Abstract
Aging science has recently drawn much attention, and discussions on the possibility of anti-aging medicine have multiplied. One potential target for the development of anti-aging drugs is the SIR2 (silent information regulator 2) family of NAD-dependent deacetylases/ADP-ribosyltransferases, called “sirtuins.” Sirtuins regulate many fundamental biological processes in response to a variety of environmental and nutritional stimuli. In mammals, the mammalian SIR2 ortholog SIRT1 has been most studied, and small molecule SIRT1 activators (STACs), including a plant-derived polyphenolic compound resveratrol, have been developed. On the other hand, sirtuin activity is regulated by NAD biosynthetic pathways, and nicotinamide phosphoribosyltransferase (NAMPT) plays a critical role in the regulation of mammalian sirtuin activity. Recent studies have provided a proof of concept for the idea that nicotinamide mononucleotide (NMN), the NAMPT reaction product, can be used a nutriceutical to activate SIRT1 activity. Based on these recent findings, the possibility of sirtuin-targeted nutriceutical development will be discussed.
Keywords: NAD-dependent deacetylases, sirtuins, SIRT1, NAD biosynthesis, nicotinamide phosphoribosyltransferase, NAMPT, anti-aging medicine, pharmaceuticals, nutriceuticals, aging, metabolism
Introduction
The 21st century is the era of pharmaceutical drug development. For example, in 2008 and 2009, the Lasker~DeBakey Clinical Medical Research Awards were given to the discovery of the statins and the development of Gleevec (a.k.a Glivec), respectively. From 2000 to 2005, the number of people purchasing statins increased from 15.8 million to 29.7 million, and the total expenditures for statins in an outpatient setting rose from $7.7 billion to $19.7 billion [1]. Based on Novartis’s media release, sales from Geevec/Glivec totaled $1.9 billion during the first half of 2009. These trends are just the tip of an iceberg. Pharmaceutical companies are now screening a myriad of compounds to target a variety of diseases, but only a tiny fraction of them become marketable drugs. In such pharmaceutical drug developments, there must always be clear target diseases or symptoms, most commonly type 2 diabetes, cancer, Alzheimer’s disease, atherosclerosis, and so on. But how about aging? Can aging per se be a decent, reasonable target for pharmaceutical drug development? The majority of scientists who are fully aware of the regulations and the hard reality of pharmaceutical drug development would answer “no.” Nonetheless, discussions pertaining to anti-aging drugs and medicine have multiplied in the recent past [2–4]. In these discussions, the concept of “anti-aging” connotes aging as something that must be corrected by pharmaceutical interventions, as if aging is one of many diseases that we face. Indeed, age-associated complications or pathological aspects of aging can be considered reasonable, treatable targets of pharmaceutical drugs, making efforts to develop drugs against age-associated complications exceedingly important in our predominantly aging society. However, the idea of “anti-aging” drugs does not seem to well suit the natural, physiological aspects of aging. Aging is a long, gradual process of functional decline which might not necessarily connect to diseases that need to be treated. One might think that it is more important to simply maintain better functionality throughout life than to try to treat individual age-associated complications with the battery of different drugs, but how can we achieve it? What scientific basis enables us to do so? To accomplish such a goal, we need a unique way of thinking and type of approach. In this review article, I will first summarize recent efforts to develop new drugs targeting the SIR2 (silent information regulator 2) family of proteins, now called “sirtuins,” for the treatment of diseases of aging. I will then discuss a new avenue of effective anti-aging interventions, namely, the possibility of neutriceuticals, based on the evolutionarily conserved connection between NAD-dependent sirtuins and NAD biosynthesis.
Development of small molecule sirtuin activators (STACs): A pharmaceutical approach for diseases of aging
Over the past decade since the discovery of NAD-dependent deacetylase activity of sirtuins [5–7], this novel connection between energy metabolism and protein deacetylation has inspired many scientists. Ranging from bacteria to humans, numerous target proteins have been identified for sirtuins, particularly for the mammalian SIR2 ortholog SIRT1. These studies have firmly established that the evolutionarily conserved NAD-dependent deacetylase activity of sirtuins regulates many fundamental biological processes in response to a variety of environmental and nutritional stimuli [8,9]. Among these key processes, the regulation of aging and longevity is clearly one of the most exciting aspects of sirtuin biology. In experimental model organisms including yeast, worms, and flies, the dosage or the activity of SIR2 and its orthologs determines the length of their life span [10–15]. In certain genetic backgrounds of these organisms, sirtuins also mediate the life span-extending effect of caloric restriction, a dietary regimen that retards aging and extends life span in a wide variety of organisms [13,16–19]. Mammals have seven sirtuin family members, SIRT1-7, but concrete evidence connecting alterations in mammalian sirtuin activity to aging and longevity is still awaited. Nonetheless, recent studies have provided support for the notion that SIRT1 is involved in the pathogenesis of age-associated complications, such as type 2 diabetes and Alzheimer’s disease, and the induction of age-associated physiological changes [9,20–23]. These unique aspects of SIRT1 function, already summarized and discussed in many review articles [20,22–25], have raised a broad interest in sirtuin-targeted pharmaceutical interventions, particularly against age-associated diseases.
