Abstract
Antibody responses against lytic and latent KSHV antigens were investigated in patients with Kaposi sarcoma (KS), multicentric Castleman’s disease (MCD), and primary effusion lymphoma. Antibodies against the lytic antigen K8.1 were 5-fold higher in MCD than KS patients, while antibodies to the sum of latent antigens v-cyclin and LANA were 27-fold higher in KS compared to MCD patients (P< 0.0001). The sum of anti-v-cyclin and anti-LANA antibody titers discriminated patients with KS from those with MCD and KS with 93% sensitivity and 83% specificity. These results suggest that antibody responses to lytic and latent KSHV antigens differ in these diseases.
Keywords: Kaposi sarcoma (KS), multicentric Castleman’s disease (MCD) and primary effusion lymphoma, antibodies
Kaposi sarcoma–associated herpes virus (KSHV), also called human herpesvirus-8 (HHV-8), is the causative agent of all forms of Kaposi sarcoma (KS) [1, 2]. In KS, most proliferating tumor spindle cells are infected with KSHV and express KSHV proteins [1, 2]. KSHV has a 170.5 kb genome encoding approximately 90 gene products and contains a number of pirated genes involved in cell proliferation, angiogenesis, and evasion of the immune system [3]. KSHV is also the causative agent of two rare B-cell lymphoproliferative disorders, primary effusion lymphoma (PEL) and multicentric Castleman’s disease (MCD), that occur primarily in patients infected with human immunodeficiency virus (HIV). PEL is characterized by its tendency to develop in body cavities such as pleural or peritoneal spaces [4-6]. MCD is characterized clinically by fevers, wasting, hypoalbuminemia, and cytopenias that result from overproduction of viral-encoded and human-encoded cytokines, especially interleukin-6 (IL-6) [7, 8].
Like other herpesviruses, KSHV has two phases of gene expression, latent and lytic. In KS, a majority of KSHV-infected spindle cells express only latent genes, while a small percentage express lytic genes [9-11]. By contrast, a substantial percentage of MCD cells express lytic KSHV genes, including a virally-encoded IL-6. The majority of PEL cells express KSHV latent genes, but in addition can show limited expression of certain lytic genes [9-11].
Luciferase immunoprecipitation systems (LIPS) is a powerful new method to quantitatively measure antibody responses to a wide range of infectious agents. LIPS screening of a panel of KSHV antigens identified v-cyclin as a useful diagnostic antigen that along with 3 other antigens, provided high sensitivity and specificity for the differentiation between patients with KS and blood bank donor controls [12]. Given the differential expression of KSHV lytic and latent proteins in KS, PEL, and MCD, we hypothesized that different antibody profiles to KSHV antigens detected by LIPS might distinguish these diseases.
Methods
Sera were from patients or volunteers under institutional review board-approved protocols at the NIH Clinical Center, NIAID, and the NCI. Serum samples from 35 patients with KS, 14 with both MCD and KS (MCD+/KS+), 6 with MCD but no KS (MCD+/KS-), and 5 with PEL, all generally taken at the time of diagnosis, and 34 KSHV-uninfected controls were tested. The median CD4 counts for KS (270/mm3, interquartile range (IQR) 137-530), MCD+/KS+ (294/mm3, IQR 185-620) and MCD+/KS- (359/mm3, IQR 123-580) were not statistically different between the paired groups. The PEL patients had the lowest median CD4 counts (111/mm3, IQR 46-368) and were statistically lower only to the MCD+/KS+ samples.
The pREN2-KSHV antigen constructs for K8.1, ORF65, v-cyclin, and LANA have already been described [12]. The LIPS assay was performed at room temperature, and all light unit (LU) data were obtained from the average of two separate experiments and corrected for background protein A/G bead binding [12]. The GraphPad Prism software (San Diego, CA) was used for statistical analyses. Antibody titers between the uninfected controls (CTRL), MCD+/KS+, MCD+/KS- and PEL patient serum samples are reported as the geometric mean titer (GMT) ± 95% confidence interval. The non-parametric Mann-Whitney U test was used for comparison of antibody titers in different groups. P values were not corrected for multiple comparisons.
