Abstract
Accurate pronunciation of phonetically irregular words (exception words) requires prior exposure to unique relationships between orthographic and phonemic features. Whether such word knowledge is accompanied by structural variation in areas associated with orthographic-to-phonemic transformations has not been investigated. We used high resolution MRI to determine whether performance on a visual word-reading test composed of phonetically irregular words, the Wechsler Test of Adult Reading (WTAR), is associated with regional variations in cortical structure. A sample of 60 right-handed, neurologically intact individuals were administered the WTAR and underwent 3T volumetric MRI. Using quantitative, surface-based image analysis, cortical thickness was estimated at each vertex on the cortical mantle and correlated with WTAR scores while controlling for age. Higher scores on the WTAR were associated with thicker cortex in bilateral anterior superior temporal gyrus, bilateral angular gyrus/posterior superior temporal gyrus, and left hemisphere intraparietal sulcus. Higher scores were also associated with thinner cortex in left hemisphere posterior fusiform gyrus and central sulcus, bilateral inferior frontal gyrus, and right hemisphere lingual gyrus and supramarginal gyrus. These results suggest that the ability to correctly pronounce phonetically irregular words is associated with structural variations in cortical areas that are commonly activated in functional neuroimaging studies of word reading, including areas associated with grapheme-to–phonemic conversion.
Keywords: Cortical Thickness, Dyslexia, Reading, Exception Words
Introduction
Substantial progress has been made in identifying the key neuroanatomical substrates of reading. In contrast to a large number of functional neuroimaging studies on word reading networks, there have been relatively few investigations of structural variations associated with reading proficiency (Richardson & Price, 2009). The current study was designed to investigate whether structural variations associated with oral reading proficiency can be observed in nonimpaired individuals and whether regional variation in cortical thickness corresponds to neuroanatomical areas previously implicated in functional and structural neuroimaging studies of normal reading pathways.
Recent advances in neuroimaging have contributed significantly to the understanding of the associations between specific brain areas and reading function. Functional imaging studies of nonimpaired individuals consistently identify several left-lateralized and bilateral regions despite variations in task design and post-processing procedures (Fiez & Petersen, 1998; Turkeltaub, Eden, Jones, & Zeffiro, 2002). These regions of the so-called ‘reading network’ include occipital and occipitotemporal cortex, the left frontal operculum, bilateral regions within the cerebellum, primary motor cortex, the superior and middle temporal cortex, and medial regions in the supplementary motor area and anterior cingulate. Meta-analytic comparison of alphabetic, syllabic, and morpho-syllabic reading systems revealed common activations in the left superior posterior temporal gyrus, the left inferior frontal gyrus, and the left occipitotemporal region (Bolger et al., 2005), regions of the reading network that were found to be functionally correlated even at rest (Koyama et al., 2010).
Comparison of specific reading strategies has identified dissociable networks for word decoding and whole word retrieval. Brain areas implicated during graphophonological conversion usually include left-hemisphere regions, such as superior temporal areas, supramarginal gyrus, and the opercular part of inferior frontal gyrus; whereas, tasks requiring lexicosemantic access consistently activate left occipitotemporal, middle temporal gyrus and the pars triangularis of the inferior frontal gyrus (Jobard et al., 2003; Pugh et al., 2000). Phonetically irregular word reading activates the left frontal operculum relative to phonetically regular word reading (Fiez & Petersen, 1998). Greater supramarginal/angular gyrus and superior temporal gyrus activation is associated with graphophonological conversion tasks (Booth et al., 2002, 2003). The latter findings suggests that inferior parietal areas maintain a specialized role in extracting statistical regularities between orthographic and phonetic word features (Booth et al., 2004).
