Abstract
Objective
To develop an in vitro strategy to support the growth of early-stage follicles and produce mature oocytes competent for fertilization.
Design
Whole ovaries from 8-day-old mice were cultured for 4 days, then secondary follicles were isolated and cultured for 12 days in a 3-dimensional (3-D) alginate or fibrin-alginate hydrogel matrix.
Setting
University-affiliated laboratory.
Animals
Mice
Intervention(s)
None
Main Outcome Measures
Histologic evaluation of follicle development, steroid hormone production, and rates of oocyte maturation, oocyte fertilization, and embryo formation.
Results
Culture of 8-day-old mouse ovaries for 4 days resulted in transition of the follicle population from primordial and primary follicles to secondary follicles, similar to that seen in a 12-day-old ovary. Isolated secondary follicles cultured for 12 days showed larger increases in oocyte diameter and more frequent antrum formation and theca cell differentiation in the FA-hydrogel matrix compared with the alginate matrix (P<0.05). Steroid hormone secretion patterns were consistent with the changes in follicle morphology and cell differentiation observed in the cultured follicles. Compared with oocytes from alginate follicle cultures, a greater number of oocytes retrieved from the FA-based follicle cultures progressed to metaphase I (MI), reached metaphase II (MII) and could be fertilized and cleaved to two-cell embryos (P<0.05). The organ culture plus FA-hydrogel follicle culture strategy produced a very high rate of oocyte progression to MII (88 ± 8.7%) and formation of 2-cell embryos (54 ± 4%).
Conclusion
A strategy combining whole ovary culture of early-stage follicles and subsequent FA hydrogel in vitro follicle culture produced a high percentage of oocytes competent for fertilization and may provide new options for fertility preservation in women and prepubertal girls facing fertility-threatening diseases or treatments.
Keywords: follicle, alginate, fibrin, 3-D, oocyte maturation, fertilization, in vitro culture
INTRODUCTION
Recent advances in ovarian tissue or oocyte cryopreservation and transplantation or in vitro follicle culture and oocyte maturation may provide options for women who are facing fertility-threatening diseases or treatments (1–2). A practical limitation to the success rate of these methods is the fact that the majority of follicles within the ovary, particularly within the cortex, are arrested at the primordial stage. Current methods limit the ability to dissect primordial follicles from ovarian tissue, leaving a significantly smaller population of secondary follicles available for collection and eventual in vitro oocyte maturation and/or in vitro fertilization (IVF). While culture of ovarian cortex tissue, with successful transition of primordial/primary follicles to secondary follicles in vitro, has been achieved in some species (mouse, bovine, baboon and human) (3–6), in vitro follicle culture methods have been successful only with secondary or later stage follicles. Collection and culture of primordial and primary follicles in vitro to produce meiotically competent, fully mature oocytes would significantly expand the number of follicles available for future use, thus increasing the statistical odds of IVF success.
Successful in vitro culture of primordial/primary follicles has been hampered by the sheer complexity of recreating the multiple signals and cell-cell/cell-stromal interactions needed to support early follicle growth and selection into the growing follicle pool. In the past decade, important advances have been made in two-dimensional (2-D) culture techniques for in vitro growth of preantral follicles (1,7–8). However, follicles cultured in these systems must attach to a flat culture surface on which somatic cells migrate away from the oocyte, thus altering the native three-dimensional structure of the follicle and disrupting the somatic cell-gamete interactions important for normal oocyte growth (9). Three-dimensional in vitro culture systems that mimic the ovary’s internal architecture appear to be optimal for supporting follicle growth and oocyte maturation. A recently developed alginate follicle culture system provides a three-dimensional (3-D) scaffold matrix for supporting the growth and maturation of multilayered secondary follicles (10–13). This system has been shown to produce meiotically competent oocytes that are able to be fertilized and produce viable offspring (14). Subsequent studies have demonstrated that the concentration of the alginate hydrogel matrix can be modified to support the growth of earlier secondary follicles (15).
Here we describe the development of a two-step method of in vitro follicle culture involving ovarian tissue culture followed by secondary follicle culture in a modified alginate matrix as described previously by our group (12–15). In this system, whole ovaries are cultured for 4 days to support early follicle growth and development. Secondary follicles recovered from the cultured ovaries are then cultured in interpenetrating fibrin-alginate (FA) beads (16) to support further follicle development and oocyte maturation. We hypothesize that the in-organ culture of primordial and primary follicles will produce a greater number of secondary follicles for subsequent in vitro culture, and that the FA gel is superior to alginate alone for producing mature oocytes competent for fertilization. The ability to culture follicles in vitro to produce mature oocytes competent for fertilization represents a major step forward in the development of fertility-sparing options for women and girls facing potentially gonadotoxic diseases or treatments.
