Abstract
The initiation and progression of several forms of retinal degenerations involve excessive, repetitive, and/or sustained oxidative stress that, in turn, mediate photoreceptor cell damage and death. Since phosphatidylinositol 3-kinase (PI3K)/Akt and mTOR/p70S6-kinase pathways are part of survival signaling in cells confronted with oxidative stress, we asked whether or not docosahexaenoic acid-derived neuroprotectin D1 (NPD1) mediates survival upon single-dose and/or repetitive oxidative stress through this pathway. For this purpose, we used human retinal pigment epithelial (ARPE-19) cells challenged by exposure to hydrogen peroxide (H2O2) plus tumor necrosis factor alpha (TNF-α). We found that in single-dose oxidative stress-induced apoptosis, phosphorylation of Akt, mTOR, and p70S6K was both time- and dose-dependent. Inhibition of PI3K or mTOR/p70S6K by wortmannin and rapamycin, respectively, increased apoptosis and inhibited phosphorylation of Akt and p70S6K induced by single-dose oxidative stress. While two exposures of a low-dose, non-damaging oxidation induced apoptosis and upregulation of Akt, mTOR, and p70S6K, longer treatment of the cells with three exposures of low dose to low-dose stress showed no changes in the levels of Akt, mTOR, or p70S6K, and resulted in enhanced apoptosis compared to higher doses. Removing the oxidative stress-inducing agents following the single-dose or short term repetitive oxidative stress at the peak of Akt, mTOR, and p70S6K phosphorylation (i.e, 30 minutes after induction) led to recovery, with no apoptosis after 16 hours of incubation. Cells that were induced with three low doses of stress did not show recovery when oxidative stress was removed 30 minutes after the last exposure. NPD1 protected the RPE cells against both single-dose and repetitive oxidative stress-induced apoptosis and promoted higher levels of phosphorylated Akt, mTOR, and p70S6K. Together, our results show that a) repetitive oxidative stress is dose dependent and may not be recovered by removing the oxidative stress-inducing agents, b) PI3K/Akt and mTOR/p70S6K pathways play a major role in the protection against oxidative stress-induced apoptosis in ARPE-19 cells, and c) NPD1 exerts protection under these conditions by inducing PI3K/Akt and mTOR/p70S6K pathways.
Keywords: phosphorylation, neurodegenerative diseases, photoreceptor cells, docosahexaenoic acid, retinal degenerations
1. Introduction
Excessive, repetitive and/or sustained oxidative stress is involved in the initiation and progression of several forms of retinal degenerations, as well as other neurodegenerations. In age-related macular degeneration (AMD), retinal pigment epithelial (RPE) cells confronted with excessive oxidative stress contribute to photoreceptor cell damage that leads to vision impairments and blindness. Omega-3 essential fatty acids, which are highly enriched in fish oil and marine algae, are required for vision and neurological functions (Bazan, 2006, 2007). Docosahexaenoic acid (22:6, n-3, DHA), quantitatively the most abundant member of this family, is an acyl group of phospholipids present in photoreceptors, RPE cells, and other parts of the central nervous system (CNS) (Fliesler and Anderson, 1983). RPE cells, the most active phagocytes of the body, support photoreceptor function and modulate the uptake, conservation, and delivery of DHA to photoreceptors (Bazan, 2007). One particular RPE cell function includes the synthesis of the DHA-derived mediator neuroprotectin D1 (NPD1), which is induced by neurotrophins (Mukherjee et al., 2007) or generated when cells are confronted with oxidative stress (Marcheselli et al., 2003; Mukherjee et al., 2004). NPD1 is a homeostatic modulator of cell survival that down-regulates pro-inflammatory signaling during oxidative stress and, consequently, promotes RPE cell survival (Bazan, 2007) through stereoselective specific binding of NPD1 with RPE cells, suggesting specific receptors for this novel mediator (Marcheselli et al., 2010).
PI3K/Akt pathway has been previously implicated in the pathophysiology of AMD-inducing oxidative stress and has been proposed to protect RPE cells against the deleterious effects of oxidative stress (Defoe and Grindstaff, 2004; Yang et al., 2006). To further clarify whether or not phosphorylation of Akt, mTOR, and p70S6K is involved in excessive, repetitive, and/or sustained oxidative stress in RPE cells, and to determine if the mediator NPD1 targets this pathway, we used hydrogen peroxide (H2O2) plus tumor necrosis factor alpha (TNF-α) to trigger oxidative stress. H2O2 serves as a source of reactive oxygen species (ROS), and TNF-α was added because it promotes the formation of excess free radicals and impairs glutathione (GSH) production, thus resulting in lowered GSH and ultimately damaging cells (Glosli et al., 2002; Ishii et al., 1992; Witkamp and Monshouwer, 2000). We show here that oxidative stress activates PI3K/Akt and mTOR/p70S6K pathways, that blocking of these pathways increases apoptosis, and that high H2O2 concentrations plus TNF-α (400 and 600 μM) rapidly up-regulates Akt, mTOR, and p70S6K phosphorylation in a transient fashion under single-dose oxidative-stress conditions, thus increasing apoptosis. In RPE cells that received a low level of oxidative stress (200 μM H2O2/TNF-α), apoptosis was unaffected and phosphorylation of Akt, mTOR, and p70S6K occurred in a sustained manner, suggesting the importance of both the PI3K/Akt and mTOR/p70S6K pathways in RPE cell survival under oxidative stress. During repetitive oxidative stress, RPE cells that were induced with two exposures of low dose H2O2/TNF-α (200 μM) over a three-hour period of time showed a rapid increase, followed by a decrease of Akt, mTOR, and p70S6K expression and less than 50% of the cells showed apoptosis. Interestingly, the RPE cells that were induced with three exposures of H2O2/TNF-α over a six-hour period of time did not show detectable phosphorylation of Akt, mTOR, or p70S6K and more than 75% of the cells that displayed apoptotic features, indicating that the cells can not cope with repetitive oxidative stress since protective pathways are not sufficiently activated. Furthermore, NPD1 promoted RPE cell survival by inhibiting both single-dose and repetitive oxidative stress-induced apoptosis by inducing the activation of PI3K/Akt and mTOR/p70S6K pathways.