Results and discussion
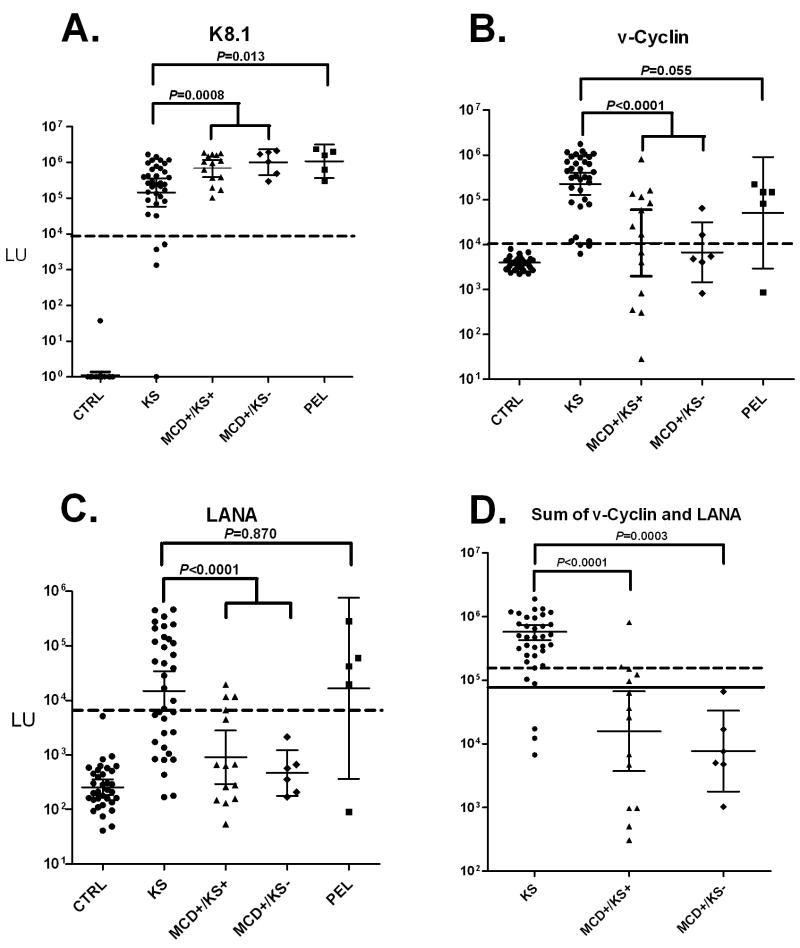
Using the LIPS assay and antigens from both the lytic and latent phase of the KSHV life cycle, we compared antibody responses among the patient cohorts. The GMT of antibodies to K8.1 early lytic antigen was 145,900 LU (95% CI; 58,490-363,700) in the 35 KS patients, 684,700 LU (95% CI: 390,100-1,202,000) in the 14 MCD/KS+ patients, 1,020,000 LU (95% CI; 437,500-2,378,000) in the 6 MCD/KS-, and 1,092,000 LU (95% CI; 366,600-3,252,000) in the 5 PEL patients (Figure 1A). Statistical analysis using the Mann Whitney U test revealed that KS patients had significantly lower anti-K8.1 antibody titers than MCD/KS+ (P = 0.009), MCD/KS- (P= 0.008) or the PEL patients (P = 0.013). Also, the difference between the anti-K8.1 antibody titers in KS versus the combined MCD/KS+ and MCD/KS- patient groups was highly significant (P= 0.0008).
Figure 1.
Anti-K8.1, anti-v-Cyclin and anti-LANA antibodies in uninfected controls, KS, MCD and PEL patients. Shown are results from 34 uninfected controls, 35 KS, 14 MCD+/KS+, 6 MCD+/KS-, and 5 PEL patients. Each symbol represents a serum sample from an individual patient. The geometric mean antibody titer and 95% CI for (A) anti-K8.1 lytic antibodies, (B) anti-v-cyclin latent antibody and (C) anti-LANA antibody titers in LU are plotted on the Y-axis using a log10 scale. The dashed line represents the cut-off level for determining seropositivity and is derived from the mean plus 5 SD of the antibody titer of the 34 uninfected controls. All the uninfected controls were negative for all KHSV antibodies and were below the established cutoff. (D) Sum of anti-v-cyclin and anti-LANA antibodies. The solid line represents the optimum cut-off (70,000 LU) for discriminating the KS from the MCD+/KS- patients, while a higher cutoff (165, 000 LU) shown by the dotted line optimally separated the KS from the MCD+/KS+ patients. All P values were calculated using the Mann Whitney U test.
The GMT of the late lytic antigen, ORF65, in the 35 KS patients was 34,380 LU (95% CI; 12,570-94,050) and was significantly lower (P < 0.006) than the GMT of 1,010,000 LU (95% CI; 317,300-3,214,000) for the PEL patients (data not shown). The MCD+/KS+ and MCD+/KS- patients showed variable antibody titers and were not significantly different from the KS patients (data not shown).