MATERIALS AND METHODS
Animals
C57BL/6j×CBA/Ca F1 hybrid mice study were housed and bred in the Central Animal House of Northwestern University. Eight-day-old F1 female mice were used in this study. All animals were housed in a temperature- and light-controlled environment (12L:12D) and were provided with food and water ad libidum. In the current study, animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the established Institutional Animal Care and Use Committee protocol at Northwestern University.
Organ culture of 8-day-old mouse ovaries
As reported previously (17), ovaries were excised from the ovarian bursa and washed twice with culture medium: αMEM supplemented with 10 mIU/ml of recombinant FSH [A. F. Parlow, National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases], 3 mg/ml of BSA, 1 mg/ml of bovine fetuin [Sigma-Aldrich, St. Louis, MO], 5 ng/ml of insulin, 5 ng/ml of transferrin, and 5 ng/ml of selenium. Ovaries were transferred into 24-well plates with tissue culture well inserts (non-tissue culture treated, Millicell-CM, 0.4-um pore size; Millipore Corp, Billerica, MA). Approximately 400 µl of culture medium was added to the compartment below the membrane insert, such that ovaries on the membrane were covered with a thin film of medium. Up to 6 ovaries were placed in each well. The ovaries were incubated at 37°C, 5% CO2 for 4 days. Every other day, 150 µl media was replaced with fresh culture media.
Histological analysis and follicle classifications
Ovaries from 8- and 12-day-old mice were fixed overnight in a 4% paraformaldehyde solution at 4 °C and then dehydrated in an ethanol series and embedded in paraffin wax. 5-µm sections were stained with hematoxylin and eosin (H&E). The number of follicles at each developmental stage was counted and averaged in three serial sections from the largest cross-sections through the center of the ovary (17,18). Only follicles that contained an oocyte nucleus were counted. Follicles were classified as primordial (stage 0), primary (stage 1), and secondary (stage 2) as previously described (19). Follicle counting results were calculated as percentages to account for differences between pre- and post-culture ovaries.
Alginate hydrogel and fibrin-alginate (FA) gel preparation
Alginate hydrogel was prepared as described in previous reports (9,14). FA gel was prepared as described in (16). Tisseel® fibrin sealant kits from Baxter were used according to the kit instructions (Deerfield, IL). Fibrinogen and thrombin were reconstituted with aprotinin (3000 KIU/mL) solution and 40 mM calcium chloride separately. Appropriate concentrations of both solutions were attained by diluting them in Tris Buffered Saline solution (TBS). The FA gel was prepared by mixing 50 mg/mL fibrinogen solution with 0.5% alginate solution at 1:1, then adding the same volume of 50 IU/mL thrombin solution to the mixture.
Isolation, encapsulation and culture in vitro of preantral follicles
After 4 days of ovary tissue culture, secondary follicles were mechanically isolated using insulin-gauge needles and placed into L15 media (Invitrogen, Carlsbad, CA) with 1% fetal calf serum (FCS), then transferred into αMEM supplemented with 1% FCS and incubated at 37 °C, 5% CO2 for 2 h. Follicles with centrally located oocytes and at least two layers of granulosa cells were encapsulated into alginate beads (0.25% [w/v]) or FA beads (0.25% alginate, 25 mg/mL fibrinogen) using the method reported by Xu et al (15). Encapsulation in FA beads was performed as described by Shikanov et al (16). Alginate and FA beads containing follicles were washed twice in culture media. One bead was placed in each well of a 96-well plate, in 100 µl culture media and incubated at 37 °C, 5% CO2 for 12 days. Every other day, 50 µl of the media was replaced by fresh culture media and follicle survival and diameter were assessed as described previously (15). At the end of the culture period, the media was replaced by 100 µl of L15 medium containing 10 units/ml alginate lyase (Sigma-Aldrich, St. Louis, MO), and the beads were incubated for 30 min at 37 °C. Follicles were then removed from the degraded alginate bead by mechanical isolation (14,15).
In vitro maturation and fertilization of oocytes
Cumulus-enclosed oocytes (CEOs) were collected from antral follicles released from alginate or FA beads. The CEOs were placed in αMEM, 10% FCS, 1.5 IU/ml hCG, and 5 ng/ml epidermal growth factor (EGF) (Sigma-Aldrich) for 18 h at 37 °C, 5% CO2 (28).
Sperm was collected from the cauda epididymis of proven CD-1 male breeder mice using Percoll gradient centrifugation (PGC) as described by others (14). The sperm was capacitated in IVF media (KSOM; Specialty Media, Phillipsburg, NJ) containing 3 mg/ml BSA and 5.36 mM D-glucose for 30 min. Approximately 5–10 MII stage oocytes were placed in a 100 µl droplet of IVF medium containing sperm, placed under mineral oil, and incubated for 7–8 h at 37 °C, 5% CO2. Fertilized oocytes were washed three times in fresh KSOM to remove all sperm and then transferred into a 50 µl fresh KSOM microdrop under mineral oil overnight. Embryos that cleaved to the two-cell stage were recorded as fertilized (8,15).
