Abstract
The genome of Potato yellow dwarf virus (PYDV; Nucleorhabdovirus type species), was determined to be 12,875 nucleotides (nt). The antigenome is organized into seven open reading frames (ORFs) ordered 3′-N-X-P-Y-M-G-L-5′, which likely encode the nucleocapsid, phospho, movement, matrix, glyco and RNA-dependent RNA polymerase proteins, respectively, except for X, which is of unknown function. The ORFs are flanked by a 3′ leader RNA of 149 nt and a 5′ trailer RNA of 97 nt, and are separated by conserved intergenic junctions. Phylogenetic analyses indicated that PYDV is closely related to other leafhopper-transmitted rhabdoviruses. Functional protein assays were used to determine the subcellular localization of PYDV proteins. Surprisingly, the M protein was able to induce the intranuclear accumulation of the inner nuclear membrane in the absence of any other viral protein. Finally, bimolecular fluorescence complementation was used to generate the most comprehensive protein interaction map for a plant-adapted rhabdovirus to date.
Keywords: rhabdovirus, GFP, TagRFP, Nicotiana benthamiana, BiFC, interactome, localization, FRAP, confocal, nuclear localization
Introduction
The Rhabdoviridae family of viruses contains members that collectively infect humans, animals, fish, insects, and plants (Ammar et al., 2009; Jackson et al., 2005; Kuzmin et al., 2009; Tordo et al., 2005). The plant-adapted rhabdoviruses are currently assigned to two genera (Tordo et al., 2005). The genus Cytorhabdovirus, for which the type species is Lettuce necrotic yellows virus (LNYV), are those plant rhabdoviruses that replicate and undergo morphogenesis in the cytoplasm of infected cells (Dietzgen et al., 2006). Potato yellow dwarf virus (PYDV) is the type species of the second genus, Nucleorhabdovirus, members of which are typified by their nucleotropic character (Jackson et al., 2005; Tordo et al., 2005).
PYDV was first reported as a highly destructive pathogen of potato (Solanum tuberosum; Barrus and Chupp, 1922), and early research of this virus contributed significantly to the arena of virus-insect interactions (Adam and Gaedigk, 1986; Chiu et al., 1970; Gaedigk et al., 1986; Hsu and Black, 1973; Nault and Ammar, 1989), as well as, defining the structure and cytopathology of plant-adapted rhabdoviruses (Adam and Hsu, 1984; Knudson and MacLeod, 1972; MacLeod et al., 1966; Reeder et al., 1972; Wagner et al., 1972), and development of sucrose-gradients as an analytical method (Brakke, 1951; Brakke et al., 1951; Scholthof et al., 2008).
Despite the historical and taxonomic importance of PYDV in research, the complete genome sequence has only been partially determined to date (Ghosh et al., 2008). However, comparative studies employing Sonchus yellow net virus (SYNV) and PYDV demonstrated that these two nucleorhabdoviruses elicit markedly different symptoms in Nicotiana benthamiana (Ghosh et al., 2008). For example, there is a strong recovery phenotype exhibited in SYNV-infected plants, with symptoms clearing from infected plants about four weeks post-inoculation. In contrast, most PYDV-infected plants die after severe symptom onset, which correlates with high virus titers (Ghosh et al., 2008). Additionally, PYDV perturbs the nuclear envelope at the periphery of the nucleus, whereas SYNV induces the intranuclear accumulation of nuclear membranes (Goodin et al., 2005; MacLeod et al., 1966; Martin et al., 2009; Martins et al., 1998). Here, we have extended the preliminary characterization of the N and P proteins of PYDV (Ghosh et al., 2008) by completing the genome sequence determination of the sanguinolenta strain of PYDV and conducting extensive protein localization and interaction studies with the encoded proteins.
Our results are consistent with the previously established taxonomic placement of PYDV. However, it is clear, as is evidenced by comparisons between this virus and SYNV, that these two genetically-related viruses have markedly different cytopathic effects on a common host, N. benthamiana. The present study provides the resources required for further investigation into the molecular associations between host and viral factors, which in turn lead to disease.
Materials and Methods
Virus maintenance and purification
All plants, including transgenic N. benthamiana lines expressing autofluorescent proteins fused to histone 2B, a nuclear marker, were maintained in the greenhouse on open benches. PYDV (American Type Culture Collection accession PV-234) was maintained by serial passage in N. benthamiana and N. rustica plants housed in insect-proof cages in a greenhouse under ambient conditions. SYNV was maintained in a similar manner in N. benthamiana. PYDV was purified on sucrose density gradients, as described previously by Falk and Weathers (1983).
Isolation of total RNA, RT-PCR
Total RNA was extracted from plant tissues using the Qiagen RNeasy Plant minikit according to the manufacturer's instructions (Qiagen). Except where noted, first strand cDNA synthesis and PCRs were carried out using Superscript reverse transcriptase III (Invitrogen) and Phusion high fidelity DNA polymerase (Finnzymes), respectively.
Rapid amplification of cDNA ends (RACE)
3′- and 5′-RACE were performed with the BD-SMART RACE cDNA Amplification kit according to the manufacturer's instructions (Clontech). For these analyses, cDNA was synthesized by MMLV reverse transcriptase, and PCRs were conducted with Advantage-II DNA polymerase (Clontech).
Determination of the terminal sequences of the PYDV genome
Definition of terminal nucleotides is essential for accurate determination of viral genomes. Therefore, we employed circular RT-PCR (cPCR), 5′- or 3′-RACE for determining the terminal sequences of PYDV. These experiments were conducted with RNA isolated from preparations of highly-enriched PYDV (Falk and Weathers, 1993).
For cPCR (Szymkowiak et al., 2003), the termini of PYDV genomic RNA were ligated together using T4 RNA ligase (Promega). The circularized RNA was used as a template for cDNA synthesis. PCR was performed using forward and reverse primers anchored in the L and N genes, respectively, to obtain a cDNA fragment that traversed the region containing the leader and trailer sequences. The resultant 3.0 kb fragment was cloned into pCR2.1-TOPO vector (Invitrogen) and sequenced. Definition of the terminal nucleotides of the leader and trailer sequences was determined by 5′ or 3′-RACE using genomic RNA as template, essentially as described above. For cloning of the leader sequence, PYDV genomic RNA was polyadenylated using poly-A tailing kit (Ambion) and cDNA was synthesized using the 3′CDS primer provided in the SMART cDNA synthesis kit (Clontech), which anchored to the poly-A tail. PCR amplification was carried out using a reverse primer anchored to the N gene and a primer complimentary to the CDS primer.
DNA sequencing
Sequencing of the PYDV-N and -P genes was reported previously (Ghosh et al., 2008). To complete the genome sequencing, a 1.5 kb fragment of the PYDV-L gene was amplified using forward and reverse primers (LCF: GAAGGTAGATTTTTCTCATTAATG and LCR: CCATCCCTTTTGCCGTAGACCTTC) targeted to the conserved “block-III” of rhabdovirus polymerase genes (Bourhy et al., 2005). Primers anchored in the P and L genes (LR1: CCATATCCTGTACAAACCATG, PeF: GACTATCCCATTGTATCCCTAG) were then used to amplify an approximately 5 kb fragment containing the intervening genes. The DNA Sequencing Core Laboratory, University of Florida, Gainesville, FL, performed all DNA sequencing.
Northern blotting
Detection of RNA species hybridizing to 32P-labeled PYDV cDNAs was performed using Northern hybridization as described by Senthil et al. (2005). Following post-hybridization washes, nylon membranes were wrapped in plastic film. Autoradiograms were captured using a Typhoon Phosphoimager and analyzed by ImageQuant Software (Molecular Dynamics).
