Abstract
Much of the cognitive decline shown by aging primates can be attributed to dysfunction of prefrontal cortex and, as shown previously, about 30% of asymmetric (excitatory) and symmetric (inhibitory) axodendritic synapses are lost from the neuropil of layer 2/3 in prefrontal area 46 with age (Peters et al., 2008). Whether there is a similar loss of inhibitory axosomatic synapses from this cortex has not been determined, but a study in primate motor cortex suggests that axosomatic synapses are not lost with age (Tigges et al., 1992). The present study is focused upon whether the remaining axon terminals forming inhibitory synapses in old monkeys hypertrophy to compensate for any age-related loss. Analysis of electron micrographs show that in layer 2/3 of area 46 in both young and old monkeys, axon terminals forming axosomatic synapses are significantly larger and contain more mitochondria than those forming axodendritic synapses and both axodendritic and axosomatic terminals become larger with age. However, while mitochondria in axodendritic terminals do not change in either size or amount with age, the mitochondria in axosomatic terminals become larger. Similarly, in terminals forming axodendritic synapses, the mean numbers of synaptic vesicle profiles is the same in young and old monkeys, whereas in terminals forming axosomatic synapses there is an increase in the numbers of synaptic vesicles with age. We also show that among these age-related changes, only the numbers of synaptic vesicles in axosomatic synapses are significantly correlated with the cognitive impairment indices displayed by the same monkeys. In summary, the data provide original evidence that axosomatic axon terminals increase in size and in their content of mitochondria and synaptic vesicles. Furthermore, based on our and previously published results, we speculate that these changes are linked to age-related cognitive decline.
Keywords: Aging, prefrontal cortex, synapses, inhibitory, axon terminals, ultrastructure
Rhesus monkeys provide an excellent model in which to study normal aging, for although they live as long as 35 years (Tigges et al., 1988), they are not subject to Alzheimer's disease. But as they age, rhesus monkeys exhibit cognitive decline, which parallels that shown by humans (e.g. Gallagher and Rapp, 1997; Herndon et al., 1997; Moore et al., 2003; Moss et al., 2007). It is possible to assess the cognitive status of monkeys using behavioral tasks that are derived and adapted from those used for humans (e.g. Bachevalier et al., 1991; Albert and Moss, 1996; Herndon et al., 1997) and then to examine their brains to determine what changes in morphology parallel the cognitive decline (e.g. Peters 2007; 2009). Much of the cognitive decline shown by primates has been attributed to dysfunction of prefrontal cortex, and age-related impairment in spatial and reversal learning tasks, as well as recognition memory tasks are considered to be a result of dysfunction of area 46 (e.g. Kojima and Goldman-Rakic, 1982; Lai et al., 1995; Fuster 1997; Moore et al., 2003; Moss et al., 1997; 2007). Earlier it was assumed that the cognitive decline was due to loss of neurons, but more recent studies show there is not a significant loss of neocortical neurons with age (e.g. Peters et al., 1994; 1998a; 1998b; Hof et al., 2000; Peters 2002), and this is also true for area 46 (e.g. Peters et al., 1994; Smith et al. 2004). However, neurons are not spared because there is a regression or loss of some dendritic branches together with a reduction in the numbers of dendritic spines (e.g., Jacobs et al., 1997; Peters et al., 1998b; Duan et al., 2003; Kabaso et al., 2009).
Since dendrites and their spines are the main recipients of axon terminals forming synapses on cortical neurons, regression or loss of these structures should result in a loss of synapses with age. Indeed, with increasing age there is an overall loss of about 30% of synapses from layer 2/3 and both asymmetric (excitatory) and symmetric (inhibitory) synapses are lost at the same rate (Peters et al., 2008). When these data are correlated with the overall cognitive impairment shown by aging monkeys, a strong inverse correlation emerges between the numerical density of asymmetric synapses in layers 2/3 and cognitive impairment, but a somewhat less strong correlation between the numerical density of symmetric synapses and cognitive impairment (Peters et al., 2008). In layer 5 the situation is different. The overall loss of synapses is only 20% and this is almost entirely due to a loss of asymmetric synapses and there is no correlation between the numerical density of synapses in layer 5 and cognitive impairment (Peters et al., 2008). These results essentially agree with those of Bertoni-Freddari et al. (2006) who examined the frontal and temporal cortices of long-tailed macaques and found the ratio of synapses to neurons in the neuropil of frontal cortex to decrease by 21% with age, although there is little change in temporal cortex.