Antibody titers were also evaluated against two latent antigens, v-cyclin and LANA (ORF73). The anti-v-cyclin GMT in the KS patients was 225,900 LU (95% CI; 128,600-396,900), which was markedly higher than the GMT of 10,840 LU (95% CI: 1964-59,840) in the MCD+/KS+ patients (P= 0.0004) or 6,751 LU (95% CI; 1446-31,530) for the MCD+/KS-patients (P= 0.0006). As shown in Figure 1B, the difference between the anti-v-cyclin antibody titers in KS versus the combined MCD/KS+ and MCD/KS- patient groups was also highly significant (P < 0.0001). The GMT for the PEL patients was 51,563 LU (95% CI; 2943-903,400) which trended lower than the GMT for the KS patients (P= 0.055). Using a cutoff value based on the uninfected controls, 91.4% (32/35) of the KS, 71.4% (10/14) of the MCD+/KS+, 40% (2/5) of the MCD+/KS-, and 80% (4/5) of the PEL patients were seropositive for anti-v-cyclin antibodies.
As shown in Figure 1C, antibodies against the latent protein, LANA, in the KS patients showed a GMT of 14,940 LU (95% CI; 6520-34,250). Lower levels of anti-LANA antibodies were found in the MCD+/KS+ patients with a GMT of 907 LU (95% CI: 292-2823) and the MCD+/KS- patients with a GMT of 465 LU (95% CI; 177-1218) (Figure 1C). Significant differences were found between the anti-LANA antibodies in KS versus the MCD+/KS+ (P= 0.0007) and MCD+/KS- (P= 0.002). Additionally, the difference between the anti-LANA antibody titers in KS versus the combined MCD/KS+ and MCD/KS- patient groups was highly significant (P<0.0001). The PEL patients showed high anti-LANA antibodies, which were not significantly different from the KS patients (P= 0.87). Using a cut-off derived from the uninfected controls (Figure 1C), 63% (22/35) of KS patients were positive versus 80% (4/5) of the PEL, 21% (3/14) of the MCD+/KS+, and none (0/6) of the MCD+/KS- patients. These results suggest that the relative absence of anti-LANA antibodies is a common feature of MCD compared to patients with KS or PEL.
The titer data were also analyzed to determine whether any single antibody or antibody combination might distinguish KS from those with MCD+/KS+ and/or MCD+/KS-. In part because the anti-LANA and anti-v-cyclin antibody titers in the KS patients tracked each other poorly (rs=0.03), even greater antibody titer differences were observed in the patient groups using the sum of the anti-v-cyclin and anti-LANA antibodies. Specifically, the sum of the anti-v-cyclin and anti-LANA antibodies was 350,700 LU (95% CI; 223,700-550,000) in the 35 KS patients, 15,880 LU (95% CI: 3746-67,330) in the 14 MCD/KS+ patients, and 7,686 LU (95% CI; 1783-33,130) in the 6 MCD/KS- (Figure 1D). As shown in Figure 1D, significant differences were found between the sum of the anti-v-cyclin and anti-LANA antibodies in KS versus the MCD+/KS+ (P< 0.0001) and MCD+/KS- (P= 0.0003). Also, the difference between the sum of the antibody titers in KS versus the combined MCD/KS+ and MCD/KS- patient groups was highly significant (P< 0.0001). From receiver operator characteristics the most informative approach to optimally separate the KS and from the MCD+/KS- patients used the sum of these latent antigens with a cut-off of 70,000 LU and discriminated KS from MCD+/KS-with 100% sensitivity and 91% specificity (Figure 1D). Although this 70,000 LU cut-off was less useful in discriminating KS from MCD+/KS+ (64% sensitivity and 91% specificity), a higher cut-off of 165,000 LU showed 93% sensitivity (13/14 of the MCD+/KS+ were below the cut-off) and 83% specificity for distinguishing KS from MCD+/KS+ (Figure 1D).
By profiling antibodies against several latent and lytic HHV-8 antigens, significant differences in antibody titers were observed between the KS, MCD and PEL patients. One of the most obvious differences was that antibody titers against the early lytic HHV-8 antigen, K8.1, were markedly higher in the PEL and MCD patients compared to the KS patients. The findings of higher anti-K8.1 in MCD are consistent with studies showing that a substantial percentage of MCD cells express lytic KSHV genes, including a virally-encoded IL-6. Similarly, the higher antibody titers against the ORF65 lytic protein in PEL as compared to KS may also reflect the greater KSHV viral load and expression of lytic antigens in PEL [9, 13]. It is not clear why antibodies to another lytic antigen, ORF65, were not significantly higher in MCD than in KS. It is possible that it relates to ORF65 being a late lytic antigen. Alternatively, it is possible that this is in part because MCD patients have some subtle defects in specific antibody production, perhaps related to cytokine dysregulation that blunts what would otherwise be an increase.