DNA sequence analysis
Assembly of nucleotide sequences into the complete PYDV genome was performed using the DNASTAR v.7 software package. Homology searches were used to compare PYDV sequences to the genomes of other rhabdoviruses using various BLAST tools provided on the National Center for Biotechnology Information (NCBI) server. Open reading frames (ORFs) were identified using ORF finder search tool (Tatusov et al., 2007). The deduced amino acid sequences of proteins encoded by PYDV were analyzed using a variety of algorithms provided by the Expasy proteomics server (Gasteiger et al., 2003), including Compute PI/MW (Bjellqvist et al., 1993), PSORT for prediction of protein localization (Nakai et al; 1991), SignalP for prediction of signal peptide cleavage sites (Bendtsen et al., 2004) and NetNGlyc for prediction of N-glycosylation sites (Blom et al., 2004).
Multiple sequence alignments
Except for PYDV, all L protein sequences used in the sequence alignment study were obtained from data deposited in the NCBI database. The deduced amino acid sequences of the L genes were aligned using the CLUSTAL W algorithm (Thompson et al., 1994), included in the MegAlign program of the DNASTAR software package. The alignments were analyzed by MEGA4.0.2 (Tamura et al., 2007). The phylogenetic tree derived from these datasets was generated using the neighbor-joining method (Saitou and Nei, 1987) with a bootstrap test with 1000 replicates (Felsenstein, 1985) to determine the percentage of replicate trees in which the taxa clustered together. The evolutionary relationship of these polymerase proteins was computed using the Dayhoff matrix-based method (Schwartz and Dayhoff, 1979). In contrast to other algorithms for determining phylogenetic relationships, the Dayhoff method is more effective when using small datasets of closely related proteins, which is the assumption made here given that only rhabdoviral sequences were considered. L gene sequences utilized in phylogenetic analyses include the following: Lettuce necrotic yellows virus (LNYV; NC_007642), Northern cereal mosaic virus (NCMV; NC_007642 ), Rabies virus (RABV; NC_001542Vesicular stomatitis Indiana virus (VSIV; EF197793.1), Maize mosaic virus (MMV; NC_005975), Taro vein chlorosis virus (TaVCV; NC_006942), Maize Iranian mosaic virus (MIMV; DQ186554), Rice yellow stunt virus (RYSV; NC_003746), SYNV (M87829), and Maize fine streak virus (MFSV; NC_005974).
Protein expression in plant cells
Sequence-validated clones in vector pDONR221 (Invitrogen) of all PYDV ORFs except L, were recombined into appropriate binary vectors for the expression of autofluorescent protein fusions in plant cell for localization and bimolecular fluorescence complementation (BiFC) assays as described previously (Chakrabarty et al., 2007; Goodin et al., 2007; Martin et al., 2009). Vectors employed in this study were pSITE-2CA (GFP fusions) and pSITEII-6C1 (TagRFP fusions) for localization experiments, and the pSITE-BiFC-nEYFP and pSITE-BiFC-cEYFP vectors for BiFC assays. Recombinant vectors were transformed into Agrobacterium tumefaciens strain LBA4404. Agroinfiltration for expression of protein fusions in plant cells was conducted essentially as described previously (Goodin et al., 2005). Each expression construct was examined in sections taken from a minimum of three leaves from each of three independent plants (nine leaves total). Several hundred cells were examined for each experiment and at least three high-resolution micrographs were acquired for each construct.
Laser scanning confocal microscopy
All microscopy was performed on an Olympus FV1000 laser-scanning confocal microscope as described previously (Goodin et al., 2005).
Fluorescence recovery after photobleaching (FRAP)
FRAP experiments were conducted in agroinfiltrated leaves expressing nuclear membrane marker proteins fused to GFP, coinfiltrated with PYDV-M fused to TagRFP. These experiments were performed essentially as described previously (Goodin et al., 2005). Imaging for FRAP experiments was conducted using a 40X objective with 488 nm laser line from a multi-line argon laser set at 0.3% of full power and a 543 nm Helium/Neon laser set at 10% of full power to excite GFP and TagRFP, respectively. Regions of interest (ROIs) were photobleached for 50 ms using a 405 nm diode laser set at 60% power, which was delivered via the FV1000 Simultaneous (SIM) scanner. Images for FRAP analyses were acquired at a resolution of 512×512 pixels at a scan rate of 2 ms pixel/s, which was necessary to monitor fast protein dynamics. Two images were acquired prior to photobleaching, followed by an additional 28 images to monitor fluorescence recovery. Quantitative fluorescence data in Microsoft Excel format and confocal images in TIFF format were exported using Olympus Fluoview software. FRAP experiments were repeated three times for each ROI, with 2 min between bleaching events in order to allow full recovery of fluorescence. The fluorescence intensity data was normalized and then averaged using data from three independent experiments.
Construction of pNIA-DEST for nuclear import assays in yeast cells
The pNIAc vector (Zaltsman et al., 2007) was modified to facilitate Gateway recombination-based cloning by blunt-end ligation of the Gateway vector conversion reading-frame cassette-B (Invitrogen) into Sma1 digested pNIAc to create pNIA-DEST. Recombinant pNIA-DEST plasmids for expression of PYDV-N, -P, -Y and -M proteins, as well as genes for glutathione-S-transferase (GST), maltose-binding protein (MBP), and histone 2B, were transformed into Saccharomyces cerevisiae strain L40 (Zaltsman et al., 2007). The transformed yeast cells were grown for 2 days at 30°C on minimal media lacking tryptophan (Trp-). Yeast colonies were then re-streaked onto minimal media lacking both tryptophan and histidine (His-) and containing 5 mM 3-amino-1, 2, 4,-triazole (3AT). Growth of yeast cultures on Trp-/His- media was indicative of a functional nuclear localization signal in proteins expressed from pNIA-DEST (Zaltsman et al., 2007).
Results
The genome sequence of PYDV
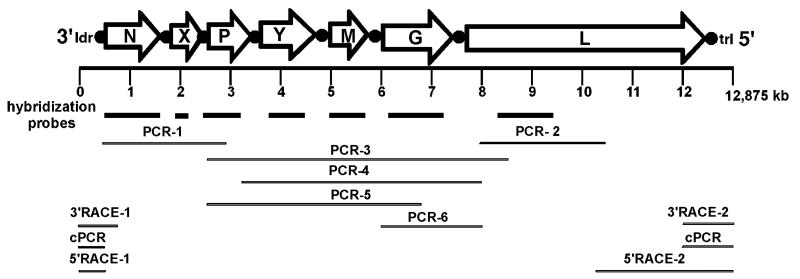
The complete 12,875 nt genome of PYDV, deposited into Genbank as accession bankit1319249, was determined from the sequence of overlapping cDNA clones generated by PCR. The sequences of the N and P genes of PYDV were determined previously using degenerate primers corresponding to sequenced peptides of PYDV proteins (Ghosh et al., 2008). To determine the reminder of the genome, we used primers reported by Bourhy et al. (2005), targeted to regions conserved in rhabdoviral L genes (Fig. 1; PCR-2). Four additional PCR products were generated to clone the portion of the genome between the P and L genes (Fig. 1; PCR-3-6). The antigenomic sequence has the coding capacity for seven ORFs, encoding proteins greater than 100 aa each.
Fig. 1.
Organization of the PYDV genome. The 12,875 nt genome encodes seven open reading frames (ORFs; open arrows) that are separated by conserved gene junctions (black circles) and flanked by short leader (ldr) and trailer (trl) sequences, respectively. Probes for each ORF used for nucleic acid hybridization are represented by bold lines. The complete genome sequence was assembled from overlapping fragments generated by standard PCR (PCR), circular PCR (cPCR) or RACE (3′RACE or 5′RACE). Each genome fragment was sequenced at least twice.