2. Materials and methods
2.1 Reagents
Monoclonal anti-actin was purchased from Sigma (St. Louis, MO). Phosphospecific Akt (Ser473), p70S6K (Thr389), and mTOR (Ser2448) antibodies, and rapamycin (mTOR inhibitor) were obtained from Cell Signaling (Carlsbad, California). HRP-conjugated anti-rabbit and anti-mouse secondary antibodies were purchased from BD Transduction Laboratories (Franklin Lakes, NJ). Wortmannin, a specific inhibitor of PI3Ks, was from Calbiochem (La Jolla, CA). NPD1 was kindly provided by Dr. Nicos A. Petasis (University of Southern California, Los Angeles, CA).
2.2 Cell culture
Human RPE (ARPE-19) cell line was obtained from American Type Culture Collection (Manassas, VA) and used at passages 25-35 in all experiments. Cultures of ARPE-19 cells were maintained at 37°C in DMEM-F12 medium supplemented with 10% fetal bovine serum (FBS) in a humidified atmosphere containing 5% CO2. For Western blot analysis, ARPE-19 cells were grown in 6-well plates from Costar (Corning, NY) to 100% confluence to mimic the monolayer properties of the RPE layer in the retina. All experiments were performed after 6- to 18-hours starvation of cell cultures in DMEM-F12 medium containing 0.5% FBS.
2.3 Single-dose and repetitive oxidative stress induction
To induce single-dose oxidative stress, ARPE-19 cells received one exposure of 200, 400, or 600 μM H2O2 plus 10 ng/ml TNF-α, and whole cell lysates were prepared at different time points up to three hours. For repetitive oxidative stress, cells were treated with 200 μM H2O2 plus 10 ng/ml TNF-α two or three times at 3-hour intervals, and whole cell lysates were prepared at different time points up to 3 hours after the last exposure. In some experiments, the oxidative stress-inducing agents were removed by disposing the medium, washing the cells with 2 ml/well tissue culture medium, and adding fresh medium to assess cell recovery.
2.4 Cell lysates and Western blot analysis
ARPE-19 cells were grown in 6-well plates for three days to get to 100% confluence, serum-starved for 18 hours to remove any stimulation from serum factors, and stimulated according to the experimental design. Cells were washed once with ice-cold phosphate buffered saline (PBS). The whole-cell lysate was prepared by scraping the cells into a 100-300 μl membrane lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 10 mM EDTA, 200 μM Na3VO4, 10 mM NaF, 5 μg/ml leupeptin, 1 mM PMSF, 10% glycerol, and 1% NP-40), and then incubated on ice for 10 minutes followed by centrifugation for 30 minutes at 14,000 rpm and 4°C. The protein content of lysates was determined using Bio-Rad protein assay. Equal amounts of cell extract protein from different conditions (10-20 μg) were loaded onto 8-16% SDS-PAGE ready-made gels from Invitrogen (Carlsbad, CA) and separated by electrophoresis according to the procedure described by the manufacturer. Proteins were then transferred to a polyvinylidene fluoride (PVDF) membrane from Invitrogen in a Novex transblot apparatus according to the manufacturer's instructions. Membranes were blocked with 5% skim milk from Bio-Rad (Hercules, CA) in phosphate-buffered saline containing 0.1% Tween 20 (PBST) for one hour at room temperature or overnight at 4°C. Membranes were incubated with primary antibody for one hour at room temperature or overnight at 4°C followed by three washes with PBST, incubation with HRP-conjugated secondary antibody for 20 minutes at room temperature, and three washes with PBST. Protein bands were visualized by electrophoresis (ECL) (Amersham, Little Chalfont Buckinghamshire, England) and autoradiography on X-OMART AR films (Kodak, Rochester, NY). The bands were scanned and quantified by a Gel Doc system using Quantity One software from Bio-Rad.
2.5 Neuroprotectin D1 treatment
ARPE-19 cells were serum starved overnight and pretreated with 50 nM NPD1 (Marcheselli et al. 2003; Mukherjee et al., 2004, 2007) for 30 minutes to protect the cells against oxidative stress, then stimulated with two or three exposures of 200 μM H2O2 plus 10 ng/ml TNF-α (repetitive stimulation) or one exposure of 200, 400, or 600 μM H2O2 plus 10 ng/ml TNF-α (single-dose stimulation). After appropriate incubation time at 37°C, the protective effects of NPD1 were investigated by Western blot, immunochemistry, or Hoechst staining.
2.6 Immunochemistry
ARPE-19 cells were cultured in 8-well chamber slides, stimulated according to the experimental design, washed once with PBS, and then fixed with methanol for six minutes at –20°C. Fixed cells were permeabilized with 0.1% Triton X-100 in PBS for five minutes. Blocking was performed with 10% bovine serum albumin (BSA) and 1% goat serum in PBS for 30 minutes at room temperature. Cells were incubated with antibody against phosphorylated Akt, mTOR, or p70S6K for one hour at room temperature. After being washed three times with 1% BSA in PBS, cells were incubated with an Alexa 546-labeled anti-mouse IgG antibody for 30 minutes at room temperature. Cells were then washed three times, mounted in fluorescent mounting medium (Vector, Burlingame, CA), and examined under a confocal microscope.
2.7 Nuclear staining with 33342 Hoechst
ARPE-19 cells were grown in 6-well plates and serum-starved for five hours before the stimulant was added. At the time points specified for each experiment, cells were fixed for 10 minutes with 100% methanol and stained for nuclei with 10 μM Hoechst 33342 (Sigma, St. Louis, MO) in PBS for 10 minutes at room temperature. The staining solution was then replaced with 2 ml fresh PBS, and cells were examined under a confocal fluorescent microscope.