The functional significance of a decreased numerical density of inhibitory synapses with age is unclear. A recent patch-clamp study has found that normal aging results in increased frequency of spontaneous inhibitory post-synaptic currents (IPSCs), but not miniature IPSCs, in layer 2/3 pyramidal neurons in area 46 of rhesus monkeys (Luebke et al., 2004). Because spontaneous IPSCs reflect activity of inhibitory inputs, this result suggests that loss of inhibitory synapses may be compensated by an increased activity of remaining axon terminals. It is not known, however, if the remaining inhibitory synapses become altered to compensate for any loss and to make this determination, we have compared the morphological features of symmetric axodendritic and axosomatic synapses in layer 2/3 of area 46 of young and aged rhesus monkeys. The following parameters were examined: axon terminal and mitochondrial sizes, numbers of synaptic vesicles and junctional lengths.
Experimental Procedures
Tissue specimens and processing
Tissue was taken from area 46 in the prefrontal cortex of ten rhesus monkeys (Macaca mulatta), 9 of which had been previously behaviorally tested to assess their cognitive status (Peters et al., 2008). The cognitive status is measured using a delayed nonmatching to sample task and a spatial delayed recognition memory span task. A cognitive impairment index (CII) for each monkey is derived from these measures (Peters et al., 2008). The identification number, age and CII of each monkey used in this study are shown in Table 1. Briefly, the monkeys were pre-anesthetized with ketamine (0.5mg/kg), after which sodium barbitol was administered i.v. (15 mg/kg to effect) until the monkey was deeply anesthetized and a state of areflexia attained. Each monkey was then intubated and artificially respired with a mixture of 5% CO2 and 95% O2. The chest cavity was opened and the monkey perfused intracardially with a warm solution of 1% paraformaldehyde and 1.25% glutaraldehyde in either 0.1M cacodylate or phosphate buffer at pH 7.4. The brain was then removed and one hemisphere stored in a cold solution of 2% paraformaldehyde and 2.5% glutaraldehyde in the same buffer used for the perfusion. The perfusions were carried out in full accordance with the approved Institutional Animal Care and Use Committee Regulations, and in accordance with the NIH Publication Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
Table 1.
Identification number, age and cognitive impairment index (CII) of monkeys used in this study.
| Monkey identification number | Age (years) | CII |
|---|---|---|
| AM16 (young) | 5 | No data |
| AM76 (young) | 6 | 0.08 |
| AM129 (young) | 7 | 1.87 |
| AM47 (young) | 9 | 0.51 |
| AM53 (young) | 10 | 0.32 |
| AM19 (old) | 25 | 1.98 |
| AM12 (old) | 27 | 3.31 |
| AM62 (old) | 27 | 3.81 |
| AM26 (old) | 29 | 1.05 |
| AM41 (old) | 32 | 4.51 |
Several pieces of cortex were removed from the lower bank inside the sulcus principalis of each monkey, at the level of the corpus callosum. This portion of the prefrontal cortex is area 46. The pieces of cortex were then osmicated, dehydrated, stained en bloc with uranyl acetate and embedded in araldite.
Preparation of sections and photography
The araldite embedded tissue blocks from area 46 were sectioned in a plane at right angles to the pial surface, so that the apical dendrites of pyramidal cells were sectioned along their lengths, as evidenced by examining 1μm thick sections that had been stained with toluidine blue for light microscopic examination. Thin sections were then taken, mounted on copper grids, and stained with lead citrate. The location of layer 2/3 was determined and electron micrographs were taken of random samples of neuropil and of axon terminals forming symmetric synapses with the perikarya of pyramidal cells in layer 2/3. Axoaxonic synapses were excluded from this study because they are too sparse to sample readily. All micrographs were taken at an initial magnification of × 6,000. Some of the micrographs of the neuropil used in this study were the same ones that had been used in our previous study of the effects of age on the numerical density of synapses in layer 2/3 (Peters et al., 2008). These micrographs had been printed photographically at a final magnification of ×12,500. Other micrographs of neuropil and the ones of axon terminals forming symmetric axosomatic synapses on the cell bodies of layer 2/3 pyramidal cells were scanned on an Epson Perfection V700 photo scanner using Epson scan plug-in software for Photoshop and saved as 150 dpi tif files. Prints from these scanned images were made at a magnification of ×12,500 using a Kodak Professional 9810 Digital Photo Printer.