The termini of the genome were determined by cPCR as well as RACE, using genomic and antigenomic templates. The sequences of twelve clones derived from each of the three methods were identical, thus confirming the leader and trailer regions (data not shown).
Gene junctions in the PYDV genome
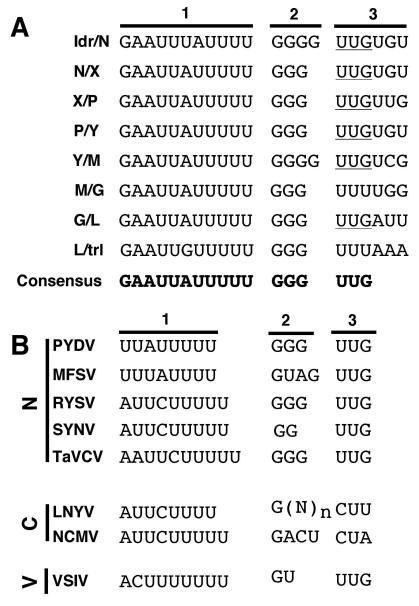
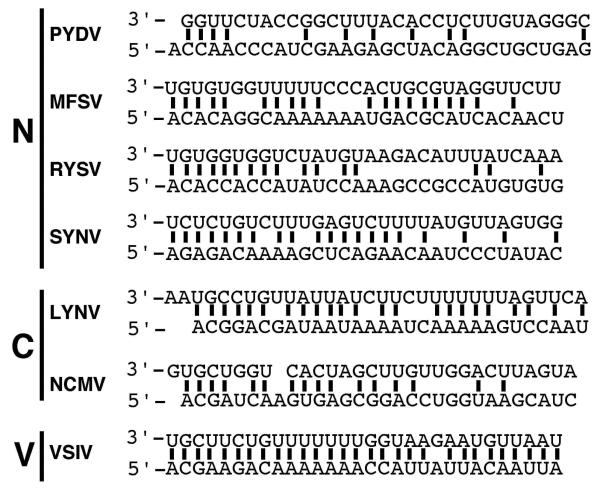
A conserved gene junction with the consensus 3′-GAAUUAUUUUUGGGUUG-5′ (Fig. 2A) was located between each of the ORFs in the PYDV genome, as well as the leader (ldr)/N gene junction. The gene junction consisted of three regions (labeled 1-3, Fig. 2A). Region 1 consisted of a poly-U track of five residues in all gene locations, except that of the ldr/N junction, which had four residues. Region 2 consisted of three non-templated guanasyl residues that were not present in 5′-RACE products of PYDV transcripts (data not shown). Region 2 in the ldr/N and Y/M junctions contained an additional G residue. Finally, Region 3, likely the transcriptional start site, began with UUG in all cases except for M/G and L/trl junctions, which began with UUU. Regions 2 and 3 of the consensus PYDV intergenic junction were identical to those of RYSV and TaVCV. However, in contrast to six other rhabdoviruses, PYDV showed minimal complementarity within its terminal sequences: 11/31 nt versus 29/30 for VSIV and 20/29 for SYNV (Fig. 3). In common with all of these viruses is that fact that the region of greatest complementarity is at the terminal nucleotides of the genome (4/5 nucleotides; Fig. 3).
Fig. 2.
(A) Sequence of each intergenic junction (IGJ) in the PYDV genomic RNA (drawn here in genomic orientation). The IGJs are divided into three sections to denote the (1) poly-adenylation signal, (2) intergenic spacer and (3) transcription start site. The consensus IGJ is provided at the bottom. (B) Consensus IGJ comparisons from rhabdoviruses in the Nucleorhabdovirus (N), Cytorhabdovirus (C) or Vesiculovirus (V) genera. Abbreviations: (N)n, variable number of nucleotides.
Fig. 3.
Complementary nucleotides (bold lines) in leader (3′) and trailer (5′) terminal sequences of selected rhabdoviruses in the Nucleorhabdovirus (N), Cytorhabdovirus (C) or Vesiculovirus (V) genera.
Detection of PYDV transcripts
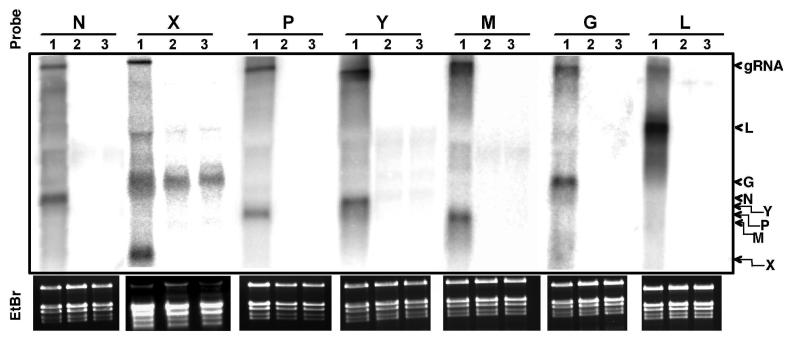
We conducted Northern hybridization analyses in order to verify that the ORFs predicted in the sequenced genome were transcribed in infected tissues (Fig. 2B). Double-stranded DNA probes up to approximately 1 kb in length were generated by PCR. Radioactively-labeled probes were hybridized to total RNA extracted from mock-inoculated or PYDV- or SYNV-infected leaves of N. benthamiana. Each probe hybridized to the PYDV genomic RNA, as well as one major transcript of length consistent with the length of the cognate gene (Fig. 4). Except for the X probe, there was no cross-hybridization to transcripts in RNA isolated from mock-inoculated or SYNV-infected leaves. The cross-hybridization of the X probe to ribosomal RNA could not be prevented by high-stringency hybridization and washes.
Fig. 4.
Detection of PYDV transcripts in infected N. benthamiana. Northern gel-blot hybridizations were conducted with ORF-specific probes shown in Fig. 1. 32P-labeled cDNA probes were hybridized to total RNA extracted from PYDV- (lane 1), SYNV-infected (lane 2) or mock-inoculated (lane 3) plants. The relative positions of the PYDV genomic RNA (gRNA) and N, X, P, Y, M, G, and L transcripts are indicated on the right side of this Figure. Ethidium-bromide (EtBr) stained gels at bottom indicate RNA quality and loading for each hybridization. The strong cross-hybridization of the X gene probe with ribosomal RNA
Predicted features of PYDV proteins
Following the demonstration that transcripts corresponding to the seven predicted PYDV ORFs were produced in vivo, we used a variety of algorithms to predict the function of each encoded protein. A subset of this information is provided in Table 1 (Bendtsen et al., 2004; Bjellqvist et al., 1993; Blom et al., 2004; Gasteiger et al., 2003; Nakai et al., 1991; Tatusov et al., 2007). The ORF1 protein, with a predicted molecular weight of 52 kDa, had homology to rhabdovirus nucleocapsid proteins (Ghosh et al., 2008). ORF2 was predicted to encode a protein of 9.7 kDa, which contained 9 proline residues in the 86-residue protein. Additionally, this protein has a predicted isoelectric point (pI) of 4.5, due primarily to the presence of a high abundance of aspartic and glutamic acid residues (23/86 aa). ORF3 was predicted to encode a protein of 31 kDa, which shares homology with rhabdoviral phosphoproteins (Ghosh et al., 2008). The predicted 33 kDa ORF Y protein did not share homology with any known proteins. The 29 kDa putative matrix protein is encoded by ORF5. In addition to homology to plant-adapted rhabdoviral matrix proteins, PYDV-M contains a YPDL sequence at amino acids 61-64, similar to the “YXXL” late domains that interact with components of the vacuolar sorting machinery to promote budding of multiple viruses including Sendai virus and Nipah virus (Chen and Lamb, 2008). The 607 amino acid ORF6 was predicted to be the PYDV glycoprotein, given that it has an 18-residue signal peptide at its amino-terminus and a Type 1a single-pass transmembrane domain at amino acid residues 576-591. Asparagine residues at positions 6, 108, 156, 169, and 464 in PYDV-G are predicted to be N-glycosylated. Threonines at positions 27, 30, 38, 39, 45, and 48, and a serine at position 42 are predicted to be O-glycosylated. Finally, ORF7 encodes a 220 kDa protein, which shares extensive homology with RNA-dependent RNA polymerases.