Fewer studies have looked at the structural correlates of reading. Group studies of dyslexic and unimpaired readers that use voxel-based morphometry (VBM) and diffusion tensor imaging (DTI) show reduced gray matter density in the left inferior, middle, and superior temporal gyri (Brown et al., 2001; Vinckenbosch, Robichon, & Eliez, 2005), decreased fractional anisotropy (FA) in bilateral fronto-temporal and left temporo-parietal white matter regions, and reduced gray matter volume in bilateral superior temporal gyri (Steinbrink et al., 2008), as well as reduced gray matter volume in bilateral fusiform gyri, anterior cerebellum, and right supramarginal gyrus (Kronbichler et al., 2008). This overlap with areas of the functional reading network indeed suggests a link between regional brain morphology and function. In a study combining functional magnetic resonance imaging (fMRI) and VBM (Hoeft et al., 2007), showed that the left parietal and bilateral fusiform cortices of individuals with dyslexia activate less compared to typically developing readers, a pattern that was accompanied by reduced gray matter volume in the left parietal, but not the fusiform regions. Similarly, PET and VBM analyses in two separate studies revealed reduced activation in left temporal and temporal-occipital regions in dyslexic adults from three different countries (Paulesu et al., 2001), along with reduced gray matter density in left middle temporal gyrus and reduced white matter density in the depth of left Broca's area, left post-central gyrus, and supramarginal gyrus (Silani et al., 2005). These findings are supported by post-mortem findings of small pathological ectopias and dysplasias in individuals with developmental dyslexia, located throughout the inferior left frontal, superior left temporal, and right frontal regions (Galaburda, Sherman, Rosen, Aboitiz, & Geschwind, 1985; Rosen, Sherman, & Galaburda, 1993).
Structural imaging studies of reading performance in nonimpaired populations are more sparse, but do support the shared structure-function hypothesis. For example, faster reading speeds have been associated with increased gray matter density and higher FA values in left frontal regions (Steinbrink et al., 2008), whereas increased local gray matter volume (LGMV) in perisylvian areas and reduced LGMV in inferior temporal areas (including fusiform gyrus) correlates with better pseudoword reading performance (Pernet, Andersson, Paulesu, & Demonet, 2009). Intriguingly, structural comparisons between individuals who learned to read as adults and matched illiterates revealed increased white matter in the splenium of the corpus callosom and increased gray matter in bilateral angular gyri, dorsal occipital, middle temporal, left supramarginal, and superior temporal gyri (Carreiras et al., 2009), suggesting that experience and proficiency can effect structural changes in areas of the reading network even in adulthood.
The current study explores whether variations in cortical thickness are associated with exception word reading, a skill that involves the acquisition of unique associations between visual and auditory features. Exception words, or phonetically irregular words, are words that do not follow typical phonetic rules (for example: gnat, porpoise, ogre, aisle, paradigm). Exception word reading, in contrast to pseudoword decoding, must involve prior exposure to unique grapheme-to-phoneme relationships and is therefore well-suited to investigate whether (a) exposure/learning is associated with structural variations in classic reading networks, and more specifically (b) whether such structural differences are located in areas associated with rapid word identification (ventral route, occipito-temporal areas) and/or areas associated with the integration of orthographic with phonological and lexical-semantic features (dorsal route, inferior parietal areas). In line with prior work that demonstrates structural differences between late-literates and illiterates in bilateral inferior parietal areas and the splenium of the corpus callosum (Carreiras et al., 2009), we predict that structural variations will be bilateral and homologous.
Materials and Methods
Participants
A sample of 60 right-handed, neurologically intact individuals (age range: 19 to 66 years; M = 36.4, SD = 13.3, 32 females) gave consent to participate in the study. All participants were determined to be right-handed with the Edinburgh Handedness Inventory, with left-handedness defined as a score < 0 (Oldfield, 1971). Participants were determined to be free of schizophrenia, neurological disorders, and reading impairment in an initial screening interview where they were asked whether they had ever been diagnosed with a psychiatric illness, neurological illness, or learning disability. Group education level ranged from an eighth grade education to a graduate degree (M = 14.0, SD = 2.4). The study was approved by the Institutional Review Board of New York University.