Measurements
To measure the sizes of axon terminals forming symmetric synapses in the neuropil of layer 2/3 in area 46, the outlines of 426 such axon terminals profiles from the five young monkeys and 323 profiles from the five old monkeys were traced from the ×12,500 photographic prints onto acetate sheets. The outlines of the mitochondria within these terminals were also drawn, as well as the lengths of their synaptic junctions. Axon terminals forming symmetric axosomatic synapses with the cell bodies of pyramidal cells in layer 2/3 were similarly examined. Some 30 axon terminals from each of the monkeys were traced, so that a total of 159 terminals forming axosomatic synapses in the young monkeys and 150 terminals from the old monkeys were traced. The numbers of sampled axon terminals forming axosomatic synapses were lower than those forming axodendritic synapses because axosomatic synapses are less frequent than axodendritic synapses. In order to test the presumption that the numbers of sampled axon terminals are sufficient for accurate and reliable statistical analysis, we carried out a post-hoc analysis of achieved power using the G*Power3 software (Faul et al., 2009). Based on the group means, standard deviations and sample sizes, the numbers of sampled axon terminals forming axodendritic synapses or axosomatic synapses are sufficient to reach a power 1-β>0.8 (axodendritic: size effect=0.3845; 1-β=0.999 and axosomatic: size effect=0.3018; 1-β=0.8415).
The tracings of axon terminals and the mitochondria they contained were scanned on an Epson Perfection V700 photo scanner using the Epson scan plug-in for Photoshop CS3 and saved as 150dip tif files. These scans were imported into ImageJ 1.40g (Wayne Rasband, National Institutes of Health) and the areas of the profiles of the terminals and their mitochondria measured and expressed in square microns. These data were copied to, and organized in Excel X for Mac (Microsoft). Ultimately analyses and graphs were constructed using Prism 4 for Macintosh (version 4.0c, GraphPad Software, Inc).
Counts of the numbers of profiles of synaptic vesicles in axon terminals, the sizes (areas) of which had been previously determined, were made in Photoshop. The images of the axon terminals were projected onto the computer screen and enlarged so that the synaptic vesicles could be seen clearly. The number of profiles of synaptic vesicles in each axon terminal profile was counted by marking the vesicles sequentially using the brush tool to superimpose a dot onto each vesicle as it was added to the count. For each monkey, the numbers of synaptic vesicles were counted in at least ten terminals forming axosomatic synapses and ten terminals forming axodendritic synapses, providing vesicle counts from 50-60 axosomatic and 50-60 axodendritic terminals in both the young and old monkeys.
Statistical differences between axodendritic and axosomatic axon terminals and between young and old monkeys were analyzed with an unpaired two-tailed Student's t-test.
Results
Morphology of synapses
Morphologically there are two types of synapses in cerebral cortex, and when brains are perfused with fixatives containing glutaraldehyde these two types are referred to as asymmetric and symmetric synapses (Colonnier, 1968). Asymmetric synapses predominate in the neuropil and it is generally agreed that they are excitatory in function (see Peters and Palay, 1996). Asymmetric synapses involve axon terminals that contain uniformly round vesicles. Their synaptic junctions have wide synaptic clefts with a prominent postsynaptic density. In layer 2/3, these synapses most commonly occur on dendritic spines (84%), with fewer on dendritic shafts (16%), and a very few on the cell bodies of nonpyramidal neurons (Peters et al., 1991).
The less common symmetric synapses are inhibitory in function and they account for only 13% of all synapses in the neuropil of layer 2/3 (Peters et al., 2008). The axon terminals forming these synapses contain somewhat smaller vesicles than terminals forming asymmetric synapses. As shown in Figure 1, the synaptic vesicles are pleomorphic, so while some of the vesicles have round profiles, others are elongate. The synaptic junctions formed by these axon terminals have narrower clefts and thinner, less prominent, postsynaptic densities than the asymmetric ones. In the neuropil of layer 2/3, about 80% of symmetric synapses are with dendritic shafts (Figure 1A), the remainder are with dendritic spines and axon initial segments. However, symmetric synapses account for the majority of axosomatic synapses (Figure 1B), since only symmetric synapses occur on the cell bodies of pyramidal neurons, while the less common nonpyramidal neurons in layer 2/3 have both symmetric and asymmetric axosomatic synapses (see Peters et al., 1991; Peters and Palay, 1996).
Figure 1.
A. Electron micrograph illustrating the morphology of an axon terminal (At) forming a symmetric synapse on the dendrite (D) of a neuron in layer 2/3 of area 46 of the cerebral cortex. The axon terminal contains pleomorphic vesicles and forms two active zones where the synaptic vesicles accumulate adjacent to the synaptic densities (arrows). B. Electron micrograph illustrating the morphology of an axon terminal (At) forming a symmetric synapse on the soma (S) of a neuron in the same cortical region. The axon terminal contains pleomorphic vesicles and forms one active zone where the synaptic vesicles accumulate adjacent to the synaptic densities (arrow). Note that the relative area covered by mitochondria is larger in the axon terminal forming the axosomatic compared to axodendritic synapse. Scale bar=1μm.