Table 1.
Features of PYDV proteins determined by predictive algorithms.
| ORF | MW (kD) |
TM | Predicted NLS** |
Putative function | Highest scoring virus/E-value (BLAST) |
|---|---|---|---|---|---|
| 1 | 52 | None | None | Nucleocapsid (N) | Ghosh et al., (2008) |
| 2 | 9.7 | None | None | unknown (X) | no significant |
| 3 | 31 | None | None | Phosphoprotein (P) | Ghosh et al., (2008) |
| 4 | 33 | None | None | Movement (Y) | no significant |
| 5 | 29 | None | None | Matrix protein (M) | RYSV-M/0.001 |
| 6 | 70 | aa 576-591 | None | Glycoprotein (G) | MIMV-G/2e-49 |
| 7 | 220 | None | None | Polymerase (L) | RYSV-L/0.0 |
TM = Transmembrane domain
NLS = nuclear localization signal
Being the type member of the Nucleorhabdovirus genus, we fully expected to find predictable nuclear localization signals (NLSs) in at least one of the PYDV proteins. However, no NLSs were predicted in any of the seven proteins using conventional protein localization algorithms.
Taxonomic assignment of PYDV based on L protein sequence comparisons
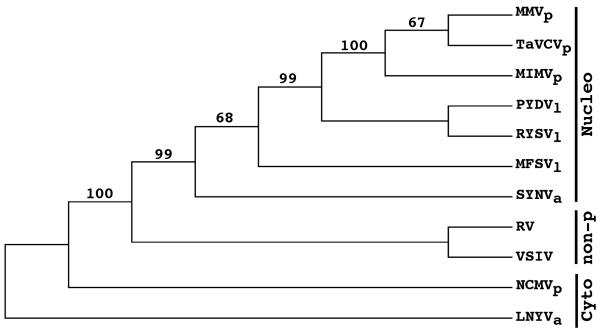
We have previously used N protein sequences to show that PYDV is most closely related to viruses assigned the genus Nucleorhabdovirus (Ghosh et al., 2008). Here we use the deduced amino acid sequence of L proteins to show that PYDV is most closely related to other leafhopper-transmitted viruses, RYSV and MFSV. Interestingly, the planthopper-transmitted MIMV and MMV clustered with TaVCV, for which planthopper transmission is suspected but not firmly established (Revill et al., 2005). SYNV, transmitted by the aphid, Aphis correopsidis, formed a separate clade to the aforementioned viruses. However, as a group, all of the nucleorhabdoviruses and MIMV clustered together and were well separated from the cytorhabdoviruses and non-plant-associated rhabdoviruses (Fig. 5).
Fig. 5.
Phylogeny of plant rhabdoviruses inferred from L protein sequences. Representative rhabdoviruses infecting a variety of hosts were used, including viruses that do not infect plants (non-p) as well as plant-adapted viruses in the Nucleorhabdovirus (Nucleo) and Cytorhabdovirus (Cyto) genera. Bootstrap values greater than 50% are shown at nodes in the tree. Vectors for the plant-adapted viruses are shown as subscripts, which are aphid (a), leafhopper (l), and planthopper (p). Virus names and Genbank accession numbers are listed in the Materials and
In planta localization of PYDV proteins
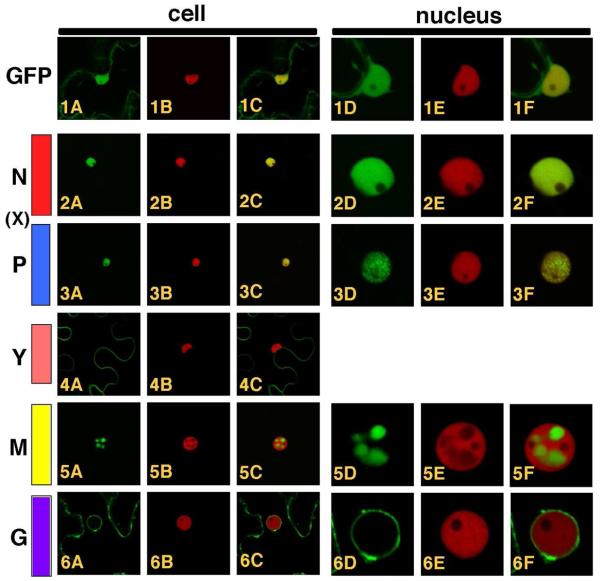
The taxonomic placement of PYDV was seemingly at odds with the protein localization prediction data, specifically that no NLSs were identified in the primary structures of any of the PYDV proteins. To resolve this discrepancy, we determined the subcellular localization patterns for five PYDV proteins in planta. Each of the N, X, P, Y, M and G proteins were expressed as GFP fusions in transgenic N. benthamiana, which expressed RFP fused to histone 2B (Fig. 6). As for previous experiments in which the DNA-selective dye DAPI was used to mark nuclei (Ghosh et al., 2008), the PYDV-N and -P proteins (Fig. 6, 2A-F and 6, 3A-F, respectively) localized exclusively to nuclei, as did PYDV-M (Fig. 6, 5A-F). Consistent with the prediction that it is a movement protein, the GFP-Y fusion localized to the cell periphery, with no detectable association with the nucleus (Fig. 6, 4A-C). The GFP-PYDV-G fusion associated with membranes, in particular the nuclear envelope (Fig. 6, 6A-F). We were unable to detect fluorescence of a GFP-X fusion.
Fig. 6.
Confocal micrographs of PYDV protein fusions expressed by agroinfiltration in leaf epidermal cells of transgenic N. benthamiana plants expressing RFP fused to histone 2B, a nuclear marker. Whole cell or nuclear views of fluorescence of GFP, RFP and overlaid images are provided. 1A-F. GFP. 2A-F. PYDV-N. 3A-F. PYDV-P. 4A-C. PYDV-Y. 5A-F. PYDV-M. 6A-F. PYDV-G. The X protein, shown in its relative genomic location, could not be expressed as a GFP fusion and therefore was omitted here.
Nuclear import of PYDV proteins
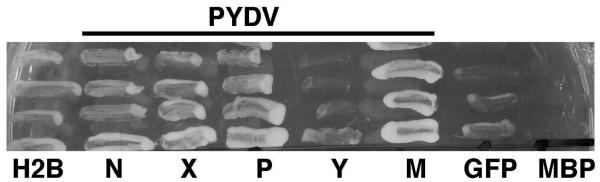
To further support the in planta localization data for the PYDV-N, -P and -M proteins, we employed a yeast-based nuclear import assay (NIA; Fig. 7). In this assay, only proteins containing a functional NLS will facilitate the nuclear import of a transcriptional activator required for expression of a reporter gene in yeast cells (Zaltsman et al., 2007). Consistent with the in planta localization patterns, the N, P and M proteins were all positive in the NIA. The Y protein was NIA-negative, consistent with in planta localization results. We did not test the L or G proteins in the yeast assay due to their size and transmembrane association, respectively. In contrast to the negative results obtained in planta, PYDV-X was shown to be nuclear localized in yeast cells.