Hormone assays
Estradiol (E2) and progesterone (P4) were measured in conditioned media collected on follicle culture days 2, 6, and 12. Conditioned media from each time point was pooled together and the average concentration at each time point was determined from three independent experiments. All measurements were carried out by electrochemoluminescent assay using an Immulite 2000 Analyzer (Roche, Indianapolis, IN) in the Endocrine Services Lab, Oregon National Primate Research Center, Oregon Health & Science University, Portland, OR. Interassay variations were 6.1% for E2 and 5.4% for P4, and the limits of sensitivity were 5 pg/ml for E2 and 0.03 ng/ml for P4.
Statistical analysis
All experiments were performed at least three times. Values are given as mean ± S.E.M. and statistical analysis was done using Student’s t-tests. Differences were considered significant at P<0.05.
RESULTS
Follicle development in organ culture
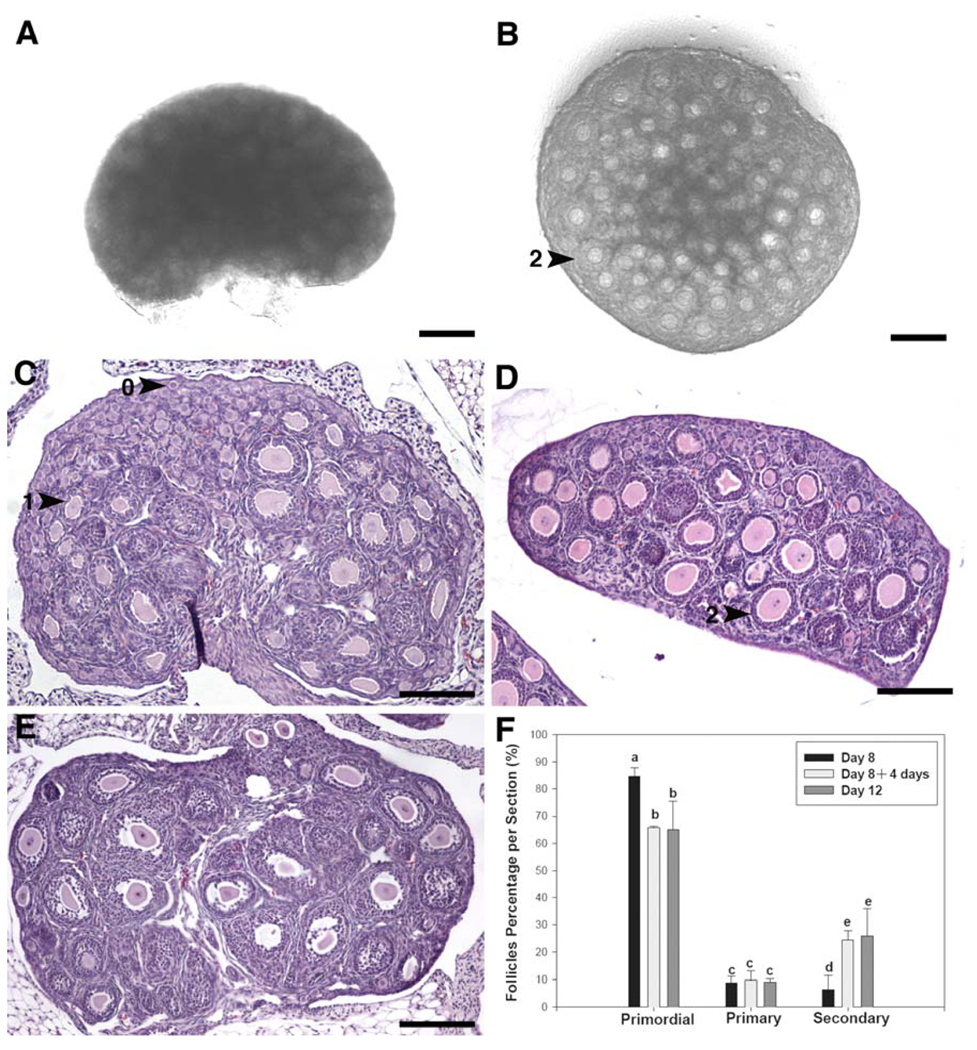
Ovaries from 8-day-old mice contained mostly primordial follicles (84.8 ± 3.2%), with a few primary (8.8 ± 2.5%) and secondary follicles (6.4 ± 5.2%) (Fig. 1A, 1C, 1F). After 4 days of organ culture in vitro (Fig. 1B, 1D), primordial follicles represented a smaller percentage of the total follicle pool (65.7 ± 0.5%), similar to the follicle distribution seen in ovaries from 12-day-old mice (65 ± 10.6%) (Fig. 1E, 1F). The proportion of secondary follicles increased significantly during the 4-day culture, from 6.4 ± 5.2% to 24.5 ± 3.3%; P<0.05 (Fig. 1A vs 1B; Fig. 1C vs 1D). The ratio of activated follicles in the cultured ovaries was similar to that of 12-day-old ovaries (25.8 ± 10%). There were no differences in the proportion of primary follicles in the 8-day-old ovaries before or after culture and in the 12-day old ovaries (Fig. 1F).