3. Results
3.1 Single-dose oxidative stress induces transient phosphorylation of Akt, mTOR, and p70S6K
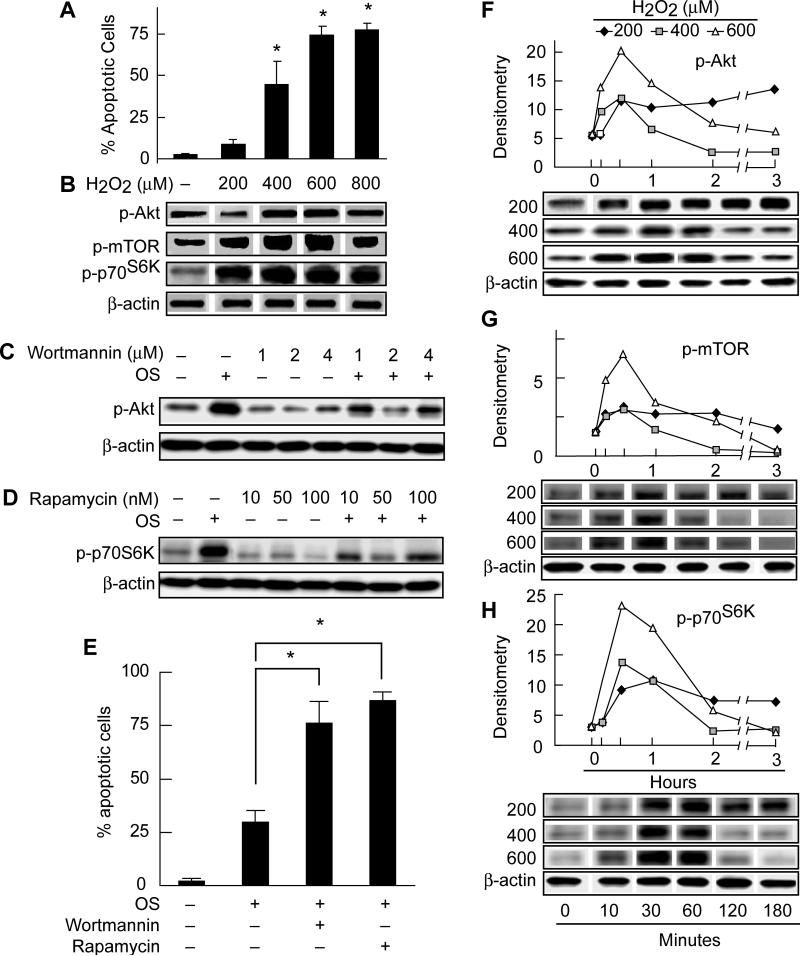
RPE cells challenged with different concentrations of H2O2 plus 10 ng/ml TNF-α showed that oxidative stress-induced apoptosis is dose dependent (Fig. 1A) and that, at lower concentrations of H2O2, RPE cells can protect themselves against apoptosis. Since both PI3K/Akt and mTOR/p70S6K pathways are involved in cell survival signaling as well as cell growth (Cantley, 2002; Wan and Helman, 2002), we studied the effect of single-dose oxidative stress on phosphorylated Akt, mTOR, and p70S6K in ARPE-19 cells exposed to H2O2 (200, 400, 600, or 800 μM) plus 10 ng/ml TNF-α for 30 minutes. The highest phosphorylation of Akt, mTOR, and p70S6K was observed with 600 μM H2O2/TNF-α (Fig. 1B). When cells were challenged by oxidative stress at 10, 30, 60, 120, and 180 minutes, the phosphorylated-Akt, -mTOR, and -p70S6K levels increased shortly after stimulation (Fig. 1F-H), and maximum levels occurred 30 minutes after challenge by H2O2/TNF-α (i.e., 200, 400 and 600 μM). This up-regulation was followed by a return to basal levels after challenge by 400 and 600 μM, but not with 200 μM of H2O2/TNF-α. We found that when we stimulated RPE cells with 200 μM H2O2/TNF-α, there was a persistent elevation of phosporylated-Akt, -mTOR, and -p70S6K at all the time points studied and no ensuing evidence of apoptosis (Fig. 1F-H). These results suggest that the persistent elevation of Akt may enhance the RPE cell's ability to resist the damaging effects of low-level oxidative stress.
Figure 1. Single-dose oxidative stress upregulates Akt, mTOR, and p70S6K phosphorylation.
In A and B, ARPE-19 cells were exposed to either 200, 400, 600, or 800 μM H2O2 plus 10 ng/ml TNF-α for 16 hr and were examined for apoptotic changes. (A) The average number of cells displaying 33342 Hoechst positive compacted nuclei in 5 different microscope fields plus SD were graphed for comparison between the conditions. * = p<0.05. (B) Western blot analysis of Akt, mTOR, and p70S6K phosphoproteins and of actin corresponding to experimental conditions in A. (C,D) Wortmannin and rapamycin are shown to block oxidative stress-induced phosphorylation of Akt and mTOR. ARPE-19 cells were serum starved overnight, pretreated with different concentrations of wortmannin (C) or rapamycin (D) for 1 hr, and stimulated with 400 μM H2O2 plus 10 ng/ml TNF-α at 37°C for 30 min. (E) ARPE-19 cells were exposed to 500 μM H2O2 plus 10 ng/ml TNF-α in the absence or after 30 min preincubation with either wortmannin (2 μM) or rapamycin (50 nM) and incubated for 16 hr. The average number of apoptotic cells in 5 different microscope fields from each condition was determined and the mean plus SD for three different experiments was graphed for comparison between different treatments. * = p<0.05 (F,G,H) ARPE-19 cells were exposed to 200, 400, or 600 μM H2O2 plus 10 ng/ml TNF-α for 10, 30, 60, 120, or 180 min. Density of Akt, mTOR and p70S6K phosphoprotein immunoreactivity bands were plotted to illustrate the time course of phosphorylation after the induction of oxidative stress.