Sizes of axon terminals
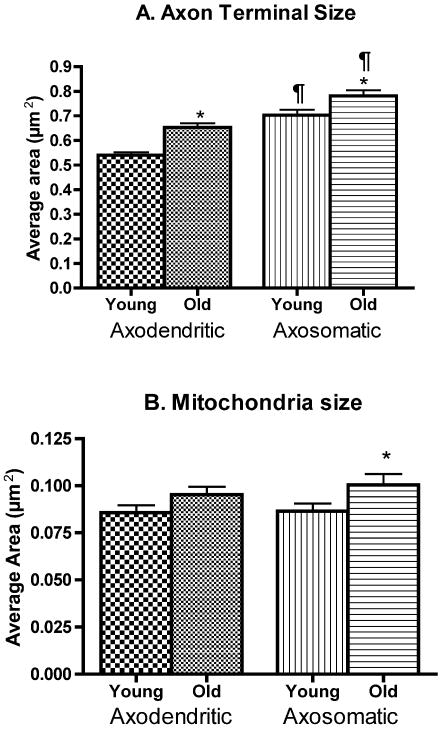
The mean size (±SEM) of axon terminals forming axodendritic synapses in the neuropil of layer 2/3 is significantly larger in old monkeys than in young ones (Figure 2A and Table 2). On average, axodendritic axon terminals are 21% larger in old than in young monkeys. The axon terminals forming axosomatic synapses are slightly, but significantly, larger than those forming axodendritic synapses, and again, axosomatic terminals are significantly larger in old monkeys than in young ones (Figure 2A and Table 2). On average, axosomatic axon terminals in old monkeys are 11% larger than in young ones.
Figure 2.
A. Average sizes of axon terminal profiles forming axodendritic and axosomatic synapses in young and old rhesus monkeys. *indicates statistically significant differences between young and old (p<0.0001 and p=0.018 for axodendritic and axosomatic axon terminals respectively; two-tailed unpaired t-test). ¶indicates statistically significant differences between axodendritic and axosomatic for each age group (p<0.0001; two-tailed unpaired t-test). B. Average sizes of mitochondria profiles in axon terminals in young and old rhesus monkeys. *indicates statistically significant differences between young and old (p=0.0456; two-tailed unpaired t-test).
Table 2.
Values represent the average area occupied by axon terminal and mitochondria profiles, the average numbers of synaptic vesicle profiles per axon terminal and per unit area and the average length of symmetric synaptic differentiations. The statistical significance shown is between young and old. Results from statistical analyses between axodendritic and axosomatic terminals are shown on Figures 2-4.
| Axodendritic | Axosomatic | |||
|---|---|---|---|---|
| Young | Old | Young | Old | |
| Axon Terminal Size (μm2) | 0.540 ± 0.013 | 0.653 ± 0.017* | 0.703 ± 0.021 | 0.781 ± 0.022* |
| p<0.0001 | p=0.018 | |||
| n=426 | n=323 | n=159 | n=150 | |
| Mitochondria Size (μm2) | 0.086±0.004 | 0.095±0.004 | 0.087±0.004 | 0.100±0.006* |
| p=0.0456 | ||||
| n=208 | n=230 | n=189 | n=219 | |
| Synaptic Vesicle (number per terminal) | 54.55±2.933 | 57.50±3.048 | 48.83±2.569 | 59.88±2.598* |
| p<0.005 | ||||
| n=55 | n=60 | n=60 | n=72 | |
| Synaptic Vesicle number per μm2 | 81.60±3.079 | 73.91±3.536 | 67.39±3.292 | 66.40±2.855 |
| n=57 | n=58 | n=50 | n=60 | |
| Synaptic length (μm) | 0.308±0.120 | 0.299±0.0731 | 0.291±0.009 | 0.292±0.011 |
| n=238 | n=210 | n=91 | n=77 | |
Mitochondria
The mitochondria in all of the axon terminals examined appeared to have normal morphologies and showed none of the broken cristae that have been associated with the oxidative damage that occurs in Alzheimer's disease and in normal old human cortices (e.g Hirai et al., 2001). As shown in Figure 2B and Table 2, the mean sizes of individual mitochondria in axodendritic terminals in old and young monkeys are not significantly different (p=0.10). In contrast, mitochondria in axon terminals forming symmetric axosomatic synapses are significantly larger in old than in young monkeys. Moreover, in the two age groups, proportionally there are more mitochondria in axon terminals forming axosomatic synapses than in terminals forming axodendritic synapses, since as shown in Figure 3 in axosomatic terminals the amount of the terminal occupied by mitochondria is about 15%, while in axodendritic terminals mitochondria only occupy about 10% of axon terminal profiles. Thus, the volume occupied by mitochondria in axosomatic terminals is about 40% greater than in axodendritic terminals in both young and old monkeys.