Fig. 1.
Representative photomicrographs of H&E stained paraffin sections of whole ovaries before and after culture. (A) Control, uncultured 8-day-old mouse ovary. (B) 8-day-old mouse ovary after 4 days of organ culture. (C) H&E staining of uncultured 8-day-old mouse ovary, which contains mainly primordial follicles with a few primary and secondary follicles. (D) H&E staining of 8-day-old mouse ovary after 4 days of organ culture. More secondary follicles were observed. (E) H&E staining of uncultured 12-day-old mouse ovary. (F) Follicle distribution in mouse ovaries before and after 4-day organ culture. 0 indicates primordial follicle; 1 indicates primary follicle; 2 indicates secondary follicle. The scale bars in A and B are 150 µM. Other scale bars are 200 µM. Letters indicate a statistically significant difference between groups (P<0.05).
Secondary follicle growth in the alginate and FA culture systems
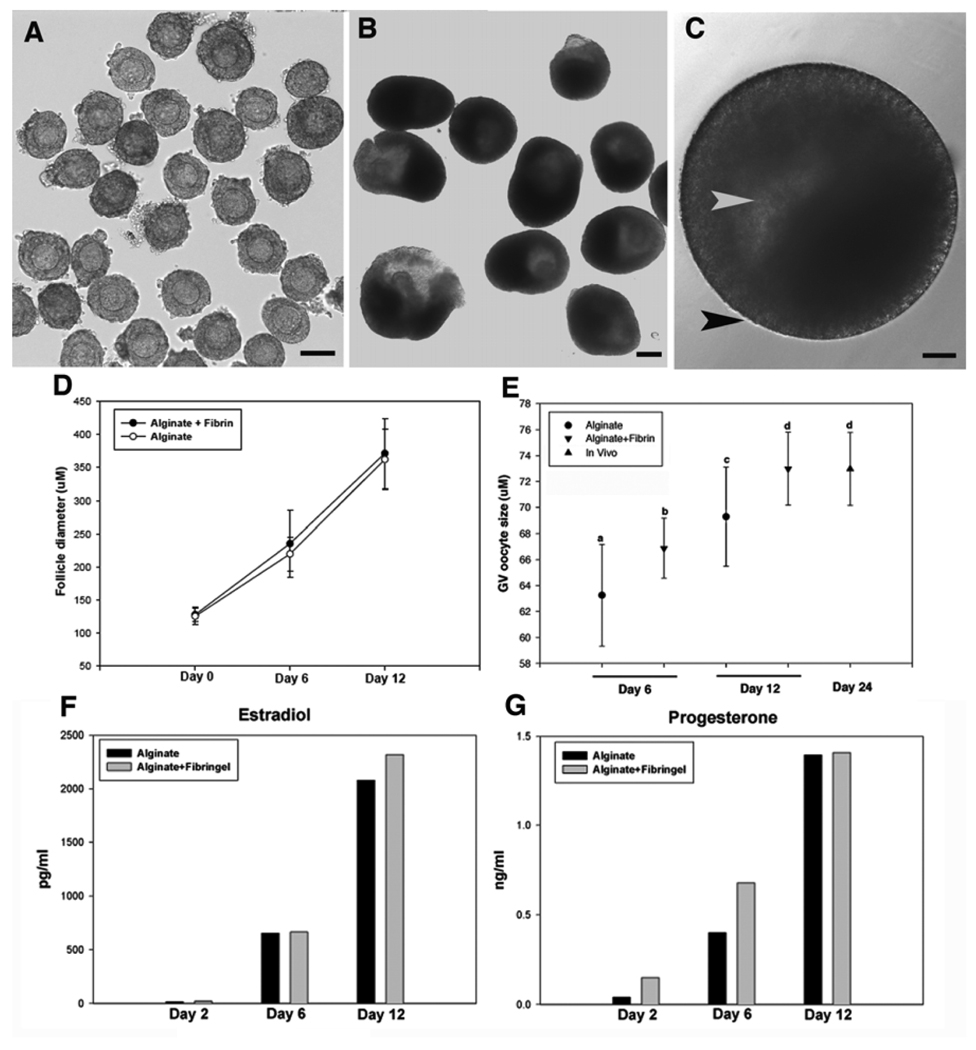
A total of 430 secondary follicles, with a diameter range of 111–137 µm, were isolated from the cultured ovaries (Fig. 2A), embedded in FA beads or alginate beads and cultured for 12 days (Fig. 2B). At day 12, the majority of follicles had survived the culture period in either FA beads (74.8 ± 4.6%) or alginate beads (68.6 ± 5.5%) (Table 1). Antrum formation and the appearance of a laminar-like theca cell layer were seen more frequently in follicles cultured in the FA system compared with follicles cultured in alginate (antrum: 72.0 ± 3.9% vs 59.7 ± 5.6%; P<0.05; theca layer: 72.3 ± 3.2% vs 64.7 ± 4.6%; P<0.05) (Fig. 2C, Table 1). Follicle diameter increased significantly, from 124 ± 2.2 µm at day 0 to 362.4 ± 10.1 µm in alginate and 371.6 ± 8.8 µm in FA (Fig. 2D). Follicle-enclosed oocytes in both groups also increased in size during the 12-day culture (Fig. 2E); however, final oocyte diameter was larger in the FA-cultured follicles compared with alginate-cultured follicles (73 ± 0.6 µm vs 69.3 ± 0.7 µm, P<0.05). By comparison, the average oocyte diameter in secondary follicles from 24-day-old mice was 73 ± 0.6 µm (Fig. 2E). As shown in Fig. 2F and 2G, the secretion patterns of E2 and P4 were consistent with observed changes in follicle morphology and cell differentiation in the cultured follicles. During the first 6 days of culture, both E2 and P4 levels rose more slowly than during the last 6 days of culture. There was no significant difference in steroid secretion between follicles cultured in alginate or FA.