By contrast to the anti-K8.1 antibody profile, markedly higher antibodies to v-cyclin, a latent KSHV gene, were found in the KS and PEL patients compared to the MCD. Anti-LANA antibodies also were also markedly higher in the KS compared to the MCD patients. Together these results are consistent with immunohistochemical studies showing that KS spindle cells express large amounts of latent HHV-8 proteins compared to MCD cells [9, 10, 14]. At present, we have no definitive explanation for why MCD+/KS+ patients have lower antibody titers to v-cyclin and LANA compared to patients with only KS. Relatively greater HIV-induced immunosuppression in the MCD patients does not seem to be the cause because the CD4 counts were similar in the KS, MCD+/KS+ and MCD+/KS- groups. As noted above, it is possible that MCD patients have a blunting of specific antibody responses to KSHV antigens, perhaps related to local cytokine dysregulation.
Since elevated anti-latent antibodies are a common feature found of KS patients compared to MCD, the sum of the anti-v-cyclin and anti-LANA antibodies was the most useful approach for optimally separating KS from MCD+/KS+ and MCD+/KS-. Using this approach with a cut-off value of 165,000 LU discriminated KS from MCD+/KS+ with 93% sensitivity and 83% specificity. This and other KSHV antibody tests may be useful in identifying KS patients who may also have developed MCD, a disease that can be difficult to diagnose. Possible explanations for the higher anti-latent antibody responses in KS as compared to MCD+/KS+ and MCD+/KS- may include greater expression of KSHV latent antigens in KS [9, 10] or possibly blunting of KSHV-specific antibody formation in MCD as postulated above. In summary, antibody responses to latent and lytic KSHV proteins are different between KS, MCD+/KS+, MCD+/KS- and PEL patients and likely reflect altered protein expression and/or immune recognition differences among these diseases.
Acknowledgments
The authors thank the patients who volunteered for these studies.
Financial support: This work was supported by in part by the Intramural Research Program of the NIH, the National Institute of Dental and Craniofacial Research, the NIH Clinical Center, and the National Cancer Institute and, in part, by a Bench to Bedside award from the NIH Clinical Center.
Footnotes
Potential conflicts of interest: Three of the authors (P.D.B., M.J.I. and J.A.K) have a patent application submitted using LIPS for detecting anti-KSHV antibodies.
References
- 1.Boshoff C, Schulz TF, Kennedy MM, et al. Kaposi’s sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1:1274–8. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- 2.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–9. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 3.Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–44. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 4.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–91. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 5.Cesarman E, Nador RG, Aozasa K, Delsol G, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus in non-AIDS related lymphomas occurring in body cavities. Am J Pathol. 1996;149:53–7. [PMC free article] [PubMed] [Google Scholar]
- 6.LaDuca JR, Love JL, Abbott LZ, Dube S, Freidman-Kien AE, Poiesz BJ. Detection of human herpesvirus 8 DNA sequences in tissues and bodily fluids. J Infect Dis. 1998;178:1610–5. doi: 10.1086/314514. [DOI] [PubMed] [Google Scholar]
- 7.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–80. [PubMed] [Google Scholar]
- 8.Sullivan RJ, Pantanowitz L, Casper C, Stebbing J, Dezube BJ. HIV/AIDS: epidemiology, pathophysiology, and treatment of Kaposi sarcoma-associated herpesvirus disease: Kaposi sarcoma, primary effusion lymphoma, and multicentric Castleman disease. Clin Infect Dis. 2008;47:1209–15. doi: 10.1086/592298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katano H, Sato Y, Kurata T, Mori S, Sata T. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi’s sarcoma, and multicentric Castleman’s disease. Virology. 2000;269:335–44. doi: 10.1006/viro.2000.0196. [DOI] [PubMed] [Google Scholar]
- 10.Parravicini C, Chandran B, Corbellino M, et al. Differential viral protein expression in Kaposi’s sarcoma-associated herpesvirus-infected diseases: Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. Am J Pathol. 2000;156:743–9. doi: 10.1016/S0002-9440(10)64940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staskus KA, Sun R, Miller G, et al. Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. J Virol. 1999;73:4181–7. doi: 10.1128/jvi.73.5.4181-4187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burbelo PD, Leahy HP, Groot S, et al. Four-antigen mixture containing v-cyclin for serological screening of human herpesvirus 8 infection. Clin Vaccine Immunol. 2009;16:621–7. doi: 10.1128/CVI.00474-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcelin AG, Motol J, Guihot A, et al. Relationship between the quantity of Kaposi sarcoma-associated herpesvirus (KSHV) in peripheral blood and effusion fluid samples and KSHV-associated disease. J Infect Dis. 2007;196:1163–6. doi: 10.1086/521625. [DOI] [PubMed] [Google Scholar]
- 14.Chiou CJ, Poole LJ, Kim PS, et al. Patterns of gene expression and a transactivation function exhibited by the vGCR (ORF74) chemokine receptor protein of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2002;76:3421–39. doi: 10.1128/JVI.76.7.3421-3439.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]