David Sinclair and his colleagues pioneered the development of small molecule sirtuin activators (STACs) and reported that a group of polyphenolic compounds, including resveratrol, activates the catalytic activity of SIR2 and its orthologs and extends the life spans in yeast, worms, and flies [11,15]. Although this sirtuin-activating activity of resveratrol has been shown to be dependent on specific peptide substrates with a covalently attached fluorophore in vitro [26,27], two independent groups have provided in vivo evidence supporting the efficacy of resveratrol to counteract detrimental effects of high-fat diet on metabolism and other physiological parameters [28,29]. Resveratrol has also been shown to induce gene expression profiles similar to those induced by every-other-day feeding, a regimen known to convey physiological effects similar to those caused by caloric restriction, particularly in liver and skeletal muscle [30]. Resveratrol has other pleiotropic effects on multiple diseases of aging, including cardiovascular and neurodegenerative diseases [25,31]. More recently, new SIRT1-activating non-polyphenolic compounds, which are 1,000-fold more potent than resveratrol, have been developed [32]. These new STACs, particularly SRT1720, have so far been proven to improve metabolic complications in several different rodent models [32–35], providing a hope for the first anti-aging pharmaceutical drugs to treat pathological aspects of aging.
However, the development, evaluation, and usage of these STACs are still bound by the pharmaceutical approach of targeting specific symptoms or diseases of aging [25,36]. Such an approach does not provide any particular rationale for ordinary, healthy people to start taking these drugs until they develop specific target diseases of aging. It is for this reason exactly why nutriceuticals might better suit the physiological aspects of aging. Nutriceuticals are compounds that naturally exist in our body and provide specific, essential systemic functions. Their use as an anti-aging intervention stems from the idea that supplementing such essential compounds is able to maintain better body functionality throughout life. While this definition might sound like many other commercially available supplements, there is a strict distinction between ordinary supplements and nutriceuticals: the requirement of solid scientific foundation and rigorous clinical assessment. Omega-3 fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are a recent case of nutriceutical development [37], and the development of equol, an isoflavone derivative, could be another [38,39]. Can we learn from such examples to develop effective sirtuin-targeting nutriceuticals to improve our body functionality and prevent its decline? An important hint comes from the absolute requirement of NAD for sirtuin enzymatic activity.