Fig. 2.
Development and differentiation of representative secondary follicles cultured in vitro. (A) Secondary follicles with centrally located immature oocytes isolated from cultured ovarian tissues. (B, C) Follicles maintained their 3-D structure with proliferation of granulosa cells, antrum formation (white arrowhead), and development of theca cell layers (black arrowhead) after 12 days of culture in 0.25% alginate or AF. (D) Follicle diameter in both culture systems increased significantly during the culture period. (E) Oocyte size increased significantly over the culture period. Statistically significant differences were observed between groups as indicated with different letters (P<.05). The scale bar represents 100 µM. (F, G) Average values of E2 (F) and P4 (G) secretion were measured in conditioned culture media from secondary follicle cultures.
Table 1.
Assessment parameters of follicle and oocyte growth cultured in two different gel
| Group | Na | Survival (%) | Theca Layer (%) | Antrum (%) | nb | DG (%) | GV (%) | GVBD (%) | MIIc (%) | Two-cell embryosd (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Alginate | 230 | 68.6 ± 5.5 | 64.7 ± 4.6a | 59.7 ± 5.6b | 96 | 11.3 ± 1.3 | 13.7 ± 1.9 | 75 ± 0.6c | 61.3 ± 2.4d | 33 ± 1.7f |
| Fibrin+Alginate | 200 | 74.8 ± 4.6 | 72.3 ± 3.2aa | 72.0 ± 3.9bb | 50 | 7.7 ± 1.2 | 6 ± 0.6 | 86.3 ± 0.9cc | 88 ± 8.7dd | 54 ± 4ff |
Note: Values are the average ± SEM of multiple follicles or oocytes from at least three independent cultures; different superscripts with each column indicate statistically significant differences (p<0.05); GV, germinal vesicle; GVBD, germinal vesicle breakdown; MII, metaphase II; DG, degenerate.
N = number of secondary follicles.
n = number of CEOs from antral follicles.
The percentage of MII oocyte was calculated as a proportion of oocytes undergoing GVBD.
Two-cell embryos/MII oocytes.