Figure 3.
Average percent area occupied by mitochondria relative to the size of axon terminal (AT) profiles in axodendritic and axosomatic synapses of young and old monkeys. ¶indicates statistically significant differences between axodendritic and axosomatic axon terminals in both young and old monkeys (p<0.005 for young and p<0.05 for old; two-tailed unpaired t-test).
Synaptic vesicles
As shown in Figure 4A and Table 2, the mean numbers of synaptic vesicles in profiles of layer 2/3 axodendritic axon terminals are not different in young and old monkeys, and the number of synaptic vesicles per unit area of axodendritic terminal is similar in the two age groups (Figure 4B and Table 2; p=0.39).
Figure 4.
A. Average number of synaptic vesicle profiles per axon terminal (AT) and B. Average number of synaptic vesicle profiles per area of axon terminal profiles in young and old rhesus monkeys. *indicates statistically significant difference between axosomatic terminals in young and old monkeys (p<0.005; two-tailed unpaired t-test). ¶indicates statistically significant difference between axodendritic and axosomatic (p<0.005; two-tailed unpaired t-test).
For axosomatic synapses the situation is somewhat different (Figure 4A and Table 2). The mean number of synaptic vesicle profiles per axon terminal profile is significantly higher in old monkeys than in young ones. But interestingly, the numbers of synaptic vesicle profiles per unit area of axon terminal forming axosomatic synapses are not significantly different in young and old monkeys (Figure 4B), because these terminals become larger with age (see Figure 2).
When comparing axodendritic and axosomatic synapses, the numbers of synaptic vesicle profiles per unit area of axon terminal are lower in axosomatic compared to axodendritic synapses. However, these differences reach significance in young monkeys only (Figure 4B).
Lengths of synaptic junctions
In our earlier publication (Peters et al., 2008) it was shown that the mean length of the symmetric axodendritic junctions is 0.29μm and does not change with age. In the present study we have also measured the lengths of axosomatic symmetric junctions, and similarly, there is no significant change in the average length of these junctions with age (Table 2).
Correlations with cognitive status
Using the cognitive impairment index (CII) of the monkeys (see Table 1), we conducted a number of linear regression analyses between axon terminal, mitochondria sizes and numbers of synaptic vesicles. The only significant correlation is between the CII and the mean number of synaptic vesicles in axon terminals forming axosomatic synapses (Figure 5A). In contrast, the numbers of synaptic vesicles in axodendritic synapses are not correlated with the CII (Figure 5B).
Figure 5.
Linear regression analyses were carried out between the mean numbers of synaptic vesicles in axon terminals forming axosomatic synapses (A) or axodendritic synapses (B) and the cognitive impairment indices of monkeys. Results show that the severity of cognitive impairment is significantly correlated with the number of synaptic vesicles in axon terminals forming axosomatic but not axodendritic synapses (r= Pearson Correlation Coefficient).
Discussion
In summary, axon terminals forming axosomatic synapses are larger than axodendritic ones, and both sets of terminals increase in size with age. However, in profiles of axodendritic terminals, the mean number of synaptic vesicles does not change with age, and neither does the number of vesicles per unit area of the terminal profiles. For axosomatic terminals the situation is somewhat different, since with age the number of synaptic vesicles in the profiles of these terminals increases by about 20%. Furthermore, these increases are correlated with the cognitive impairment indices of the same monkeys. However, the numbers of vesicles per unit area of the terminal profiles does not change significantly with age, due to the increase in size of the terminals. Also, the synaptic junctions are similar in length at axodendritic and axosomatic synapses and there is no change in the lengths of the junctions with age in either set of terminals. The other component of axon terminals is the mitochondria, which provide the energy for sustained neurotransmitter secretion. The mean size of mitochondrial profiles does not change with age in axodendritic terminals, but in axosomatic terminals, there is a significant increase in mitochondria volume of about 14% with age.