3.2 Enhanced oxidative stress-induced apoptosis by inhibition ofPI3k/Akt and mTOR/p70S6K pathways
Since Akt and mTOR phosphorylation appear to be an early event associated with oxidative stress-induced apoptosis, and since we wanted to explore the protective effects of PI3K/Akt and mTOR/p70S6K pathways against H2O2/TNF-α-induced apoptosis in RPE cells, we pretreated ARPE-19 cells with wortmannin (a PI3K inhibitor that functions upstream of Akt) or rapamycin (mTOR inhibitor that functions upstream of mTOR/p70S6K) for 30 minutes before stimulation with 400 μM H2O2/TNF-α for 16 hour. Cells were then examined for the rate of apoptosis by Hoechst staining (Fig. 1E). Results showed that inhibition of PI3K or mTOR significantly increased the oxidative stress-induced apoptosis as compared to cells that received oxidative stress in the absence of the inhibitors.
In the same set of experiments, we also showed by Western blot analysis that pretreatment of cells with wortmannin or rapamycin abolishes oxidative stress-induced phosphorylation of Akt and mTOR, respectively (Fig. 1C & D). It is noteworthy that wortmannin and rapamycin were not cytotoxic to the cells at the concentrations used, nor did they cause the phosphorylation of Akt, mTOR, or p70S6K in the absence of oxidative stress (Fig. 1C & D). These findings suggest that PI3K and mTOR activity are essential to the survival of RPE cells during the cellular response to oxidative stress.
3.3 Effect of repetitive oxidative stress on PI3K/Akt and mTOR/p70S6K pathways
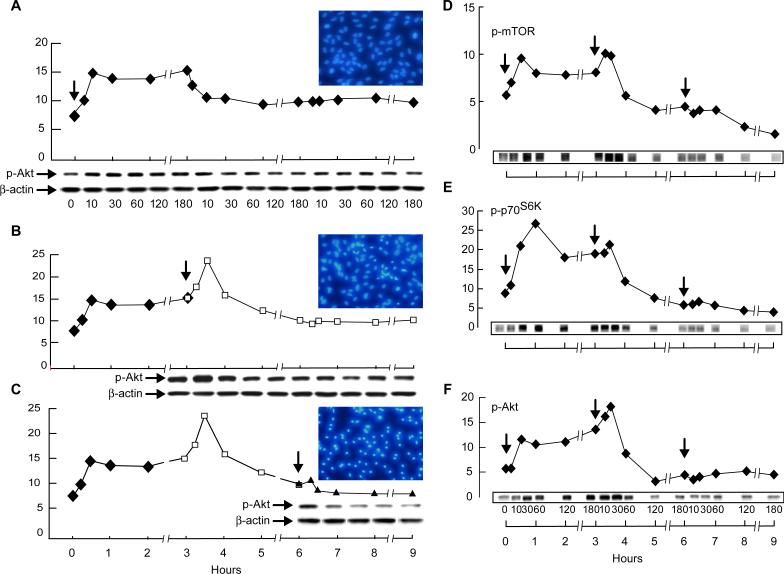
To define whether prolonged and repetitive oxidative stress alters phosphorylation of Akt, mTOR, or p70S6K, as in the case of single-dose oxidative-stress events (Bailey et al., 2004; Honda et al., 2001), we challenged ARPE-19 cells with two or three exposures of low concentrations of H2O2 (200 μM) plus TNF-α (10 ng/ml) with three-hour intervals.
Akt phosphorylation after the first, second, and third challenge to H2O2/TNF-α revealed a trend of up-regulation and subsequent down-regulation, but little pAkt expression occurred after the third challenge (Fig. 2A-C). Phosphorylation of Akt as well as mTOR and p70S6K after the second challenge to a low dose of H2O2/TNF-α yielded a comparable profile of phosphorylated Akt, mTOR, and p70S6K expression to that obtained after a single exposure of RPE cells to 400 μM H2O2/TNF-α (Figs. 1F-H & 2D-F), suggesting an additive effect from two consecutive doses of 200 μM H2O2/TNF-α. However, a third challenge to the low dose of H2O2/TNF-α did not result in a phosphorylation profile similar to that obtained with a high dose of 600 μM H2O2/TNF-α; none of the phosphorylated proteins were up-regulated (Figs. 1F-H & 2D-F), indicating that RPE cells cannot activate these pathways after undergoing prolonged, low-level oxidative stress.
Figure 2. Akt, mTOR and p70S6K phosphorylation after repetitive oxidative stress challenges.
ARPE-19 cells were challenged once, twice, or three times with 200 μM H2O2 (plus 10 ng/ml TNF-α) with 3-hr consecutive intervals, and whole cell extracts were prepared at various time points after the last exposure. Ordinate depicts densitometry values. Arrows indicate the time of addition of 200 μM H2O2 plus 10 ng/ml TNF-α: in A and D-F, three times; in B, twice; in A, once. The density of Western blot bands were plotted to illustrate the time course of phosphorylation of Akt up to 9 hr after the first exposure in each series. Hoechst staining was performed 16 hr after the last exposure (see inserts in A-C). Phosphorylation of Akt, mTOR, and p70S6K after the first, second, and third challenge of oxidative stress are illustrated in D-F.