Fig. 7.
Yeast-based assay for identification of proteins containing a functional NLS. Positive- (H2B) and negative-control (GFP, MBP) proteins or PYDV proteins (N, X P, Y, and M) were expressed from pNIA-DEST in yeast strain L40. Only those proteins containing a functional NLS (H2B, N, X, P and M) were able to facilitate yeast growth on media lacking histidine.
M protein-induced membrane relocalization
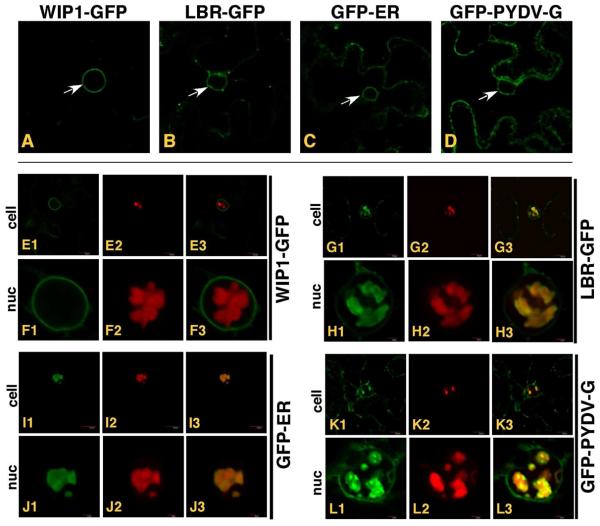
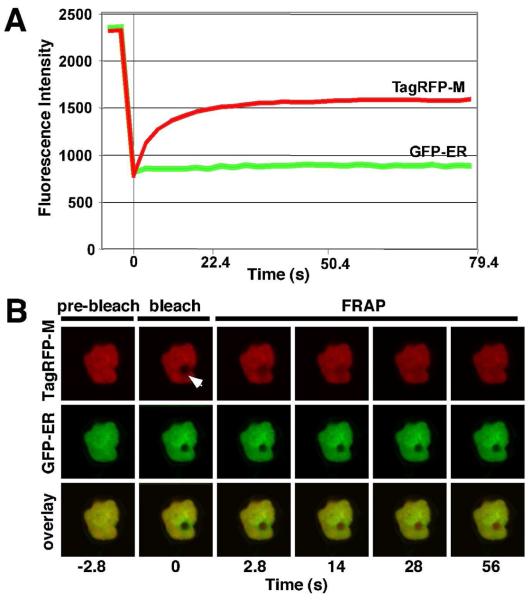
While conducting experiments to determine the subcellular localization of PYDV proteins, we observed that the M protein was capable of inducing the intranuclear accumulation of plant membranes (Fig. 8). To further define which membranes were being affected, we coexpressed the M protein as an RFP fusion in plant cells with GFP fusions targeted to the outer nuclear membrane (WIP1-GFP; Xu et al., 2007; Fig. 8, E1-3, F1-3 A; 51), inner nuclear membrane (LBR-GFP; Irons et al. 2003; Fig. 8B, G1-3, H1-3; 27), endoplasmic reticulum (GFP-ER; Fig. 8C, I1-3, J1-3) or multiple membranes (PYDV-G; Fig. 8D, K1-3, L1-3). Interestingly, all membrane markers, except WIP1-GFP, accumulated in the nucleus when coexpressed with RFP:PYDV-M. FRAP analyses of RFP-M-induced intranuclear membranes in cells of transgenic plants showed that the GFP-ER marker was not mobile, in contrast to the M protein (Fig. 9).
Fig. 8.
Single plane confocal micrographs of protein fusions of cells in which TagRFP-PYDV-M was co-expressed with various membrane markers fused to GFP by agroinfiltration in leaf epidermal cells of transgenic N. benthamiana plants. White arrows indicate the accumulation of markers on nuclear membranes in the absence of TagRFP-PYDV-M; A. WIP1-GFP (outer nuclear membrane marker), B. LBR-GFP (inner nuclear membrane marker), C. GFP-ER (endoplasmic reticulum) and D. GFP-PYDV-G (endomembranes). All remaining panels show coexpression of the membrane markers and TagRFP-PYDV-M. E1-3/F1-3, WIP1-GFP. G1-3/H1-3, LBR-GFP. I1-3/J1-3, GFP-ER. K1-3/L1-3, GFP-PYDV-G.
Fig. 9.
(A) Normalized FRAP data following photobleaching of TagRFP-PYDV-M expressed transiently in leaf epidermal cells of transgenic N. benthamiana plants expressing GFP-ER. A selected region of interest (arrow) was photobleached using a 50 ms pulse of a 405 nm laser set at 60% of full power. The micrograph acquired immediately after photobleaching was considered to be the zero time point. The relative fluorescence intensity of GFP and TagRFP were monitored for 28 frames post-bleaching using sequential imaging. The mean fluorescence intensity measurements for three experiments are plotted. (B) Single-plane confocal images of TagRFP (top panel), GFP (middle panel) fluorescence and their overlay (lower panel), used to generate the plots shown in A. Representative micrographs corresponding to the timepoints in the FRAP
Interaction matrix for PYDV proteins
BiFC was used to define the interaction and localization patterns of PYDV proteins. We chose to use BiFC given that it provided simultaneous interaction and localization data in planta (Citovsky et al., 2006; Martin et al., 2009). The PYDV-N, -P, -Y, -M and -G proteins were tested in all pair-wise interactions and against GST, which served as a non-binding control (Fig. 10). The L protein was not included in these experiments due to the inability to express this 220 kDa protein in planta. None of the PYDV proteins showed interaction with GST in either of the reciprocal fusions in which interactions can be tested (Citovsky et al., 2006; Martin et al., 2009; Fig. 10 1A-C, 2A-C, 3A-C, 4A-C, 5A-C, 7A-C). No interactions could be detected for the P/P (Fig. 10, 6A-C), G/Y (Fig. 10, 8A-C), G/P (Fig. 10, 9A-C), G/N (Fig. 10, 10A-C), P/M (Fig. 10, 19A-C), or Y/N (Fig. 10, 20A-C) combinations. Positive BiFC interactions were detected for the Y/Y (Fig. 10, 11A-C), Y/M (Fig. 10, 12A-C), M/M (Fig. 10, 13A-C), N/M (Fig. 10, 14A-C), N/N (Fig. 10, 15A-C), N/P (Fig. 10, 16A-C), G/G (Fig. 10, 17A-C) and G/M (Fig. 10 18A-C) interactions. The Y/M interaction resulted in the intranuclear accumulation of Y, which was restricted to the periphery of cells in a self-interaction. Likewise, the G/M interaction resulted in the relocalization of M to cytoplasmic membranes (compare Fig. 10, 13A-C to 18A-C). The N/M interaction resulted in the uniform distribution of M within the nucleus instead of punctate loci observed for the M/M interaction. The results of the BiFC experiments were used to generate a protein interaction map for PYDV (Fig. 11).
Fig. 10.