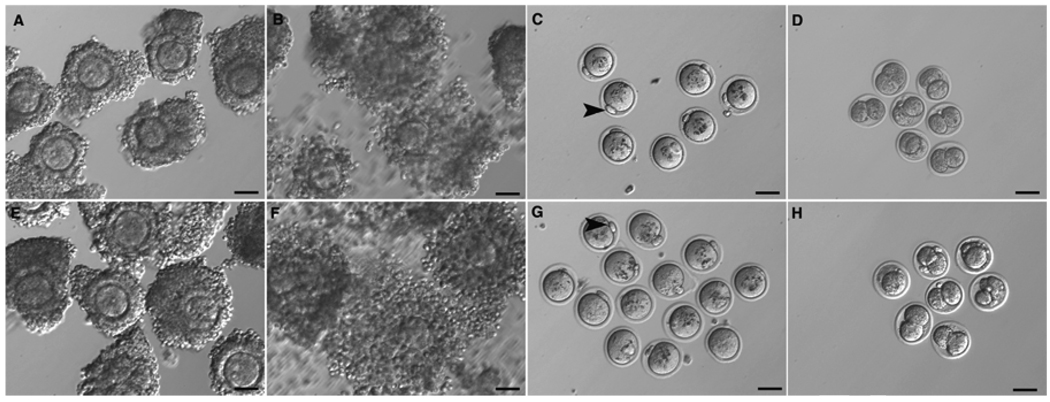
Oocyte meiosis and fertilization competence
CEOs (n=96 from alginate-cultured follicles [Fig. 3A] and n=50 from FA-cultured follicles [Fig. 3E]) were stimulated with hCG and EGF for 18 hours. After treatment, significant cumulus cell expansion was observed in both groups (Fig. 3B, 3F). Most of the oocytes in both groups resumed meiosis, underwent germinal vesicle breakdown (GVBD), and matured to MII with extrusion of a first polar body (Fig. 3C, 3G, Table 1). In the FA-cultured group, 86 ± 0.9% of the oocytes progressed to MI, compared with 75 ± 0.6% in the alginate-cultured group (P<0.05). Moreover, the percentage of oocytes that reached MII was higher in the FA-cultured group than in the alginate-cultured group (88 ± 8.7% vs 61.3 ± 2.4%, P<0.05; Table 1). In the alginate-cultured group, 33 ± 1.7% of the MII oocytes could be fertilized and cleaved to two-cell embryos, whereas in the FA-cultured group, 54 ± 4% of MII oocytes formed two-cell embryos (P<0.05; Fig. 3D vs 3H, Table 1).
Fig. 3.
Meiotic and fertilization competence of oocytes from follicles cultured for 12 days in alginate (A–D) or FA (E–H) were assessed by IVM and IVF. CEOs isolated from antral follicles retrieved from alginate (A) or FA (E) culture systems were induced with hCG for 18 h in vitro. (B, F) In both environments, cumulus cells around the oocytes expanded. (C, G) Oocytes resumed meiosis and extruded the first polar body (arrowhead). (D, H) Two-cell embryos were obtained by IVF of MII oocytes. The scale bar represents 50 µM.
DISCUSSION
In addition to providing key insights into early follicle growth and its impact on later oocyte maturation, these studies bring us one step closer to improving current in vitro follicle culture methods, which may ultimately have a clinical application in the preservation of fertility (15).
Individual early-stage follicle culture in vitro is not yet feasible, as the complete set of factors that drive progression of primordial or primary follicles toward secondary stages has not been identified. However, organ culture maintains the in vivo microenvironment of the follicles, including the surrounding stromal cells and their intercommunication with early-stage follicles, and the connectivity between cellular compartments within the follicle. The growth of primordial and primary follicles in vitro has been accomplished using organ culture (3–6), but the efficiency has been low (20–21) and it is generally accepted that organ culture alone is not able to support complete growth and development of follicles and oocytes competent for fertilization. For this reason, we chose to develop a two-step culture system that combined early follicle growth within the intact ovary with a hydrogel-based follicle culture system to support the further growth and development of secondary follicles.
Previous reports have described the growth of preantral follicles using a non-spherical (2-D) in vitro culture system. Ola et al achieved a 48% follicle survival rate and 38% antrum formation (22), and Oktem et al reported a survival rate of 47% in a standard 2-D culture system (23). Haidari et al showed a follicle survival rate of 68% and antrum formation rate of 54% (24). Here, we achieved a follicle survival rate of 68% and antrum formation rate of 59% using an alginate-based 3-D follicle culture system, and even higher rates of follicle survival (74%) and antrum formation (72%) using an FA culture system. Gomes et al showed that a flat, 2-D, adhesive environment leads to a distortion of follicle morphology, marked extracellular matrix modifications, and high rates of spontaneous follicle disruption (25). In contrast, 3-D gel environments are able to maintain follicular structure with an in vivo-like basal lamina architecture that minimizes spontaneous disruption. Oktem et al demonstrated that 3-D culture with extracellular matrix provides a better milieu for in vitro growth and survival of immature mouse preantral follicles compared with conventional 2-D culture (23).