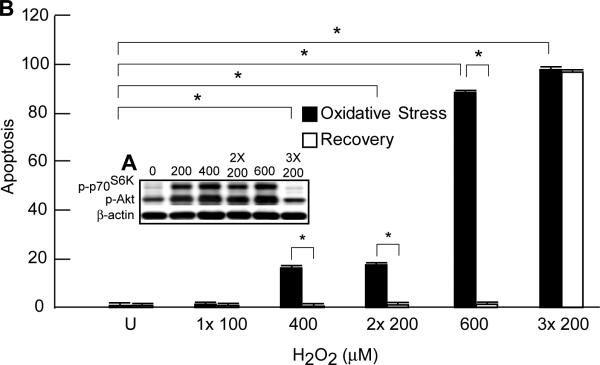
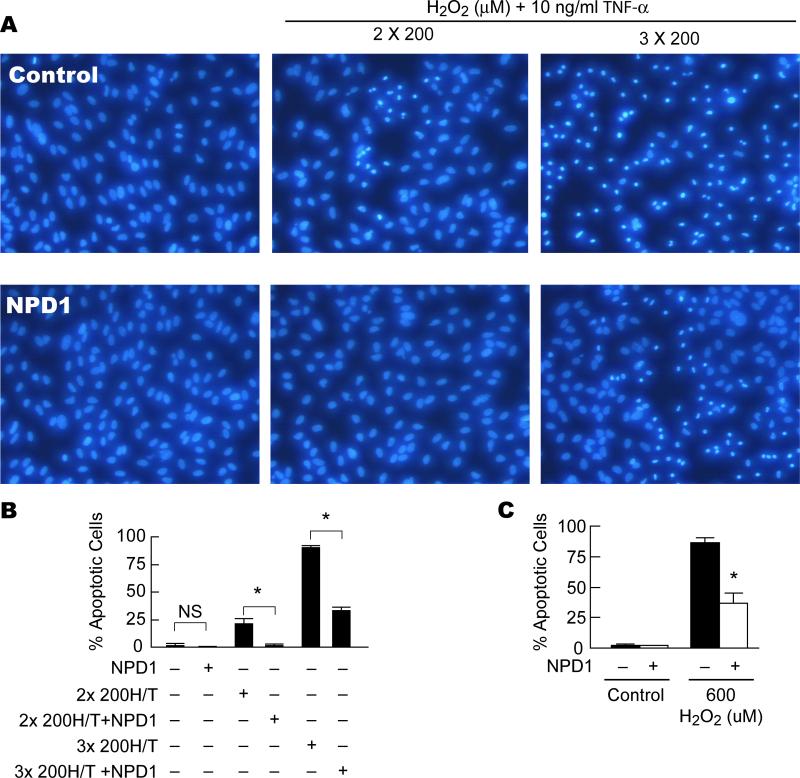
On the other hand, two challenges of ARPE-19 cells to a low dose of H2O2/TNF-α (200 μM) induced a similar degree of apoptosis as a single dose of 400 μM H2O2/TNF-α, while three exposures to the low-dose oxidative stress resulted in a higher percentage of apoptotic cells than a single exposure to 600 μM H2O2/TNF-α (Fig. 3), indicating that prolonged oxidative-stress conditions cause breakdown in the protective mechanisms, which is more damaging to RPE cells than single-dose oxidative stress.
Figure 3. Effect of sustained oxidative stress on Akt and p70S6K phosphorylation, apoptosis and recovery.
A. Western blot analysis on cell extracts 30 min after the single-dose or the last exposure of sustained oxidative stress indicates the phosphorylation of Akt and p70S6K using actin as loading control. B. ARPE-19 cells were challenged with oxidative stress (2 or 3 exposures of 200 μM H2O2 plus 10 ng/ml TNF-α with 3-hr intervals) and compared with single-dose oxidative stress (400, or 600 μM H2O2 plus 10 ng/ml TNF-α) with regard to the potential recovery of the cells from consequence of oxidative stress upon removing the oxidative stress-inducing agents. Tissue culture medium was replaced with fresh, 30 min after the last challenge to remove the oxidative stress, and the number of apoptotic cells was evaluated 16 hr later. The average number of apoptotic cells in 5 different microscope fields from each condition was determined and the mean plus SD for three different experiments was graphed for comparison between different treatments. * = p<0.05; NS = not significant
3.4 Differential effect of single-dose and repetitive oxidative stress on the recovery of RPE cells
The highest levels of Akt, mTOR, and p70S6K phosphorylation were observed 30 minutes after oxidative stress was induced by any of the different single-dose or repetitive conditions, except in long-term repetitive stimulation where cells received three exposures of oxidative stress at three-hour intervals (no phosphorylation was observed for any of the three proteins under this stimulation).
To further our understanding of how RPE cells respond to oxidative stress, we investigated the possibility that ARPE-19 cells can recover and survive if the oxidative stress is halted at different time points after single-dose oxidative stress or at peak of changes in the phosphorylation status of Akt, mTOR, and p70S6K, which was 30 minutes after the last exposure during repetitive oxidative stress. Thus, we induced single-dose oxidative stress by treating cells with one exposure of a high dose of H2O2/TNF-α (i.e., 400 or 600 μM), or induced repetitive oxidative stress through two or three exposures of 200 μM H2O2/TNF-α at three-hour intervals (10 ng/ml TNF-α for each instance). In single-dose and repetitive oxidative stress, RPE cells were either incubated for 16 hours, or the media was replaced without inducing further oxidative stress 30 minutes after treatment and then incubated for 16 hours. ARPE-19 cells treated with either 400 or 600 μM H2O2/TNF-α (single-dose oxidative stress) or two exposures of 200 μM H2O2 (short term repetitive oxidative stress) showed a complete recovery, since the percentage of apoptotic cells was comparable to untreated cells (Fig. 3). However, RPE cells exposed to three oxidative-stress events displayed enhanced apoptosis despite removal of oxidative-stress conditions. When the single-dose oxidative-stress condition were removed at later time points, recovery was achieved up to three hours after the stimulation started, but the cells could not be rescued after longer exposures of four or six hours (data not shown). Both long-term repetitive stimulation (three exposures) and single-dose oxidative stress that lasts longer than three hours are consistent with the lack or decline to the basal levels of Akt, mTOR, and p70S6K phosphorylation, respectively, and further underscore the imperative requirement for activation of PI3K/Akt and mTOR/p70S6K pathways for the survival of RPE cells that encounter an excessive oxidative-stress event.