Single-section confocal micrographs showing PYDV protein interactions determined by BiFC. Interaction assays were conducted in leaf epidermal cells of transgenic N. benthamiana expressing CFP fused to the nuclear marker Histone 2B (CFP-H2B). Shown in panels A, B and C are micrographs of CFP, YFP (BiFC) fluorescence and the resultant overlay, respectively. Proteins listed first in the pair of interactors were expressed as fusions to the amino-terminal half of YFP. Those listed second were expressed as fusions to the carboxy-terminal half of YFP. However, protein fusions to each half of YFP were tested in all pairwise interactions of which a subset is shown here. GST served as a non-binding control that did not interact with itself (1A-C) or Y (2A-C), M (3A-C), N (4A-C), P (5A-C) or G (7A-C). Pairs of PYDV proteins that did not interact were P/P (6A-C), G/Y (8A-C), G/P (9A-C), G/N (10A-C), P/M (19A-C) and Y/N (20A-C). BiFC-positive interactions were observed for Y/Y (11A-C), Y/M (12A-C), M/M (13A-C), N/M (14A-C), N/N (15A-C), N/P (16A-C), G/G (17A-C), G/M (18A-C). Panel labels in orange text indicate non-binding controls while assays conducted with two PYDV proteins are shown in blue. Boxed in red is the P/P interaction that was expected to be positive. The X protein could not be expressed
Fig. 11.
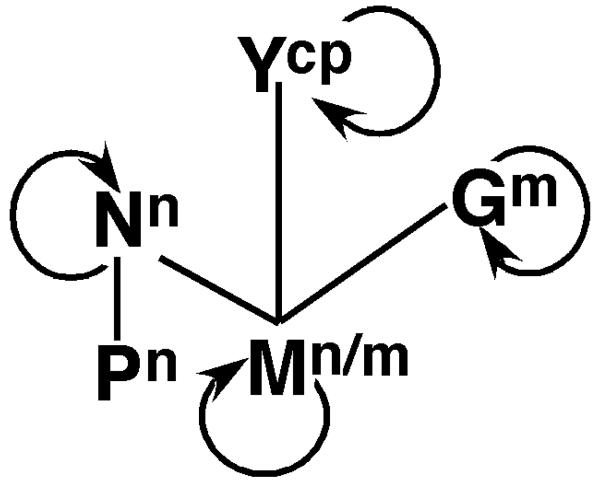
Integrated protein interaction and localization map for PYDV. Self-interactions are indicated by curved arrow. Lines indicate heterologous interactions. Superscripts indicate subcellular localization: n = nucleus, n/m = nucleus/membrane, m = membrane, cp = cell periphery.
Discussion
In this report we provided the complete sequence determination of PYDV, the types species the Nucleorhabdovirus genus. Additionally, we have determined that transcripts for all seven major ORFs are produced in infected tissues. Moreover, we have defined the subcellular localization and pair-wise interactions for five PYDV proteins. We have also described the novel activity of PYDV-M, which is capable of inducing the intranuclear accumulation of the inner nuclear membrane in the absence of any other viral protein.
The overall genome organization of PYDV corresponds to that of a generalized nucleohabdovirus with five genes (N, P, M, G and L), similar to those found in prototypical animal rhabdoviruses such as VSV, with additional “X” and “Y” genes located between the N/P and P/M genes (Jackson et al., 2005). The extra gene that we have named Y is in a similar genomic context to the sc4 gene of SYNV, and P3 of RYSV, both of which have been implicated in cell-to-cell movement in these viruses (Huang et al., 2005; Melcher, 2000). The subcellular localization pattern of PYDV-Y is on the periphery of the cells and in this respect is highly similar to that of sc4 (Goodin et al., 2007). These data taken together suggest perhaps that PYDV-Y may be a cell-to-cell movement protein. The functions of N, P, M, G and L could all be predicted based upon sequence homology to previously characterized rhabdoviruses using a variety of sequence alignment tools. Unfortunately, the inability to detect fluorescence corresponding to the GFP-X fusion prevents speculation on the function of this protein. However, the yeast-based NIA suggests that PYDV-X localizes to nuclei. The discrepancy between the localization results for PYDV-X determined in planta or in yeast may be explained in part by its small size, (11 kDa), high proline content (10%), and acidic nature (pI = 4.5). Additionally, it is likely that the yeast-based assay is more sensitive than in planta localization. Based upon molecular weight and isoelectric point, it is possible that the PYDV-X protein is the homologue of the ORF3 protein of MFSV, which is 10 kDa with a pI of 5.4. In contrast, MFSV-ORF3 contains very few proline residues (3 proline/93 aa). YFP-ORF-3 fusions accumulated in cytoplasmic loci of an undefined nature. As for PYDV-X, the function of MFSV-ORF3 is presently unknown (Tsai et al., 2005).
Interestingly, although no NLSs could be identified in any of the PYDV proteins using computational methods, the N, P and M proteins were found to localize to nuclei in both yeast- and plant-based functional assays. No regions of similarity were common among these proteins, suggesting that each employs unique non-canonical NLSs to mediate nuclear transport. Interestingly, we also observed that among all other sequenced nucleorhabdoviruses, TaVCV is the only other virus that lacks predictable NLSs in any of its proteins (Revill et al., 2005). However, no in planta characterization of TaVCV proteins has been reported, and so we are not presently able to make further comparisons between these viruses. It should be noted however, that despite a lack of predictable NLSs it is possible that importin-α may play a role in the nuclear import of PYDV-N given that these proteins were shown to interact using BiFC (Martin et al., 2009). In contrast, PYDV-P does not interact with importin-α and therefore multiple nuclear import receptors may be employed by PYDV proteins.
Additionally, once imported into nuclei, the sites of nuclear accumulation also differ among these proteins, with N and P accumulating uniformly or in dispersed speckles, respectively, within nuclei (Ghosh et al., 2008). The localization of the M protein is unique among rhabdoviral proteins, given that it is both nuclear localized and membrane-associated. In contrast to the PYDV-M protein, SYNV-M is associated with membranes only in the context of infected cells and does not alter nuclear membranes in the absence of other viral proteins (Goodin et al., 2007). Moreover, M appears to selectively interact with the inner nuclear membrane, given that M did not affect localization of WIP1-GFP, a marker for the outer nuclear membrane. Further support that only the inner nuclear membrane moves in response to M is that fact that the GFP-ER marker, which is targeted to the lumen of the ER, also accumulated in the nucleus. The association of M with membranes may be via the presence of a YPDL late domain, located at residues 61-64 in the primary structure. This domain is also found in p9 and p6 proteins of Equine infectious anemia virus and Human immunodeficiency virus-1, respectively. Related motifs YLDL and YMYL are found in the matrix proteins of Sendai virus and Nipah virus, respectively (Chen and Lamb, 2008). Importantly, these YXXL-containing proteins all bind the AIP1/Alix protein, which plays a role in vacuolar protein sorting (Chen and Lamb, 2008; Irie et al., 2008). AIP1/Alix is a highly conserved protein with homologues in animals, fungi and plants, suggesting that a similar interaction could occur in PYDV-infected N. benthamiana. Additionally, other interactions of PYDV are consistent with its function as a matrix protein, such as its interaction with the G protein. The N/M interaction could be essential for condensation of the PYDV nucleocapsid, a process that would then be followed by budding into the perinuclear space (Goodin et al., 2007; Martins et al., 1998; van Beek et al., 1985). Interestingly, M also interacted with Y, the putative movement protein, suggesting that PYDV-M may form complexes similar to the ER-associated SYNV-M-containing complexes and which were postulated to be rhabdoviral cell-to-cell movement complexes (Goodin et al., 2007). Taken together our localization and protein interaction data are consistent with current models for rhabdoviral morphogenesis (Goodin et al., 2007; Jackson et al., 2005; van Beek et al., 1985). The singular exception to this is the lack of a P/P interaction. Cognate P proteins of viruses in the Mononegavirales typically form oligomeric complexes (Basak et al., 2004; Chenik et al., 1998; Goodin et al., 2001; Masters and Banerjee, 1998). However, we have not been able to detect a P/P interaction by protein interaction assays including BiFC and yeast two-hybrid analyses. Both BiFC constructs used to determine the P/P interaction could be used to detect the N/P interaction, an association common to all members of the Mononegavirales, suggesting that fusion of reporter proteins to P did not render it non-functional.