Word reading assessment
All subjects completed The Wechsler Test of Adult Reading™ (WTAR™). This reading test is composed of a list of 50 words that have atypical grapheme to phoneme relationships. The WTAR was developed as a tool to estimate premorbid intellectual functioning in neurologically diseased populations. Words with irregular pronunciations were chosen in order to minimize the participant's ability to apply standard pronunciation rules, as well as to assess previous learning of the word. In a stratified US sample of 1,134 individuals ranging in age from 16-89, mean raw scores for each age group ranged from 24.8 (SD = 7.9) to 42.3 (SD = 6.6). The distribution of mean scores indicated a curvilinear pattern with gradual performance increases from the 16-17 age group to the 45-54 age group and gradual performance decreases from the 45-54 age group to the 84-89 age group. Internal consistency reliability coefficients were strong for each age group with an average of r = 0.93., as was test-retest stability for each age group with correlations ranging from r = 0.92 to r = 0.94 (Wechsler, 2001).
MRI scanning and image processing
Imaging was performed at the New York University Center for Brain Imaging on a 3T Siemens Allegra head-only MR scanner. Image acquisitions included a conventional 3-plane localizer and two T1-weighted volumes (TE = 3.25 ms, TR = 2530 ms, TI = 1.100 ms, flip angle = 7 deg, field of view (FOV) = 256 mm, voxel size = 1×1×1.33 mm). Acquisition parameters were optimized for increased gray/white matter image contrast. The imaging protocol was identical for all subjects studied. The image files in DICOM format were transferred to a Linux workstation for morphometric analysis. The two T1-weighted images were rigid body registered to each other and reoriented into a common space, roughly similar to alignment based on the AC-PC line. Images were automatically corrected for spatial distortion due to gradient nonlinearity (Jovicich et al., 2006) and B1 field inhomogeneity (Sled, Zijdenbos, & Evans, 1998), registered, and averaged to improve signal-to-noise ratio. Images were further processed with the FreeSurfer (4.0.2) software package (http://surfer.nmr.mgh.harvard.edu).
Surface reconstruction and thickness measurements
Cortical thickness is a measure derived from automated MRI post-processing procedures that segment the cerebral cortex and measure the distance between the pial surface and gray/white matter boundary (Dale, Fischl, & Sereno, 1999). An advantage of surface- over volume-based approaches is the possibility of spherical averaging, which aligns sulcal and gyral patterns and thus allows for improved coregistration of brain structures across individual brains (Fischl, Sereno, & Dale, 1999). To this end, the averaged volumetric MRI scan was used to construct models of each subject's cortical surface using an automated procedure that involves (1) segmentation of the white matter, (2) tessellation of the gray/white matter boundary, (3) inflation of the folded surface tessellation, and (4) automatic correction of topological defects. These steps are described in detail elsewhere (Dale et al., 1999; Fischl, Liu, & Dale, 2001; Fischl et al., 1999). From this reconstructed surface, measures of cortical thickness were obtained by constructing an estimate of the gray/white matter boundary by classifying all white matter voxels in the MRI volume (Fischl & Dale, 2000). The white matter surface was refined in order to obtain submillimeter accuracy in delineating the gray/white matter junction. The surface was then deformed outward to locate the pial surface (Dale et al., 1999). Estimates of cortical thickness were made by measuring (1) the shortest distance from each point on the white matter surface to the pial surface, and (2) the shortest distance from each point on the pial surface to the white matter surface. Cortical thickness at each vertex was computed as the average of the two values. Maps were smoothed with a Gaussian kernel (15mm FWHM) across the surface and averaged across participants using a spherical averaging technique (Fischl et al., 1999), which accurately matches anatomically homologous regions across participants while minimizing metric distortions. For each hemisphere, a general linear model was used to estimate the effects of WTAR performance on cortical thickness at each vertex along the cortical surface. Significance maps were corrected for multiple comparisons with cluster-based Monte-Carlo simulations with 10,000 permutations (Hayasaka & Nichols, 2003). Corrected significance values of thickness correlations with WTAR scores were mapped onto the white matter surface of the average brain reconstruction for visual display.
