.pneurobio.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Uranishi H, Tetsuka T, Yamashita M, Asamitsu K, Shimizu M, Itoh M, Okamoto T. Involvement of the pro-oncoprotein TLS (translocated in liposarcoma) in nuclear factor-kappa B p65-mediated transcription as a coactivator. J. Biol. Chem. 2001;276:13395–13401. doi: 10.1074/jbc.M011176200. [DOI] [PubMed] [Google Scholar]
- Urushitani M, Sik A, Sakurai T, Nukina N, Takahashi R, Julien JP. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat. Neurosci. 2006;9:108–118. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer's disease: a review. Prog. Brain Res. 2007;161:303–316. doi: 10.1016/S0079-6123(06)61021-2. [DOI] [PubMed] [Google Scholar]
- Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Pehar M, Diaz-Amarilla PJ, Beckman JS, Barbeito L. Transcriptional profile of primary astrocytes expressing ALS-linked mutant SOD1. J. Neurosci. Res. 2008;86:3515–3525. doi: 10.1002/jnr.21797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek MP, Brown CM, Colton CA. APOE genotype-specific differences in the innate immune response. Neurobiol. Aging. 2009;30:1350–1360. doi: 10.1016/j.neurobiolaging.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schuffer W, et al. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer's disease. Cell. Physiol. Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- Wang X, Arai S, Song X, Reichart D, Du K, Pascual G, Tempst P, Rosenfeld MG, Glass CK, Kurokawa R. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454:126–130. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am. J. Geriatr. Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, Lucius R. Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson's disease. FASEB J. 2003;17:500–502. doi: 10.1096/fj.02-0314fje. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat. Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Boillee S, Roberts EA, Garcia ML, McAlonis-Downes M, Mikse OR, Cleveland DW, Goldstein LS. Mutant SOD1 in cell types other than motor neurons and oligodendrocytes accelerates onset of disease in ALS mice. Proc. Natl. Acad. Sci. USA. 2008a;105:7594–7599. doi: 10.1073/pnas.0802556105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 2008b;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamin G. NMDA receptor-dependent signaling pathways that underlie amyloid beta-protein disruption of LTP in the hippocampus. J. Neurosci. Res. 2009;87:1729–1736. doi: 10.1002/jnr.21998. [DOI] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]