These data indicate that while RPE cells can be rescued after short-time exposure to strong oxidative stress stimulations, longer exposures or several exposures to a weak stimulant can cause extensive apoptosis that might not be reversed by removing the oxidative stress-inducing stimuli.
3.5 NPD1-mediated protection against repetitive oxidative stress-induced apoptosis through activation of PI3K/Akt and mTOR/p70S6K pathways
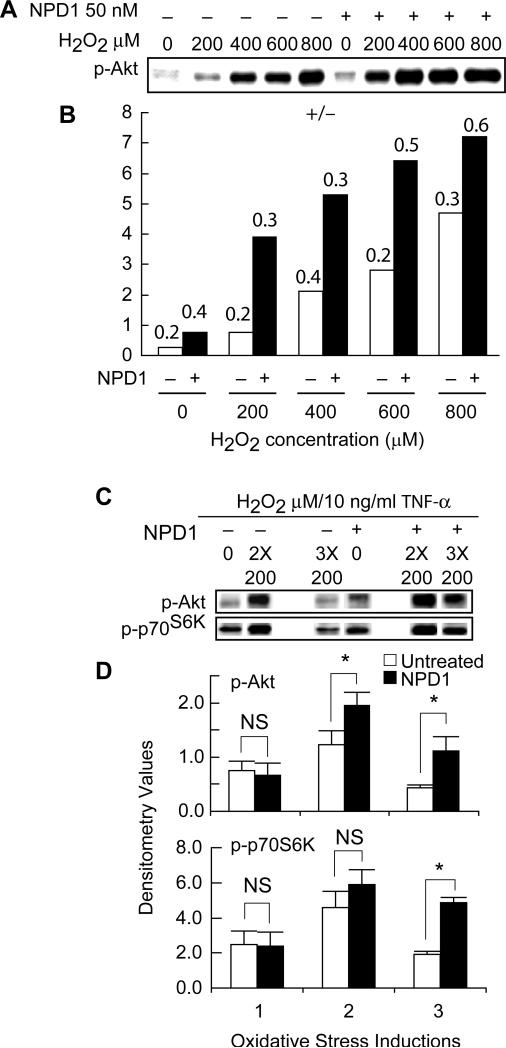
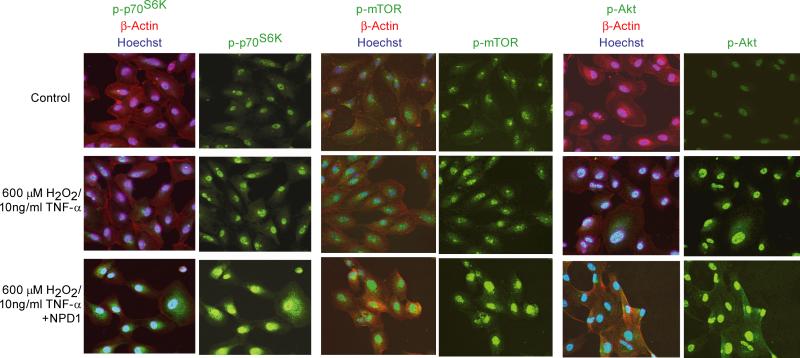
Since recovery of RPE cells from oxidative stress is correlated with their ability to up-regulate the phosphorylation of Akt, mTOR, and p70S6K, it is of interest to explore whether NPD1 increases the phosphorylation levels of these proteins. NPD1 protects RPE cells against single-dose oxidative stress-induced apoptosis (Mukherjee et al., 2004). However, AMD repetitive excessive oxidative stress involves the RPE cell (Liang and Godley, 2003). We therefore studied the protective effect of NPD1 on PI3K/Akt and mTOR/p70S6K during repetitive oxidative stress-induced apoptosis. In these experiments, RPE cells were treated with 50 nM NPD1 for 30 minutes and then stimulated with either a single dose of 600 μM H2O2/TNF-α as single-dose oxidative stress, or two or three exposures of 200 μM H2O2/TNF-α with three-hour intervals between exposures as short-term or long-term repetitive oxidative stress, respectively. NPD1 prevented apoptosis of ARPE-19 cells induced by single-dose as well as repetitive oxidative stress and significantly reduced the number of apoptotic cells (Fig. 4). We then investigated whether the inhibitory effect of NPD1 during oxidative stress is correlated with activation of cell survival signaling pathways involving PI3K/Akt and/or mTOR/ p70S6K. We examined the phosphorylation of Akt and p70S6K by Western blot analysis 30 minutes after a single exposure of different concentrations of H2O2/TNF-α or after the last exposure of a two- or three-exposure repetitive oxidative stress. We found that phosphorylation of Akt is enhanced by NPD1 for all the concentrations of H2O2/TNF-α (Fig. 5). Furthermore, during repetitive oxidative stress, both Akt and p70S6K phosphorylation were elevated in the presence of NPD1 (Fig. 5). Immunostaining of induced RPE cells confirmed the upregulation of phosphorylated-mTOR, -Akt, and -p70S6K in the presence of NPD1 (Fig. 6). These findings indicate that NPD1 protects RPE cells against both single-dose and repetitive oxidative stress by promoting the activation of Akt and p70S6K pathways.
Figure 4. Protective effect of NPD1 on chronic oxidative stress-induced apoptosis.