Results
Age and Gender
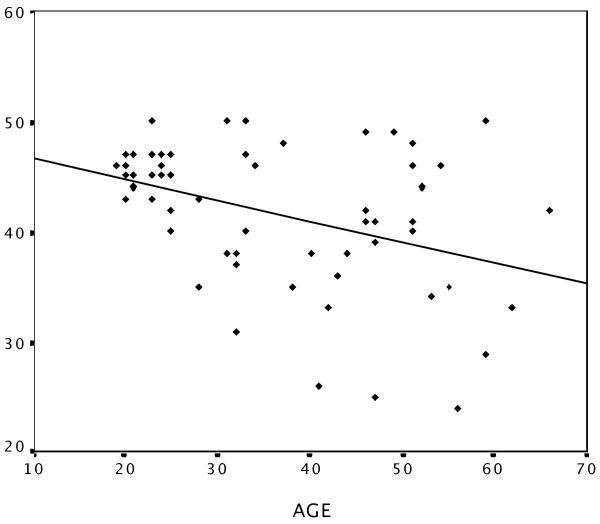
WTAR scores in our sample ranged from 24 to 50 (M = 41.72, SD = 6.43). Given the adequate range and normal distribution of these scores, raw scores, as opposed to normatively corrected scores were used in additional analyses. Performance on the WTAR showed a moderate negative correlation with age (r = -.39, p < .01) (Figure 1), a moderate positive correlation with education (r = .36, p < .01) and no difference between genders (t(58) = -1.3, p = .2; females: M = 42.72, SD = 6.5; males: mean = 40.57, SD = 6.27). Given the negative correlation between WTAR scores and age in our sample, as well as prior evidence of age-related decline in cortical thickness (Salat et al., 2004), an additional vertex-wise analysis of cortical thickness and WTAR was performed which controlled for age-related effects.
Figure 1.
Correlation of WTAR performance and age
Cortical thickness
Several brain areas showed distinct structural differences associated with performance on a test of irregular word pronunciation (Figure 2). Higher test performance was associated with thicker cortex, independent of age, in the intraparietal sulcus of the left hemisphere and the angular gyrus/posterior superior temporal gyrus area (AG/pSTG) and anterior STG (aSTG) bilaterally. Better performance was associated with decreased cortical thickness in left posterior fusiform gyrus (pFG), as well as inferior frontal gyrus (IFG) and central sulcus.
Figure 2.
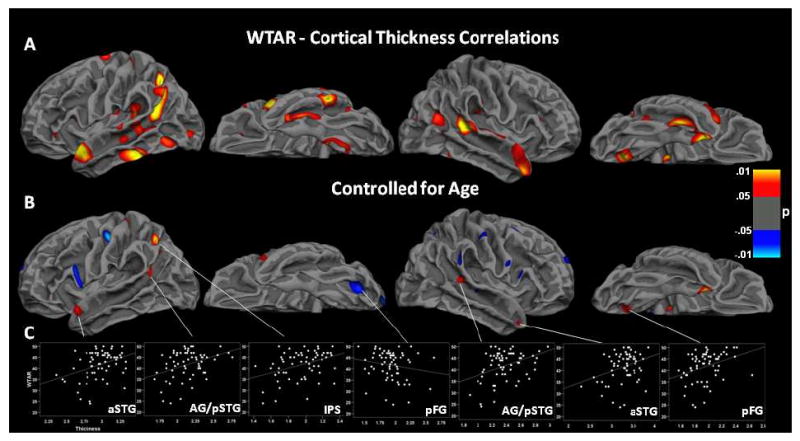
Areas with significant WTAR and cortical thickness correlations displayed on the group-averaged white matter surface. Regions in red are positive (better performance with increased thickness) and regions in blue are negative (better performance with decreased thickness). Statistical P maps thresholded at p < .0.5, cluster-corrected. A. Uncorrected for age-related changes B. Corrected for age. C. Scatter plots with best fit line showing thickness (x-axis) and WTAR score (y-axis) for each participant in areas of significant correlation. (aSTG=anterior superior temporal gyrus; AG/pSTG=angular gyrus/posterior STG; IPS=intraparietal sulcus; pFG=posterior fusiform gyrus)
Discussion
Our results demonstrate that better performance on a phonetically irregular oral word reading task is associated bilaterally with increased cortical thickness in the angular gyrus/posterior superior temporal gyrus (AG/p-STG) and anterior superior temporal gyrus (aSTG), two well-established oral reading areas. The first is consistently implicated in orthographic-to-phonemic translation (Booth et al., 2002) and the second in the analysis and categorization of speech sounds (Leff et al., 2009; Obleser et al., 2006). We also found a relationship between exception word knowledge and cortical thickness in the left superior parietal area/intraparietal sulcus (IPS), an area previously implicated in the spatial attention aspects of word reading (Baciu et al., 2002; Bitan, Manor, Morocz, & Karni, 2005).