Promoting sirtuin activity by manipulating NAD biosynthesis: lessons from yeast
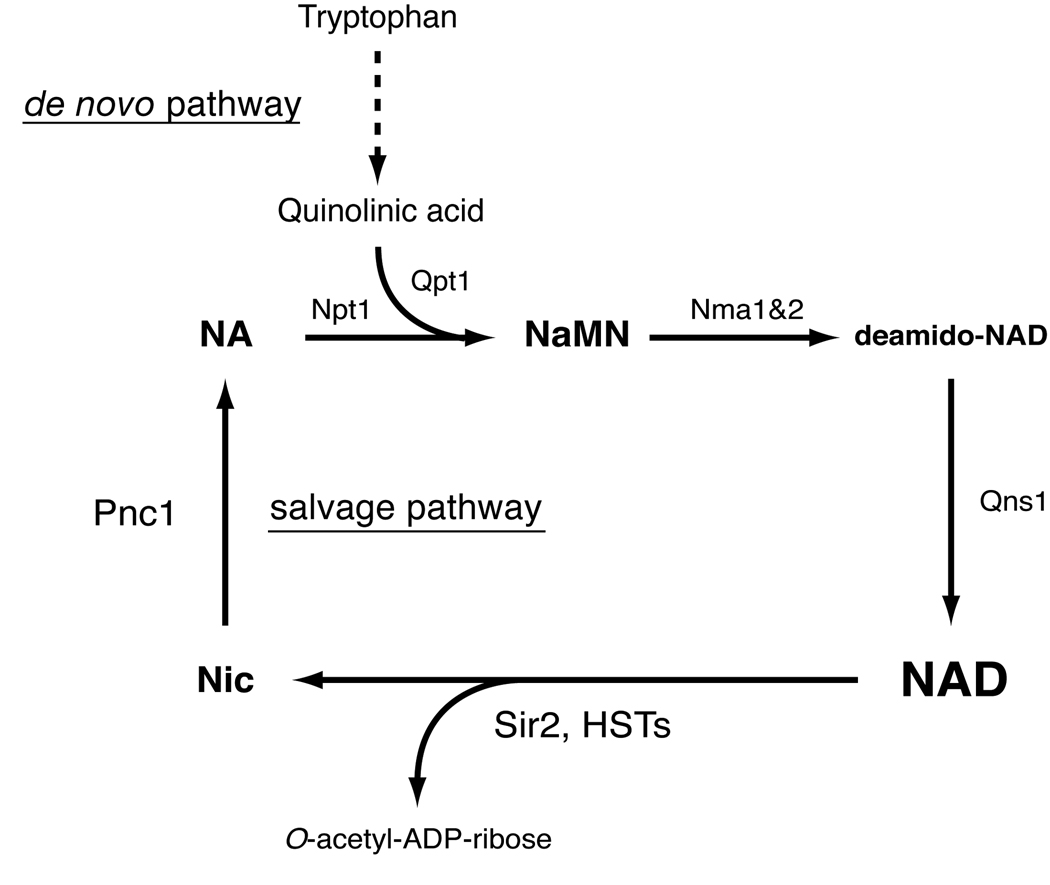
The NAD-dependent nature of sirtuin deacetylase activity is highly conserved from bacteria to mammals [40], suggesting an ancient and fundamental connection between NAD and sirtuins. NAD biosynthetic pathways have been well characterized in prokaryotes [41] and yeast [42,43]. In these organisms, NAD is synthesized by the de novo pathway via quinolinic acid and by the salvage pathway via nicotinic acid [41] (Figure 1). In yeast, the de novo pathway begins with tryptophan, which is converted to nicotinic acid mononucleotide (NaMN) through six enzymatic steps and one non-enzymatic reaction [43]. Upon NaMN synthesis, the de novo pathway converges with the salvage pathway. The salvage pathway begins with the breakdown of NAD into nicotinamide and ADP-ribose that is primarily catalyzed by yeast sirtuins, including SIR2 and HST1-4. Nicotinamide is then deamidated to nicotinic acid by a nicotinamidase encoded by the PNC1 gene. Nicotinic acid phosphoribosyltransferase (NPT1) converts nicotinic acid to NaMN, which is eventually converted to NAD through the sequential reactions of nicotinamide/nicotinic acid mononucleotide adenylyltransferase (NMA1 and NMA2) and NAD synthetase (QNS1).
Figure 1.
The de novo and salvage NAD biosynthetic pathways in the budding yeast Saccharomyces cerevisiae. Pnc1, Npt1, Nma1 and Nma2, Qns1, and Qpt1 are nicotinamidase, nicotinic acid phosphoribosyltransferase, nicotinic acid mononucleotide adenylyltransferase 1 and 2, NAD synthetase, and quinolinic acid phosphoribosyltransfease, respectively. This pathway is also conserved in C. elegans, Drosophila and other invertebrates. Nic, nicotinamide; NA, nicotinic acid; NaMN, nicotinic acid mononucleotide.
In yeast, the NAD salvage pathway has been shown to play an important role in regulating SIR2 activity [16,18,44]. Increased dosage of NPT1 increases SIR2-dependent transcriptional silencing and extends the life span of yeast mother cells [44]. Consistent with this finding, deletion of NPT1 causes a loss of SIR2-dependent silencing [45]. Importantly, PNC1 is induced by different types of stress, including glucose restriction, low amino acids, heat stress, and osmotic stress, and plays a critical role in promoting SIR2 activity and thereby life span in yeast [16], suggesting that PNC1 is a master regulator translating nutritional and environmental stimuli to the regulation of aging in yeast [46]. It has recently been reported that nicotinamide riboside (NR), another NAD precursor, promotes SIR2-dependent silencing and extends life span in yeast through two novel NR salvage pathways [47,48]. These findings support the intriguing idea that sirtuin activity can be manipulated by controlling NAD biosynthetic pathways.