A. ARPE-19 cells were serum starved overnight and left untreated or stimulated with different concentrations of H2O2 plus 10 ng/ml TNF-α in the absence or presence of 50 nM NPD1 and incubated at 37°C for 30 min. B. Densitometry results of Western blot from three different experiments are shown as the average plus SD of the density of the bands. C. ARPE-19 cells were serum starved overnight, stimulated with two or three exposures of 200 μM H2O2 plus 10ng/ml TNF-α with 3-hr intervals, in the absence or presence of 50 nM NPD1, and Western blot analysis was performed on whole cell extracts prepared 30 min after the last exposure to examine the phosphorylation of Akt and p70S6K. D. Average density of the bands plus SD from three different experiments was graphed for comparison.
Figure 5. Phosphorylation of mTOR, Akt, and p70S6K were enhanced by NPD1 in single-dose oxidative stress.
ARPE -19 cells were serum starved overnight and left untreated or, stimulated with 600 μM H2O2 plus 10ng/ml TNF-α in the absence or presence of 50 nM NPD1 and incubated at 37°C for 30 min. Cells were immunostained for phosphorylated mTOR, Akt, and p70S6K (Green) and actin (Red). Nuclei were visualize by Hoechst staining (Blue). NPD1 didn't induce the phosphorylation of Akt, mTOR, and p70S6K in the absence of oxidative stress (data not shown). The experiment was repeated three times with similar results.
Figure 6. Protective effect of NPD1 on single-dose and chronic oxidative stress-induced apoptosis.
ARPE-19 cells were serum starved overnight and left untreated or, stimulated with two or three exposures of 200 μM H2O2 (chronic, A and B) or 600 μM H2O2 (single-dose, C) plus 10ng/ml TNF-α in the absence or presence of 50 nM NPD1 and incubated at 37°C for 16 hr. Nuclear staining with Hoechst 33258 was performed to examine the rate of apoptosis (A). The average number of apoptotic cells in 5 different fields of microscope plus SD were graphed for comparison (B). For the single-dose oxidative stress the average number of apoptotic cells in 5 different fields of the microscope from each condition was determined and the mean plus SD for three different experiments was graphed for comparison between different treatments (C). * = p<0.05; NS = not significant. H/T = H2O2 plus TNF-α.
4. Discussion
To define how long RPE cells can tolerate oxidative stress without undergoing apoptosis, cells received fresh tissue culture medium three hours after oxidative stress induction (the time that phosphorylated-Akt, -mTOR, and -p70S6K return to the basal levels). Cells that were stimulated with either a high dose of single-dose oxidative stress or two-exposure repetitive oxidative stress showed almost complete recovery. On the other hand, there was no recovery in RPE cells that received three-exposure repetitive oxidative stress, and no activation of Akt or mTOR pathways was observed in these cells. These results indicate that the normal cycle of protein phosphorylation (up- and down-regulation of cell survival proteins) is necessary for cell survival and recovery against oxidative stress-induced damages. The results presented here suggest that activation of PI3K/Akt and mTOR/p70S6K pathways are a part of oxidative stress-induced cell survival signaling. NPD1 inhibits RPE apoptosis and promotes cell survival by up-regulation of Akt and p70S6K phosphorylation and activation of PI3K/Akt and/or mTOR/p70S6K pathways during single-dose and sustained oxidative stress.
The retina is prone to oxidative damage due to its high oxygen consumption and its constant exposure to light (Shen et al., 2007; Sun and Nathans, 2001). Excessive formation of reactive oxygen species activate an array of intracellular signaling cascades closely associated with both cell survival and cell death pathways (Kamata and Hirata, 1999). Excessive oxidative stress in RPE cells has been implicated in the pathogenesis of AMD (Nicolas et al., 1996; Sun and Nathans, 2001). Our results indicate that single-dose and short term repetitive (two exposures of low dose H2O2/TNF-α) oxidative stress induces phosphorylation of Akt and p70S6K, indicating the activation of both PI3K/Akt and mTOR/p70S6K pathways in RPE cells. The selective PI3K inhibitor, wortmannin, and the mTOR inhibitor, rapamycin, prevented H2O2/TNF-α-induced phosphorylation of Akt and p70S6K and significantly enhanced apoptosis. By inhibiting PI3K/Akt and mTOR/p70S6k pathways, we showed that phosphorylated Akt and p70S6K have a protective role in RPE cells during oxidative-stress induced apoptosis.
Furthermore, our data shows that both single-dose oxidative stress and short-lasting repetitive oxidative stress induced by two exposures of low-dose H2O2/TNF-α caused a maximum level of phosphorylated Akt, mTOR, and p70S6K at 30 minutes after exposure, as well as a gradual return to the basal levels at 180 minutes. We also have shown that if the oxidative stress is removed in these conditions at different time points between 30-180 minutes, the rate of apoptosis is comparable to that of untreated cells. On the other hand, in a prolonged repetitive oxidative stress model using three exposures of low-dose H2O2/TNF-α, there was no increase in phosphorylation of Akt, mTOR, or p70S6K 30 minutes after the last exposure, and the removal of oxidative stress did not cause any recovery. This implicates the lack of PI3K/Akt and/or mTOR/p70S6K pathway activity/ies as the underlying mechanism of the inability of RPE cells to survive during oxidative stress. These findings further indicate that in RPE cells 1) the damage caused by oxidative stress may be reversible depending on the severity and duration of the damage, and 2) the activation of both PI3K/Akt and mTOR/p70S6K pathways are required for cell recovery. In conditions where these pathways fail to be activated, permanent and irreversible damage will ensue.