These results are consistent with prior imaging studies that demonstrate greater volume or density in inferior parietal association cortex related to reading skill acquisition (Carreiras et al., 2009), vocabulary acquisition (Lee et al., 2007), the ability to pronounce novel words (Golestani & Pallier, 2007), and second language proficiency (Mechelli et al., 2004). In a comparison of late-literates to matched illiterates, inferior parietal areas were found to be greater in volume bilaterally, suggesting that this area may be critical for adult reading skill acquisition. Furthermore, increased functional connectivity between the left and right angular gyri was demonstrated during reading tasks in early literates, relative to object naming (Carreiras et al., 2009). This highlights the importance of inferior parietal regions in normal reading, despite inconsistent findings between functional and structural investigations. Specifically, in contrast to findings of reduced task-related activation in the left angular gyrus of dyslexic readers (Price & Mechelli, 2005; Richlan, Kronbichler, & Wimmer, 2009; Shaywitz et al., 1998), this area is not found to be strongly activated in functional neuroimaging of nonimpaired readers (Fiez & Petersen, 1998; Turkeltaub, Gareau, Flowers, Zeffiro, & Eden, 2003). Our results provide novel evidence that knowledge of unique grapheme-to-phoneme relationships is associated with increased cortical thickness in inferior and superior parietal areas, even in the absence of reading impairment.
Contrary to our hypotheses, we found an inverse relationship between skilled exception word reading and cortical thickness in bilateral inferior frontal gyri, right hemisphere lingual and supramarginal gyrus, and left hemisphere posterior fusiform gyrus and central sulcus. Activation of inferior frontal and primary motor areas is a consistent finding in functional neuroimaging studies of word reading (Fiez & Petersen, 1998; Turkeltaub et al., 2002), and the supramarginal gyrus is another region implicated in orthographic to phonemic translations (Booth et al., 2002); however, it is not clear why reduced thickness in these areas might be associated with stronger performance. The fusiform gyrus is a key area implicated in studies involving rapid letter and word identification (McCandliss, Cohen, & Dehaene, 2003). Notably, an inverse relationship between fusiform gyrus volume and pseudoword reading performance was also found in a prior investigation (Pernet et al., 2009). Although different performance measures were used, this intriguing convergence between our morphological findings strengthens the claim that decreased gray matter in the the fusiform area is associated with improved functional intregrity.
When considered in the context of prior functional imaging studies, our findings provide strong evidence for structural variations in classic reading areas associated with performance on an exception word reading test; however, the nature of these variations remains unclear. The underlying cellular properties that mediate the relationship between cortical thickness variations and word reading performance need to be investigated with post-mortem or post-resection histological studies. The cortical mantle contains cell bodies of various neuron types, neuronal synapses, axons, etc., and variation in any of these could influence both structural and functional properties of an area. Cortical thickening in some regions may reflect experience-dependent dendritic branching; however, such mechanisms remain unknown. Although there is evidence that cortical thickening is associated with learning/practice within a specific domain (Haier, Karama, Leyba, & Jung, 2009), it is also possible that early structural differences in gray matter thickness may potentiate more efficient learning and memory of novel grapheme-to-phoneme relationships.
Likewise, the relationship between cortical thinning and stronger performance on cognitive tasks has been documented in prior studies (Dickerson et al., 2008; Lu et al., 2007; Shaw et al., 2006); however, it remains unclear why stronger performance may be associated with thinner cortex in select areas. Such relationships may be associated with the myelination process or synaptic pruning, particularly in earlier stages of development (Lu et al., 2007; Sowell et al., 2008; Sowell, Thompson, & Toga, 2004). Increased myelination in tertiary cortex, such as fusiform and lingual areas, may reflect the early development of expert systems for letter and word identification, while gray matter thickening in association cortex may reflect lifetime learning/acquisition of unique relationships between visual, auditory, and articulatory word features. Further research investigating how developmental changes in gray matter properties and white matter tracts relate to oral reading skill acquisition is needed to clarify the complex relationships between regional cortical thickness and reading skill acquisition.