The development of a culture system that supports follicle growth and oocyte maturation beginning at the early follicle stage may make it possible to access a significantly greater number of follicles for in vitro maturation and IVF. Eppig and O’Brien performed 8-day organ culture of ovaries isolated from newborn mice followed by 2-D culture of CEOs (3). In their optimized protocol, a GVBD rate of 62% and MII rate of 44% were achieved (3). Here, we describe a two-step protocol that combines traditional organ culture and a novel hydrogel-based 3-D follicle culture technique. Whole ovaries from 8-day-old mice, which contained primarily primordial follicles with a few primary and secondary follicles, were cultured to support early-stage follicle growth and development into the secondary follicle stage. We believe that the cultured ovary acts as an incubator, where important stroma-cell and cell-cell interactions remain intact and the presence of local paracrine and autocrine factors support primordial and primary follicle growth. Indeed, with 4-day culture of 8-day-old ovaries, we were able to achieve a similar degree of early-stage follicle development and transition to secondary follicles as in 12-day-old ovaries. In the second step, secondary follicles were isolated from the cultured ovaries and grown in alginate beads for 12 days to support further follicle development, as described previously (12–16). During this time, follicles significantly increased in mean diameter, with formation of an antral cavity and proliferation and differentiation of granulosa cells and theca cells. The mean diameter of oocytes also increased and cumulus cells expanded significantly in response to hCG. The majority of oocytes resumed meiosis and were competent to undergo GVBD and polar body extrusion, and fertilized oocytes developed to two-cell embryos. With this novel protocol, we demonstrated the ability to produce embryos starting from early-stage follicles from 8-day-old mice.
Furthermore, we found that FA hydrogel was superior to alginate with regard to follicle growth and differentiation, producing a larger percentage of oocytes competent for fertilization and a greater number of two-cell embryos than alginate alone. Studies have shown that the efficiency of producing fertilizable oocytes in vitro is influenced by many factors (26–27). Fibrin is naturally derived, and commercial fibrin consists of thrombin and fibrinogen that is cryoprecipitated from blood plasma, as well as small amounts of fibronectin, transforming growth factor-1 (TGF-1), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF) and other biomolecules (28). Some of these factors play an important role in follicle development (29), and fibrin itself supports a number of cellular processes, including growth, proliferation and differentiation (28). The FA hydrogel also has unique dynamic mechanical properties, as cell-secreted proteases degrade the fibrin in the surrounding bead and remodel the local environment. Alginate is produced by brown algae and permits diffusion of hormones and other molecules from the surrounding environment (30). Thus, the combination of alginate and fibrin not only maintains the 3-D architecture of follicles, but also provides an environment that supports follicle growth.
In conclusion, the present study introduces a novel, robust, 2-step culture strategy for in vitro growth of early-stage follicles. Organ culture provided follicles with an in situ growth environment and the hydrogel-based 3-D culture scaffold promoted further development to terminally differentiated follicles. The maintenance of the follicle architecture supports the critical cellular interactions between adjacent somatic cells and between somatic and germ cells, which we believe facilitated coordinated growth and differentiation of granulosa and theca cells and the oocyte in culture. This two-step in vitro culture system opens up new possibilities for preserving the fertility of women who must undergo life-saving but potentially fertility-threatening treatments. In particular, the ability to successfully culture follicles to produce mature oocytes in vitro starting at the primordial follicle stage provides prepubertal female cancer patients with a new fertility-sparing option. For adult patients, this technique eliminates the need to wait for the menstrual cycle or to expose patients with hormone-sensitive cancers to exogenous hormones for IVF, and thus has the potential for preserving fertility in a much greater number of female cancer patients.
ACKNOWLEDGEMENTS
The authors would like to thank Tyler Wellington for sectioning all tissue analyzed in this study.
Financial support:
Supported by Oncofertility Consortium: NIH RL1-HD058295, PL1EB008542 and T90 grant 1TL1CA133837
S.Y.J has nothing to disclose.
L.L has nothing to disclose.
A.S has nothing to disclose.
L.D.S has nothing to disclose.
T.K.W has nothing to disclose.
Footnotes
Presented at a meeting:
Presented at American Society for Reproductive Medicine 64th Annual Meeting, San Francisco, California, USA, November 8-12, 2008
Capsule: A novel, 2-step strategy involving ovary culture followed by in vitro culture in a fibrin-alginate hydrogel matrix supports growth and differentiation of early-stage follicles to produce oocytes competent for fertilization.