The difference in axon terminal and mitochondria size between axodendritic and axosomatic synapses in young monkeys is consistent with similar observations reported for symmetric synapses in the adult rat hippocampus (Miles et al., 1996) and in the rat substantia nigra, pars reticulata (von Krosigk et al., 1992). This suggests that these differences are widespread throughout the brain and occur in different species, differences that may be linked to a higher metabolism of axosomatic compared to axodendritic terminals. The finding that the size of axon terminals and mitochrondria remains larger in old monkeys in axosomatic compared to axodendritic symmetric synapses suggests that axosomatic synapses are also metabolically more active in aged animals.
For axodendritic inhibitory terminals, the only morphological change with age is a 21% increase in mean size. In our earlier study (Peters et al., 2008) it was found that there is a 22% loss of axodendritic symmetric synapses in area 46 with age. Therefore, the increase in axodendritic axon terminal size may be occurring in response to the net loss of inhibitory input to dendrites in area 46. It is possible that the loss involves only one subclass of axon terminals, since axodendritic terminals originate from a variety of inhibitory neurons such as double bouquet cells, bitufted cells, a variety of smooth multipolar cells, and neurogliaform cells (e.g. Somogyi et al., 1998), but this has not been established. The only study comparable to the present one appears to be that of Bertoni-Freddari et al. (2007) who compared the mitochondria in the neuropil of frontal and temporal cortices of adult (mean age 10.3 years) and old (mean age 21 years) long-tailed macaques. However, they examined all axon terminals in the neuropil of these cortices and found that there were no significant differences with age in the volume density (number of mitochondria per μm3 of tissue), numeric density, average volume of individual mitochondria, or average long diameter of mitochondria in axon terminals in the two cortical areas. They conclude that there is a preservation of mitochondria in axon terminals with increasing age. This conclusion is consistent with our results, since no differences in the sizes of mitochondria or ratio of mitochondria area per axon terminal area were found for axodendritic synapses.
Compared to axodendritic terminals, axosomatic terminals forming symmetric synapses are more affected by age. Axosomatic terminals not only increase in size, but they also show an increase in the mean number of synaptic vesicles and in the sizes of individual mitochondria. Presumably this reflects the interaction between these two structural elements, since mitochondria are involved in providing energy for the cycling of synaptic vesicles and for neurotransmitter synthesis and uptake in synaptic vesicles. The age-related increase in size of axosomatic terminals could occur in response to the loss of other axosomatic terminals, but as far as can be ascertained there is no information about whether axosomatic terminals in prefrontal cortex are lost with age, or even about the number of axosomatic terminals present on layer 2/3 pyramidal cells. The only study of axosomatic terminals and the effects of age appears to be that of Tigges et al. (1992), who examined Betz cells in motor cortex of the rhesus monkey. They concluded that the total number of axosomatic terminals does not change with age and found no change in their sizes or in their mitochondrial content. Our results appear to conflict with this previous report, although it is possible that aging affects axosomatic inhibitory synapses in the motor cortex and in area 46 differently.
The main source of axosomatic synapses on pyramidal cells is the fast-spiking parvalbumin-positive basket cell of the cortex (e.g. Somogyi et al., 1998). It is possible that the changes affecting axosomatic synapses with age are part of a homeostatic mechanism aimed at maintaining the high-energy needs involved in neurotransmitter release by fast-spiking neurons. Interestingly, it has been recently shown that, in area 46 of old monkeys, the frequency of spontaneous but not miniature IPSCs recorded in pyramidal neurons is increased (Luebke et al., 2004). Our finding that aging results in an increased size of axosomatic terminals, mitochondria area and synaptic vesicle numbers suggests that basket cells providing axosomatic inhibition become more active with age, an effect that could explain the increased frequency of spontaneous IPSCs (Luebke et al., 2004). On the other hand, the lack of effect of aging on the frequency or amplitude of miniature IPSCs (Luebke et al., 2004) suggests that the increased number of synaptic vesicles per terminal reported here may not be paralleled by an increased loading of inhibitory neurotransmitter per synaptic vesicle and frequency and amplitude of quantal neurotransmitter release.