Several structural correlates in our study were bilateral and homologous, a finding which is consistent with other investigations of cortical thickness variations associated with language processing (Golestani & Pallier, 2007; Lee et al., 2007; Mechelli et al., 2004). Functional imaging studies commonly demonstrate bilateral activations in homologous areas during reading tasks, particularly in the superior temporal gyri (Fiez & Petersen, 1998; Turkeltaub et al., 2002). Although bilateral networks are activated during oral reading, left-hemisphere areas appear to be necessary while right hemisphere sites play a complementary or compensatory role (Roux et al., 2004). This is demonstrated in several studies where disruption of left hemisphere temporo-parietal networks is associated with reading impairment and an increase in reliance on right hemisphere and frontal networks (Coslett & Monsul, 1994; Horwitz, Rumsey, & Donohue, 1998; Pugh et al., 2000; Rumsey et al., 1997).
Dissociable reading networks have been proposed for reading strategies that involve effortful rule-based analysis (dorsal temporo-parietal stream) and automatic memory based whole word identification (occipito-temporal ventral stream) (Pugh et al., 2000). Our results demonstrate cortical thickening related to skilled pronunciation of phonetically irregular words in the angular gyrus/posterior superior temporal gyrus, an area previously associated with rule-based orthographic-to-phonemic analysis (Booth et al., 2004). This finding is consistent with a single area for acquisition of relationships between orthographic and phonemic word features, regardless of whether such relationships are unique, as in the case of exception words, or rule-based, as in the case of regular words or pseudowords. However, the current study was limited in that only a measure of exception word reading was administered. Future work should include comparison between structural correlates of psuedoword reading and exception word reading to determine whether regional variations overlap or can be dissociated. This would be relevant for characterizing morphometric variations associated with different profiles of reading impairment, such as surface dyslexia or phonological dyslexia.
Limitations of the current study include a reliance on a single performance measure. This precludes any claim that the structural variations we found are related specifically to exception word reading and not other measures of reading and/or language acquisition. In fact, given results from other studies (Golestani & Pallier, 2007; H. Lee et al., 2007; Mechelli et al., 2004)(Goestani et al., 2002, Mechelli et al. 2004, Lee et al., 2007) it is likely that structural variation in the inferior parietal region is associated with many different aspects of language processing. Further limitations include minimal information regarding the level of intellectual functioning of our participants, their socioeconomic status, their reading history and habits (i.e. age of reading onset, reading frequency) as well as any family history of reading disabilities. It is recommended that future studies in this area include analyses of the relationship between such demographic variables and reading performance, as they may independently contribute to variance in structural and performance measures.