Transcripts corresponding to all ORFs were detected in infected tissues. Sequence determination of each PYDV mRNA showed that each contained a common 5′-AAC sequence corresponding to Region 3 of the intergenic junctions flanking each gene. Thus, the UUG of Region 3 appears to be a bona fide transcription start site. The tri- or tetra-guanosyl non-transcribed spacer region (Region 2) of the intergenic junction is similar to that found in TaVCV and RYSV. Finally, the Region 1 poly-adenylation signal, while common among PYDV genes, is most similar to that of MFSV. However, all Region 1 sequences examined contained a poly-U track, which serves to function in template-dependent polyadenylation of rhabdoviral transcripts (Barr et al., 2002).
When considered together, our characterization of PYDV suggests a likely gene order of 3′-ldr-N-X-P-Y-M-G-L-trl-5′. Interestingly, of the seven viruses compared, PYDV showed the lowest degree of complementarity between its terminal sequences. How this may affect transcription and replication of PYDV relative to these other viruses is presently unknown. However, we note that the PYDV-L transcript was surprisingly abundant, relative to that reported for other rhabdoviruses (Tordo et al., 2005; Tsai et al., 2005). Whether this is related to transcription efficiency or RNA stability has not been investigated.
In conclusion, we note that determination of the PYDV genome sequence and characterization of its encoded proteins fully supports its taxonomic placement in the Nucleorhabdovirus genus. Moreover, we note that three clades within the genus Nucleorhabdovirus were identified that could be distinguished by the particular vector for each virus. Thus, it appears that insect vectors may have a major influence on the evolutionary trajectories of plant-adapted rhabdoviruses. Moreover, our continued comparative studies with SYNV and PYDV will contribute to a better understanding of the common and unique molecular requirements for infection of a common host by aphid- and leafhopper-vectored viruses.
Acknowledgements
We wish to express our sincere appreciation to Hei-ti Hsu and Andrew Jackson for providing stocks of PYDV and antisera. We thank Vitaly Citovsky for providing pNIAc and Byoung-Eun Min for converting it to Gateway compatibility. We are grateful to Ralf Dietzgen for providing a critical review of the manuscript prior to submission. We also thank the Kentucky Tobacco Research and Development Center, The National Institutes of Health and The National Science Foundation for grant support to MG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam G, Gaedigk K. Inhibition of Potato Yellow Dwarf Virus infection in vector cell monolayers by lysosomotropic agents. J. Gen. Virol. 1986;67:2775–2780. [Google Scholar]
- Adam G, Hsu HT. Comparison of structural proteins from two Potato yellow dwarf viruses. J. Gen. Virol. 1984;65:991–994. [Google Scholar]
- Ammar el-D., Tsai CW, Whitfield AE, Redinbaugh MG, Hogenhout SA. Cellular and molecular aspects of rhabdovirus interactions with insect and plant hosts. Annu. Rev. Entomol. 2009;54:447–468. doi: 10.1146/annurev.ento.54.110807.090454. [DOI] [PubMed] [Google Scholar]
- Barr JN, Whelan SP, Wertz GW. Transcriptional control of the RNA-dependent RNA polymerase of Vesicular stomatitis virus. Biochem. Biophys. Acta. 2002;1577:337–353. doi: 10.1016/s0167-4781(02)00462-1. [DOI] [PubMed] [Google Scholar]
- Barrus MF, Chupp CC. Yellow dwarf of potatoes. Phytopathology. 1922;12:122–133. [Google Scholar]
- Basak S, Polley S, Basu M, Chattopadhyay D, Roy S. Monomer and dimer of Chandipura virus unphosphorylated P-protein binds leader RNA differently: implications for viral RNA synthesis. J. Mol. Biol. 2004;339(5):1089–1101. doi: 10.1016/j.jmb.2004.03.081. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, Heijne GV, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Bjellqvist B, Hughes GJ, Pasquali Ch., Paquet N, Ravier F, Sanchez J.-Ch., Frutiger S, Hochstrasser DF. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis. 1993;14:1023–1031. doi: 10.1002/elps.11501401163. [DOI] [PubMed] [Google Scholar]
- Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- Bourhy H, Cowley JA, Larrous F, Holmes EC, Walker PJ. Phylogenetic relationships among rhabdoviruses inferred using the L polymerase gene. J. Gen. Virol. 2005;86:2849–2858. doi: 10.1099/vir.0.81128-0. [DOI] [PubMed] [Google Scholar]
- Brakke M. Density gradient centrifugation: a new separation technique source. J. Am. Chem. Soc. 1951;73(4):1847–1848. [Google Scholar]
- Brakke MK, Black LM, Wyckoff RWG. The sedimentation rate of Potato Yellow-Dwarf Virus. Am. J. Bot. 1951;38:332–342. [Google Scholar]
- Chakrabarty R, Banerjee R, Chung SM, Farman M, Citovsky V, Hogenhout SA, Tzfira T, Goodin MM. Vector for stable integration or transient expression of autofluorescent protein fusions in plants: probing Nicotiana benthamiana-virus interaction. Mol. Plant Microbe Interact. 2007;20:740–750. doi: 10.1094/MPMI-20-7-0740. [DOI] [PubMed] [Google Scholar]
- Chen BJ, Lamb RA. Mechanisms for enveloped virus budding: can some viruses do without an ESCRT? Virology. 2008;372(2):221–232. doi: 10.1016/j.virol.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenik M, Schnell M, Conzelmann KK, Blondel D. Mapping the interacting domains between the rabies virus polymerase and phosphoprotein. J. Virol. 1998;72(3):1925–1930. doi: 10.1128/jvi.72.3.1925-1930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu RJ, Liu HY, MacLeod R, Black LM. Potato yellow dwarf virus in leafhopper cell culture. Virology. 1970;40:387–396. doi: 10.1016/0042-6822(70)90416-2. [DOI] [PubMed] [Google Scholar]
- Citovsky V, Lee LY, Vyas S, Glick E, Chen MH, Vainstein A, Gafni Y, Gelvin SB, Tzfira TJ. Subcellular localization of interacting proteins by bimolecular fluorescence complementation in planta. Mol. Biol. 2006;362:1120–1131. doi: 10.1016/j.jmb.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Dietzgen RG, Callaghan B, Wetzel T, Dale JL. Completion of the genome sequence of Lettuce necrotic yellows virus, type species of the genus Cytorhabdovirus. Virus Res. 2006;118:16–22. doi: 10.1016/j.virusres.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Falk BW, Weathers LG. Comparison of Potato yellow dwarf virus serotypes. Phytopathology. 1983;73:81–85. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Gaedigk K, Adam G, Mundry K-W. The spike protein of Potato Yellow Dwarf Virus and its functional role in the infection of insect vector cells. J. Gen. Virol. 1986;67:2763–2773. [Google Scholar]
- Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Brooks RE, Wang R, Lesnaw J, Goodin MM. Cloning and subcellular localization of phosphoprotein and nucleocapsid proteins of Potato yellow dwarf virus, type species of the genus Nucleorhabdovirus. Virus Res. 2008;135:26–35. doi: 10.1016/j.virusres.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Goodin MM, Austin J, Tobias R, Fujita M, Morales C, Jackson AO. Interactions and nuclear import of the N and P proteins of Sonchus yellow net virus, a plant nucleorhabdovirus. J. Virol. 2001;75(19):9393–9406. doi: 10.1128/JVI.75.19.9393-9406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin M, Yelton S, Ghosh D, Mathews S, Lesnaw J. Live-cell imaging of rhabdovirus induced morphological changes in plant nuclear membranes. Mol. Plant Microbe Interact. 2005;18:703–709. doi: 10.1094/MPMI-18-0703. [DOI] [PubMed] [Google Scholar]
- Goodin MM, Chakrabarty R, Banerjee R, Yelton S, DeBolt S. Membrane and protein dynamics in live plant nuclei infected with Sonchus yellow net virus, a plant adapted rhabdovirus. J. Gen. Virol. 2007;88:1810–1820. doi: 10.1099/vir.0.82698-0. [DOI] [PubMed] [Google Scholar]
- Hsu HT, Black LM. Inoculation of vector cell monolayers with Potato yellow dwarf virus. Virology. 1973;52:187–198. doi: 10.1016/0042-6822(73)90408-x. [DOI] [PubMed] [Google Scholar]
- Huang YW, Geng YF, Ying XB, Chen XY, Fang RX. Identification of a movement protein of rice yellow stunt rhabdovirus. J. Virol. 2005;79(4):2108–2114. doi: 10.1128/JVI.79.4.2108-2114.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie T, Nagata N, Yoshida T, Sakaguchi T. Recruitment of Alix/AIP1 to the plasma membrane by Sendai virus C protein facilitates budding of virus-like particles. Virology. 2008;371(1):108–20. doi: 10.1016/j.virol.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Irons SL, Evans DE, Brandizzi F. The first 238 amino acids of the human lamin B receptor are targeted to the nuclear envelope in plants. J. Ex. Bot. 2003;54:943–950. doi: 10.1093/jxb/erg102. [DOI] [PubMed] [Google Scholar]
- Jackson AO, Dietzgen RG, Goodin MM, Bragg JN, Deng M. Biology of plant rhabdoviruses. Annu. Rev. Phytopathol. 2005;43:623–660. doi: 10.1146/annurev.phyto.43.011205.141136. [DOI] [PubMed] [Google Scholar]
- Knudson DL, MacLeod R. The proteins of Potato yellow dwarf virus. Virology. 1972;47:285–295. doi: 10.1016/0042-6822(72)90264-4. [DOI] [PubMed] [Google Scholar]
- Kuzmin IV, Novella IS, Dietzgen RG, Padhi A, Rupprecht CE. The rhabdoviruses: biodiversity, phylogenetics, and evolution. Infect. Genet. Evol. 2009;9:541–553. doi: 10.1016/j.meegid.2009.02.005. [DOI] [PubMed] [Google Scholar]
- MacLeod R, Black LM, Moyer FH. The fine structure and intracellular localization of Potato yellow dwarf virus. Virology. 1966;29:540–552. doi: 10.1016/0042-6822(66)90278-9. [DOI] [PubMed] [Google Scholar]
- Martin K, Kopperud K, Chakrabarty R, Banerjee R, Brooks R, Goodin MM. Transient expression in Nicotiana benthamiana fluorescent marker line provides enhanced definition of protein localization, movement and interactions in planta. Plant J. 2009;59:150–162. doi: 10.1111/j.1365-313X.2009.03850.x. [DOI] [PubMed] [Google Scholar]
- Martins CR, Johnson JA, Lawrence DM, Choi TJ, Pisi AM, Tobin SL, Lapidus D, Wagner JD, Ruzin S, McDonald K, Jackson AO. Sonchus yellow net rhabdovirus nuclear viroplasms contain polymerase-associated proteins. J. Virol. 1998;72:5669–5679. doi: 10.1128/jvi.72.7.5669-5679.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters PS, Banerjee AK. Resolution of multiple complexes of phosphoprotein NS with nucleocapsid protein N of Vesicular stomatitis virus. J Virol. 1988;62(8):2651–2657. doi: 10.1128/jvi.62.8.2651-2657.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher U. The ‘30K’ superfamily of viral movement proteins. J. Gen. Virol. 2000;81:257–266. doi: 10.1099/0022-1317-81-1-257. [DOI] [PubMed] [Google Scholar]
- Nakai K, Kanehisa M. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- Nault LR, Ammar ED. Leafhopper and planthopper transmission of plant viruses. Annu. Rev. Entomol. 1989;34:503–529. [Google Scholar]
- Reeder GS, Knudson DL, Macleod R. The ribonucleic acid of Potato yellow dwarf virus. Virology. 1972;50:301–304. doi: 10.1016/0042-6822(72)90378-9. [DOI] [PubMed] [Google Scholar]
- Revill P, Trinh X, Dale J, Harding R. Taro vein chlorosis virus: characterization and variability of a new nucleorhabdovirus. J. Gen. Virol. 2005;86:491–499. doi: 10.1099/vir.0.80591-0. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schwartz RM, Dayhoff MO. Protein and nucleic acid sequence data and phylogeny. Science. 1979;205(4410):1038–1039. doi: 10.1126/science.205.4410.1038. [DOI] [PubMed] [Google Scholar]
- Senthil G, Liu H, Puram GV, Clark A, Stromberg A, Goodin MM. Specific and common changes in Nicotiana benthamiana gene expression in response to infection by enveloped viruses. J. Gen. Virol. 2005;86:2615–2625. doi: 10.1099/vir.0.81043-0. [DOI] [PubMed] [Google Scholar]
- Scholthof KB, Jackson AO, Van Etten JL. Myron Kendall Brakke: 1921 to 2007. Phytopathology. 2008;98:1056–1059. doi: 10.1094/PHYTO-98-10-1056. [DOI] [PubMed] [Google Scholar]
- Szymkowiak C, Kwan W-S, Su Q, Toner TJ, Shaw AR, Youil R. Rapid method for the characterization of 3′ and 5′ UTRs of influenza viruses. J. Virol. Methods. 2003;107:15–20. doi: 10.1016/s0166-0934(02)00184-2. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tatusov T, Tatusov R. Open reading frame finder. National Center for Biotechnology Information. National Institute of Health. 2007 http://www.ncbi.nlm.nih.gov/projects/gorf/
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordo N, Benmansour A, Calisher C, Dietzgen RG, Fang R-X, Jackson AO, Kurath G, Nadin-Davis S, Tesh RB, Walker PJ. Rhabdoviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy: Classification and Nomenclature of Viruses. 8th Report of the International Committee on the Taxonomy of Viruses. Elsevier Academic Press; London: 2005. pp. 623–644. [Google Scholar]
- Tsai C-H, Redinbaugh MG, Willie KJ, Reed S, Goodin M, Hogenhout S. Complete genome sequence and in planta subcellular localization of Maize fine streak virus proteins. J. Virol. 2005;9:5304–5314. doi: 10.1128/JVI.79.9.5304-5314.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek NAM, Lohuis D, Dijkstra J, Peters D. Morphogenesis of Sonchus yellow net virus in cowpea protoplasts. J. Ultrastruct. Res. 1985;90:294–303. [Google Scholar]
- Wagner RR, Prevec L, Brown F, Summers DF, Sokol F, MacLeod R. Classification of rhabdovirus proteins: a proposal. J. Virol. 1972;10:1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Meulia T, Meier I. Anchorage of plant RanGAP to the nuclear envelope involves novel nuclear-pore-associated hproteins. Curr. Biol. 2007;17:1157–1163. doi: 10.1016/j.cub.2007.05.076. [DOI] [PubMed] [Google Scholar]
- Zaltsman A, Yi BY, Krichevsky A, Gafni Y, Citovsky V. Yeast-plant coupled vector system for identification of nuclear proteins. Plant Physiol. 2007;145(4):1264–1271. doi: 10.1104/pp.107.105973. [DOI] [PMC free article] [PubMed] [Google Scholar]