Axosomatic inhibition by parvalbumin-expressing GABAergic axon terminals on pyramidal neurons is critically involved in the generation and temporal organization of gamma and theta cortical oscillatory rhythms, which are indispensable for cortical processing (Freund, 2003; Freund and Katona, 2007). Axosomatic synapses are thought to be especially important in controlling the output of pyramidal neurons, as well as synchronizing the activity of groups of pyramidal cells (e.g. Freund, 2003). In mice, it was recently found that parvalbumin-positive neurons modulate gamma-frequency and signal transmission in neocortex by reducing noise and amplifying circuit signals (Sohal et al., 2009). Therefore, the increased size and, presumably, increased metabolic activity of axosomatic axon terminals in aging could have a critical impact on the actions of fast-spiking neurons and their modulation of gamma oscillatory rhythms. Furthermore, the finding that the numbers of synaptic vesicles in axon terminals forming axosomatic, but not axodendritic, synapses are correlated with impaired cognitive performance suggests that altered axosomatic inhibition with age has a detrimental effect on the function of neocortical circuits. Interestingly, studies in aged humans show that reduced cognitive function, as assessed in the auditory oddball paradigm, is associated with increased gamma synchrony in frontal regions (Paul et al., 2005). In addition, recent evidence indicates that compared to children or young adults, aged adults have increased gamma synchrony during a visual simple choice-reaction task (Werkle-Bergner et al., 2009). Based on our and these previous findings, it is tempting to speculate that enhanced axosomatic GABAergic inhibition with age would increase gamma synchrony and decrease cognitive function.
In conclusion, the age-related increases in axon terminal size, mitochondria size and synaptic vesicle numbers in axosomatic terminals suggest an enhanced axosomatic GABAergic input to pyramidal neurons in the aged cerebral cortex and this may represent one neuronal mechanism contributing to decreased cognitive performance and, possibly, increased gamma synchrony detected in older individuals. In this context, it would be of interest to determine if similar morphological alterations of axosomatic terminals can be detected in cortical areas involved in sensory processing and if these correlate with specific cognitive alterations.
Acknowledgments
Supported by NIH/NIA grant P01 AG.000001 (A. Peters).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert M, Moss MB. Neuropsychology of aging: findings in humans and monkeys. In: Schneider E, Rowe AH, Morris JH, editors. Handbook of the Biology of Aging. 4th. San Francisco: Academic Press; 1996. pp. 217–233. [Google Scholar]
- Bachevalier J, Landis LS, Walker LC, Brickso M, Mishkin M, Price DL. Aging monkeys exhibit behavioral deficits indicative of widespred cerebral dysfunction. Neurobiol Aging. 1991;12:99–111. doi: 10.1016/0197-4580(91)90048-o. [DOI] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Fattoretti P, Giogetti B, Grossi Y, Balietti M, Casoli T, Di Stafano G, Perretta G. Synatic pathology in the brain cortex of old monkeys as an early alteration in senile plaque formation. Rejuvination Res. 2006;9:85–88. doi: 10.1089/rej.2006.9.85. [DOI] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Fattoretti P, Giorgetti B, Grossi Y, Balietti M, Casoli T, Di Stefano G, Perretta G. Preservation of mitochondrial volume. Homeostasis at the early stages of age-related synaptic deterioration. Ann NY Acad Sci. 2007;1096:138–146. doi: 10.1196/annals.1397.079. [DOI] [PubMed] [Google Scholar]
- Colonnier M. Synaptic patterns on different cell types in the different laminae of the cat visual cortex. Brain Res. 1968;9:268–287. doi: 10.1016/0006-8993(68)90234-5. [DOI] [PubMed] [Google Scholar]
- Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cerebral Cortex. 2003;19:950–961. doi: 10.1093/cercor/13.9.950. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Freund TF. Interneuron diversity series: Rhythm and mood in perisomatic inhibition. Trends in Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56(1):33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. 3rd. New York: Lippincot-Raven; 1997. [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Ann Rev Psychology. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PLR, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon J, Moss MB, Killiany RJ, Rosene DL. Patterns of cognitive decline in early, advanced and oldest of the old aged rhesus monkeys. Behav Res. 1997;87:25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- Hof PR, Nimchinsky EA, Young WG, Morrison JH. Numbers of Meynert and layer IVB cells in area V1: a stereologic analysis in young and aged macaque monkeys. J Comp Neurol. 2000;420:113–126. doi: 10.1002/(sici)1096-9861(20000424)420:1<113::aid-cne8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Driscoll I, Schall M. Age related dendritic and spine changes in area 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol. 1997;386:661–680. [PubMed] [Google Scholar]