In sum, the current study is one of very few demonstrating structural correlates of reading performance in nonimpaired individuals and shows a clear association between exception word reading and cortical thickness in classic reading areas. Contrary to the assumption that more is better, stronger performance was associated with thinner cortex in critical reading areas such as the fusiform gyrus and the inferior frontal region. Such findings are valuable for designing future longtitudinal work investigating structural changes accompanying normal reading skill acquisition or remediation efforts in individuals with reading impairment. As the nature of such regional structure-performance correlates becomes better defined, structural markers may predict the success of remediation efforts in individuals with developmental or acquired reading impairment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Baciu M, Ans B, Carbonnel S, Valdois S, Juphard A, Pachot-Clouard M, et al. Length effect during word and pseudo-word reading. An event-related fMRI study. Neuroscience Research Communications. 2002;30(3):155–165. doi: 10.1002/nrc.10027. [DOI] [Google Scholar]
- Bitan T, Manor D, Morocz IA, Karni A. Effects of alphabeticality, practice and type of instruction on reading an artificial script: an fMRI study. Brain Research Cognitive Brain Research. 2005;25(1):90–106. doi: 10.1016/j.cogbrainres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited: Universal structures plus writing system variation. Human Brain Mapping. 2005;25(1):92–104. doi: 10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Functional Anatomy of Intra- and Cross-Modal Lexical Tasks. NeuroImage. 2002;16(1):7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Development of Brain Mechanisms for Processing Orthographic and Phonologic Representations. Journal of Cognitive Neuroscience. 2004;16(7):1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Modality independence of word comprehension. Human Brain Mapping. 2002;16(4):251–261. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Relation between brain activation and lexical performance. Human Brain Mapping. 2003;19(3):155–169. doi: 10.1002/hbm.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WE, Eliez S, Menon V, Rumsey JM, White CD, Reiss AL. Preliminary evidence of widespread morphological variations of the brain in dyslexia. Neurology. 2001;56(6):781–783. doi: 10.1212/wnl.56.6.781. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Seghier ML, Baquero S, Estevez A, Lozano A, Devlin JT, et al. An anatomical signature for literacy. Nature. 2009;461(7266):983–986. doi: 10.1038/nature08461. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Monsul N. Reading with the right hemisphere: evidence from transcranial magnetic stimulation. Brain and Language. 1994;46(2):198–211. doi: 10.1006/brln.1994.1012. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dickerson B, Fenstermacher E, Salat D, Wolk D, Maguire R, Desikan R, et al. Detection of cortical thickness correlates of cognitive performance: Reliability across MRI scan sessions, scanners, and field strengths. NeuroImage. 2008;39(1):10–18. doi: 10.1016/j.neuroimage.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(3):914–921. doi: 10.1073/pnas.95.3.914. doi: VL - 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Annals of Neurology. 1985;18(2):222–233. doi: 10.1002/ana.410180210. 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Golestani N, Pallier C. Anatomical correlates of foreign speech sound production. Cerebral Cortex (New York, NY: 1991) 2007;17(4):929–934. doi: 10.1093/cercor/bhl003. 10.1093/cercor/bhl003. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Karama S, Leyba L, Jung RE. MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual-spatial task. BMC Research Notes. 2009;2:174. doi: 10.1186/1756-0500-2-174. 10.1186/1756-0500-2-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. Validating cluster size inference: random field and permutation methods. NeuroImage. 2003;20(4):2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, et al. Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Sciences. 2007;104(10):4234–4239. doi: 10.1073/pnas.0609399104. 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(15):8939–8944. doi: 10.1073/pnas.95.15.8939. doi: VL - 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. NeuroImage. 2003;20(2):693–712. doi: 10.1016/S1053-8119(03)00343-4. 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. NeuroImage. 2006;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046. 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Koyama MS, Kelly C, Shehzad Z, Penesetti D, Castellanos FX, Milham MP. Reading Networks at Rest. Cereb Cortex. 2010:bhq005. doi: 10.1093/cercor/bhq005. 10.1093/cercor/bhq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Wimmer H, Staffen W, Hutzler F, Mair A, Ladurner G. Developmental dyslexia: gray matter abnormalities in the occipitotemporal cortex. Human Brain Mapping. 2008;29(5):613–625. doi: 10.1002/hbm.20425. 10.1002/hbm.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Devlin JT, Shakeshaft C, Stewart LH, Brennan A, Glensman J, et al. Anatomical traces of vocabulary acquisition in the adolescent brain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27(5):1184–1189. doi: 10.1523/JNEUROSCI.4442-06.2007. 