REFERENCES
- 1.Von Wolff M, Donnez J, Hovatta O, Keros V, Maltaris T, Montag M, et al. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy--a technique in its infancy but already successful in fertility preservation. Eur J Cancer. 2009;45:1547–1553. doi: 10.1016/j.ejca.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Varghese AC, du Plessis SS, Falcone T, Agarwal A. Cryopreservation/transplantation of ovarian tissue and in vitro maturation of follicles and oocytes: challenges for fertility preservation. Reprod Biol Endocrinol. 2008;6:47. doi: 10.1186/1477-7827-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 4.Wandji SA, Srsen V, Voss AK, Eppig JJ, Fortune JE. Initiation in vitro of growth of bovine primordial follicles. Biol Reprod. 1996;55:942–948. doi: 10.1095/biolreprod55.5.942. [DOI] [PubMed] [Google Scholar]
- 5.Wandji SA, Srsen V, Nathanielsz PW, Eppig JJ, Fortune JE. Initiation of growth of baboon primordial follicles in vitro. Hum Reprod. 1997;12:1993–2001. doi: 10.1093/humrep/12.9.1993. [DOI] [PubMed] [Google Scholar]
- 6.Hovatta O, Silye R, Abir R. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12:1032–1036. doi: 10.1093/humrep/12.5.1032. [DOI] [PubMed] [Google Scholar]
- 7.Cortvrindt R, Smitz J, Steirteghem ACV. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepubertal mice in a simplified culture system. Hum Reprod. 1996;11:2656–2666. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Van der Elst J, Van den Broecke R, Dhont M. Live offspring by in vitro fertilization of oocytes from cryopreserved primordial mouse follicles after sequential in vivo transplantation and in vitro maturation. Biol Reprod. 2001;64:171–178. doi: 10.1095/biolreprod64.1.171. [DOI] [PubMed] [Google Scholar]
- 9.West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439–4448. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9:1013–1021. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 11.Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73:942–950. doi: 10.1095/biolreprod.105.042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–723. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009;103:378–386. doi: 10.1002/bit.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu M, Kreeger PK, Shea LD, et al. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75:916–923. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- 16.Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–5485. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson E, Rogers N, Skinner MK. Actions of anti-Mullerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduction. 2007;134:209–221. doi: 10.1530/REP-07-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein Inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- 19.Yang MY, Fortune JE. The capacity of primordial follicles in fetal bovine ovaries to initiate growth in vitro develops during mid-gestation and is associated with meiotic arrest of oocytes. Biol Reprod. 2008;278:1153–1161. doi: 10.1095/biolreprod.107.066688. [DOI] [PubMed] [Google Scholar]
- 20.Abir R, Roizman P, Fisch B, Nitke S, Okon E, Orvieto R, et al. Pilot study of isolated early human follicles cultured in collagen gels for 24 hours. Hum Reprod. 1999;14:1299–1301. doi: 10.1093/humrep/14.5.1299. [DOI] [PubMed] [Google Scholar]
- 21.Hovatta O. Cryopreservation and culture of human primordial and primary ovarian follicles. Mol Cell Endocrinol. 2000;169:95–97. doi: 10.1016/s0303-7207(00)00359-2. [DOI] [PubMed] [Google Scholar]
- 22.Ola SI, Ai JH, Liu JH, Wang Q, Wang ZHB, Chen DY, et al. Effects of gonadotrophins, growth hormone, and activin A on enzymatically isolated follicle growth, oocyte chromatin organization, and steroid secretion. Mol Reprod Dev. 2008;75:89–96. doi: 10.1002/mrd.20762. [DOI] [PubMed] [Google Scholar]
- 23.Oktem O, Oktay K. The role of extracellular matrix and activin-A in in vitro growth and survival of murine preantral follicles. Reprod Sci. 2007;14:358–366. doi: 10.1177/1933719107303397. [DOI] [PubMed] [Google Scholar]
- 24.Haidari K, Salehnia M, Rezazadeh VM. The effect of leukemia inhibitory factor and coculture on the in vitro maturation and ultrastructure of vitrified and nonvitrified isolated mouse preantral follicles. Fertil Steril. 2008;90:2389–2397. doi: 10.1016/j.fertnstert.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 25.Gomes JE, Correia SC, Oliveira AG, Cidadao AJ, Plancha CE. Three-dimensional environments preserve extracellular matrix compartments of ovarian follicles and increase FSH-dependent growth. Mol Reprod Dev. 1999;54:163–172. doi: 10.1002/(SICI)1098-2795(199910)54:2<163::AID-MRD8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod. 1989;41:268–276. doi: 10.1095/biolreprod41.2.268. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Sun QY. Evaluation of oocyte quality: morphological, cellular and molecular predictors. Reprod Fertil Dev. 2007;19:1–12. doi: 10.1071/rd06103. [DOI] [PubMed] [Google Scholar]
- 28.des Rieux A, Shikanov A, Shea LD. Fibrin hydrogels for non-viral vector delivery in vitro. J Control Release. 2009;136:148–154. doi: 10.1016/j.jconrel.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight GP, Glister C. TGF-β superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- 30.West ER, Shea LD, Woodruff TK. Engineering the follicle microenvironment. Semin Reprod Med. 2007;25:287–299. doi: 10.1055/s-2007-980222. [DOI] [PMC free article] [PubMed] [Google Scholar]