- Kabaso D, Coskren PJ, Henry BL, Hof PR, Wearne S. The electrotonic structure of pyramidal neurons contributing to prefrontal cortical circuits in macaque monkeys is significantly altered in aging. Cerebral Cortex. 2009;19:2248–2268. doi: 10.1093/cercor/bhn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Goldman-Rakic P. Delay-related activity of prefrontal neurons in rhesus monkeys performing delayed response tasks. Brain Res. 1982;248:43–49. doi: 10.1016/0006-8993(82)91145-3. [DOI] [PubMed] [Google Scholar]
- Lai Z, Moss MB, Rosene DL, Herndon J, Killiany R. Executive system dysfunction in aged monkeys: spatial and object reversal learning. Neurobiol Aging. 1995;16:947–954. doi: 10.1016/0197-4580(95)02014-4. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Chang YM, Moore TL, Rosene DL. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2004;125(1):277–88. doi: 10.1016/j.neuroscience.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Miles R, Tóth K, Gulyás AI, Hájos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16(4):815–23. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Impairment in abstraction and set shifting in aged rhesus monkeys. Neurobiol Aging. 2003;24:125–134. doi: 10.1016/s0197-4580(02)00054-4. [DOI] [PubMed] [Google Scholar]
- Moss MB, Killiany RJ, Lai C, Rosene DL, Herndon JG. Recognition memory span in rhesus monkeys of advanced age. Neurobiol Aging. 1997;18:13–19. doi: 10.1016/s0197-4580(96)00211-4. [DOI] [PubMed] [Google Scholar]
- Moss MB, Moore TL, Schettler SP, Killiany R, Rosene DL. Successful versus unsuccessful aging in the rhesus monkey. In: Riddle DR, editor. Brain Aging Models, Methods and Mechanisms. Baton Rouge: CRC Press; 2007. pp. 21–38. [PubMed] [Google Scholar]
- Paul RH, Clark CR, Lawrence J, Goldberg E, Williams LM, Cooper N, Cohen RA, Brickman AM, Gordon E. Age-dependent change in executive function and gamma 40 Hz phase synchrony. J Integr Neurosci. 2005;4(1):63–76. doi: 10.1142/s0219635205000690. [DOI] [PubMed] [Google Scholar]
- Peters A. Structural changes in the normally aging cerebral cortex of primates. Progr Brain Res. 2002;136:455–465. doi: 10.1016/s0079-6123(02)36038-2. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on nerve fibers and neuroglia in the central nervous system. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Baton Rouge: CRC Press; 2007. pp. 97–125. [PubMed] [Google Scholar]
- Peters A. Degeneration and regeneration of myelin in the central nervous system of the aging monkey. In: Ribak CE, de la Hoz CA, Jones EG, Lariva Sahd JA, Swanson L, editors. From Development to Degeneration and Regeneration of the Nervous System. Oxford University Press; New York: 2009. pp. 145–169. [Google Scholar]
- Peters A, Leahu D, Moss MB, McNally KJ. The effects of aging on area 46 of the frontal cortex of the rhesus monkey. Cerebr Cortex. 1994;4:621–635. doi: 10.1093/cercor/4.6.621. [DOI] [PubMed] [Google Scholar]
- Peters A, Morrison JH, Rosene DL, Hyman BT. Are neurons lost from the primate cerebral cortex during normal aging? Cerebral Cortex. 1998a;8:295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL. The morphology of synapses. J Neurocytol. 1996;25:687–700. doi: 10.1007/BF02284835. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HdeF. The fine structure of the nervous system Neurons and their supporting cells. New York: Oxford University Press; 1991. [Google Scholar]
- Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cerebral Cortex. 1998b;8:671–684. doi: 10.1093/cercor/8.8.671. [DOI] [PubMed] [Google Scholar]
- Smith DE, Rapp PR, McKay HM, Roberts JA, Tuszynski MH. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J Neurosci. 2004;24:4373–4381. doi: 10.1523/JNEUROSCI.4289-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organization in the cerebral cortex. Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Tigges J, Gordon TP, Hall HM, Peters A. Survival rate and life span of the rhesus monkey. Am J Primatol. 1988;15:263–272. doi: 10.1002/ajp.1350150308. [DOI] [PubMed] [Google Scholar]
- Tigges J, Herndon JG, Peters A. Axon terminals on Betz cell somata of area 4 in rhesus monkey throughout adulthood. Anat Rec. 1992;232:305–315. doi: 10.1002/ar.1092320216. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Smith Y, Bolam JP, Smith AD. Synaptic organization of GABAergic inputs from the striatum and the globus pallidus onto neurons in the substantia nigra and retrorubral field which project to the medullary reticular formation. Neuroscience. 1992;50(3):531–49. doi: 10.1016/0306-4522(92)90445-8. [DOI] [PubMed] [Google Scholar]
- Werkle-Bergner M, Shing YL, Müller V, Li SC, Lindenberger U. EEG gamma-band synchronization in visual coding from childhood to old age: evidence from evoked power and inter-trial phase locking. Clin Neurophysiol. 2009;120(7):1291–302. doi: 10.1016/j.clinph.2009.04.012. [DOI] [PubMed] [Google Scholar]