10.1523/JNEUROSCI.4442-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff AP, Iverson P, Schofield TM, Kilner JM, Crinion JT, Friston KJ, et al. Vowel-specific mismatch responses in the anterior superior temporal gyrus: an fMRI study. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2009;45(4):517–526. doi: 10.1016/j.cortex.2007.10.008. 10.1016/j.cortex.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Leonard C, Thompson P, Kan E, Jolley J, Welcome S, et al. Normal Developmental Changes in Inferior Frontal Gray Matter Are Associated with Improvement in Phonological Processing: A Longitudinal MRI Analysis. Cereb Cortex. 2007;17(5):1092–1099. doi: 10.1093/cercor/bhl019. 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. 0.1016/S1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O'Doherty J, Ashburner J, Frackowiak RS, et al. Neurolinguistics: structural plasticity in the bilingual brain. Nature. 2004;431(7010):757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Obleser J, Boecker H, Drzezga A, Haslinger B, Hennenlotter A, Roettinger M, et al. Vowel sound extraction in anterior superior temporal cortex. Human Brain Mapping. 2006;27(7):562–571. doi: 10.1002/hbm.20201. 10.1002/hbm.20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Démonet JF, Fazio F, McCrory E, Chanoine V, Brunswick N, et al. Dyslexia: cultural diversity and biological unity. Science (New York, NY) 2001;291(5511):2165–2167. doi: 10.1126/science.1057179. 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- Pernet C, Andersson J, Paulesu E, Demonet JF. When all hypotheses are right: A multifocal account of dyslexia. Human Brain Mapping. 2009;30(7):2278–2292. doi: 10.1002/hbm.20670. 10.1002/hbm.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Mechelli A. Reading and reading disturbance. Current Opinion in Neurobiology. 2005;15(2):231–238. doi: 10.1016/j.conb.2005.03.003. 10.1016/j.conb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Mental Retardation and Developmental Disabilities Research Reviews. 2000;6(3):207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Richardson F, Price C. Structural MRI studies of language function in the undamaged brain. Brain Structure and Function. 2009;213(6):511–523. doi: 10.1007/s00429-009-0211-y. 10.1007/s00429-009-0211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Human Brain Mapping. 2009;30(10):3299–3308. doi: 10.1002/hbm.20752. 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen GD, Sherman GF, Galaburda AM. Neuronal subtypes and anatomic asymmetry: changes in neuronal number and cell-packing density. Neuroscience. 1993;56(4):833–839. doi: 10.1016/0306-4522(93)90131-x. [DOI] [PubMed] [Google Scholar]
- Roux F, Lubrano V, Lauwers-Cances V, Trémoulet M, Mascott CR, Démonet J. Intra-operative mapping of cortical areas involved in reading in mono- and bilingual patients. Brain: A Journal of Neurology. 2004;127(Pt 8):1796–1810. doi: 10.1093/brain/awh204. 10.1093/brain/awh204. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Donohue BC, Brady DR, Nace K, Giedd JN, Andreason P. A magnetic resonance imaging study of planum temporale asymmetry in men with developmental dyslexia. Archives of Neurology. 1997;54(12):1481–1489. doi: 10.1001/archneur.1997.00550240035010. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the Cerebral Cortex in Aging. Cereb Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(5):2636–2641. doi: 10.1073/pnas.95.5.2636. doi: VL - 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani G, Frith U, Demonet J, Fazio F, Perani D, Price C, et al. Brain abnormalities underlying altered activation in dyslexia: a voxel based morphometry study. Brain: A Journal of Neurology. 2005;128(Pt 10):2453–2461. doi: 10.1093/brain/awh579. 10.1093/brain/awh579. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Kan E, Yoshii J, Thompson PM, Bansal R, Xu D, et al. Thinning of sensorimotor cortices in children with Tourette syndrome. Nat Neurosci. 2008;11(6):637–639. doi: 10.1038/nn.2121. 10.1038/nn.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2004;10(4):372–392. doi: 10.1177/1073858404263960. 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Müller H, Juengling F, Kassubek J, et al. The contribution of white and gray matter differences to developmental dyslexia: Insights from DTI and VBM at 3.0 T. Neuropsychologia. 2008;46(13):3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. 10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6(7):767–773. doi: 10.1038/nn1065. 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-Analysis of the Functional Neuroanatomy of Single-Word Reading: Method and Validation. NeuroImage. 2002;16(3, Part 1):765–780. doi: 10.1006/nimg.2002.1131. 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Vinckenbosch E, Robichon F, Eliez S. Gray matter alteration in dyslexia: converging evidence from volumetric and voxel-by-voxel MRI analyses. Neuropsychologia. 2005;43(3):324–331. doi: 10.1016/j.neuropsychologia.2004.06.023. 10.1016/j.neuropsychologia.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler test of adult reading. San Antonio, Texas: The Psychological Corporation; 2001. [Google Scholar]