RPE cells are very active in the uptake, conservation, and delivery of DHA to photoreceptors (Bazan, 2005, 2006) and have the capacity to synthesize NPD1 (Calandria et al., 2009; Mukherjee et al., 2004; Rotstein et al., 2003). Here we showed that NPD1 protects RPE cells against single-dose or repetitive oxidative stress-induced apoptosis concomitant with enhanced phosphorylation of Akt and p70S6K. This was especially noticeable when repetitive oxidative stress was induced by three exposures of a non-damaging low concentration of H2O2 plus TNF-α; the increased apoptosis of ARPE-19 cells coincided with the failure in phosphorylation of Akt and p70S6K (Figs. 3 & 5C, D). While the removal of the repetitive oxidative stress did not rescue the cells (Fig. 3), exogenous NPD1 successfully protected the cells against apoptosis (Fig. 4) and reinstated the phosphorylation of both Akt and p70S6K (Fig. 5C, D), indicating that the activation of PI3K/Akt and/or mTOR/p70S6K pathways may be the underlying mechanism of NPD1-mediated survival of RPE cells during oxidative stress. Since NPD1 caused upregulation of pAkt in the absence or presence of oxidative stress-inducing agents, it may be concluded that NPD1 interference is upstream of PI3K and mTOR in these pathways. The response of primary cultures of RPE cells to oxidative stress and NPD1 may vary depending on the age of the donor; this requires further investigation.
Our results show that low (nanomolar) amounts of NPD1 generated are able to protect RPE cells against oxidative stress. Therefore, NPD1 also may be used therapeutically in the future to counteract excessive single-dose or repetitive oxidative stress in retinal degenerations.
Acknowledgements
This work was supported by National Institutes of Health, National Eye Institute Grant EY005121 (to N.G.B.). We thank Dr. Nicos A. Petasis (University of Southern California, Los Angeles, CA), who kindly provided us with neuroprotectin D1 for these experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ, Cheetham ME. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 2004;45:675–684. doi: 10.1167/iovs.03-0351. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–271. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Homeostatic regulation of photoreceptor cell integrity: significance of the potent mediator neuroprotectin D1 biosynthesized from docosahexaenoic acid: the Proctor Lecture. Invest. Ophthalmol. Vis. Sci. 2007;48:4866–4881. doi: 10.1167/iovs.07-0918. [DOI] [PubMed] [Google Scholar]
- Calandria JM, Marcheselli VL, Mukherjee PK, Uddin J, Winkler JW, Petasis NA, Bazan NG. Selective survival rescue in 15-lipoxygenase-1-deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J. Biol. Chem. 2009;284:17877–17882. doi: 10.1074/jbc.M109.003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Defoe DM, Grindstaff RD. Epidermal growth factor stimulation of RPE cell survival: contribution of phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways. Exp. Eye. Res. 2004;79:51–59. doi: 10.1016/j.exer.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Glosli H, Tronstad KJ, Wergedal H, Muller F, Svardal A, Aukrust P, Berge RK, Prydz H. Human TNF-alpha in transgenic mice induces differential changes in redox status and glutathione-regulating enzymes. FASEB J. 2002;16:1450–1452. doi: 10.1096/fj.01-0948fje. [DOI] [PubMed] [Google Scholar]
- Honda S, Hjelmeland LM, Handa JT. The use of hyperoxia to induce chronic mild oxidative stress in RPE cells in vitro. Mol. Vis. 2001;7:63–70. [PubMed] [Google Scholar]
- Ishii Y, Partridge CA, Del Vecchio PJ, Malik AB. Tumor necrosis factor-alpha-mediated decrease in glutathione increases the sensitivity of pulmonary vascular endothelial cells to H2O2. J. Clin. Invest. 1992;89:794–802. doi: 10.1172/JCI115658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata H, Hirata H. Redox regulation of cellular signaling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp. Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem. 2003;78:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- Marcheselli VL, Mukherjee PK, Arita M, Hong S, Antony R, Sheets K, Winkler JW, Petasis NA, Serhan CN, Bazan NG. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82:27–34. doi: 10.1016/j.plefa.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc. Natl. Acad. Sci. USA. 2007;104:13158–13163. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas MG, Fujiki K, Murayama K, Suzuki MT, Shindo N, Hotta Y, Iwata F, Fujimura T, Yoshikawa Y, Cho F, Kanai A. Studies on the mechanism of early onset macular degeneration in cynomolgus monkeys. II. Suppression of metallothionein synthesis in the retina in oxidative stress. Exp. Eye Res. 1996;62:399–408. doi: 10.1006/exer.1996.0045. [DOI] [PubMed] [Google Scholar]
- Rotstein NP, Politi LE, German OL, Girotti R. Protective effect of docosahexaenoic acid on oxidative stress-induced apoptosis of retina photoreceptors. Invest. Ophthalmol. Vis. Sci. 2003;44:2252–2259. doi: 10.1167/iovs.02-0901. [DOI] [PubMed] [Google Scholar]
- Shen JK, Dong A, Hackett SF, Bell WR, Green WR, Campochiaro PA. Oxidative damage in age-related macular degeneration. Histol. Histopathol. 2007;22:1301–1308. doi: 10.14670/HH-22.1301. [DOI] [PubMed] [Google Scholar]
- Sun H, Nathans J. ABCR, the ATP-binding cassette transporter responsible for Stargardt macular dystrophy, is an efficient target of all-trans-retinal-mediated photooxidative damage in vitro. Implications for retinal disease. J. Biol. Chem. 2001;276:11766–11774. doi: 10.1074/jbc.M010152200. [DOI] [PubMed] [Google Scholar]
- Wan X, Helman LJ. Effect of insulin-like growth factor II on protecting myoblast cells against cisplatin-induced apoptosis through p70 S6 kinase pathway. Neoplasia. 2002;4:400–408. doi: 10.1038/sj.neo.7900242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkamp R, Monshouwer M. Signal transduction in inflammatory processes, current and future therapeutic targets: a mini review. Vet. Q. 2000;22:11–16. doi: 10.1080/01652176.2000.9695016. [DOI] [PubMed] [Google Scholar]
- Yang P, Peairs JJ, Tano R, Jaffe GJ. Oxidant-mediated Akt activation in human RPE cells. Invest. Ophthalmol. Vis. Sci. 2006;47:4598–4606. doi: 10.1167/iovs.06-0140. [DOI] [PubMed] [Google Scholar]