Nicotinamide phosphoribosyltransferase (NAMPT): The key connecting NAD biosynthesis and sirtuin activity in mammals
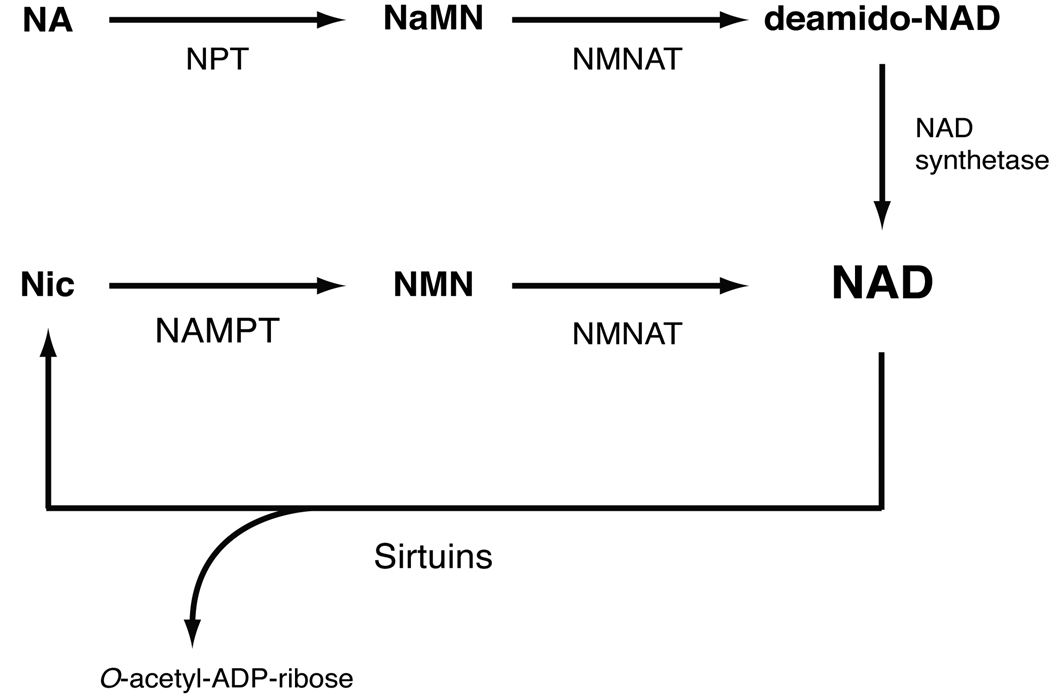
Vertebrate NAD biosynthesis markedly differs from that of yeast and invertebrates. Mammals predominantly use nicotinamide (a form of vitamin B3) rather than nicotinic acid (another form of vitamin B3) as a major precursor for NAD biosynthesis [49,50]. To initiate the NAD biosynthetic pathway directly from nicotinamide, mammals utilize the unique enzyme nicotinamide phosphoribosyltransferase (NAMPT) [51,52] (Figure 2). NAMPT is the rate-limiting enzyme in the NAD biosynthesis pathway from nicotinamide, catalyzing the conversion of nicotinamide and 5’-phosphoribosyl-1-pyrophosphate (5’-PRPP) to nicotinamide mononucleotide (NMN). NMN is then converted to NAD by the second enzyme in this pathway, nicotinamide/nicotinic acid mononucleotide adenylyltransferase (NMNAT). In mammals, NMNAT has three different isoforms, NMNAT1-3, that are localized in the nucleus, cytoplasm, and mitochondria, respectively [53].
Figure 2.
NAD biosynthetic pathways from nicotinamide and nicotinic acid in mammals. The de novo pathway from tryptophan is not shown in this scheme. These pathways, are conserved throughout vertebrates. Nicotinamide is the main precursor for NAD biosynthesis in mammals. NPT, NAMPT, and NMNAT are nicotinic acid phosphoribosyltransferase, nicotinamide phosphoribosyltransferase, and nicotinamide/nicotinic acid mononucleotide adenylyltransferase, respectively. Multiple enzymes break NAD down to nicotinamide and ADP-ribose, but only sirtuins are shown in this scheme. Nic, nicotinamide; NA, nicotinic acid; NaMN, nicotinic acid mononucleotide; NMN, nicotinamide mononucleotide.
The enzymatic activity of NAMPT was originally reported by Preiss and Handler in 1957 [54]. However, the gene encoding NAMPT was first identified in Haemophilus ducreyi in 2001 [55], revealing a surprising finding that bacterial NAMPT shows very high homology to mammalian NAMPT (a.k.a. PBEF or visfatin). Indeed, NAMPT has an ancient origin as an NAD biosynthetic enzyme. The entire pyridine nucleotide salvage cycle containing NAMPT, NMNAT, and SIR2 homologues exists even in the vibriophage [56]. Despite its ancient origin, NAMPT has a peculiar phylogenetic distribution. No other organisms between bacteria and vertebrates have obvious homologs of NAMPT, with several exceptions [57]. Interestingly, the organisms lacking NAMPT homologs, including yeast, worms, and flies, unanimously have nicotinamidase (PNC1) homologues [58]. It is likely that the organisms that have nicotinamidase use nicotinic acid as a precursor for NAD biosynthesis, while the organisms that have NAMPT use nicotinamide as the main precursor for NAD biosynthesis. Because no obvious homologues of PNC1 have been found in vertebrates [50], the presence of NAMPT, which allows a more direct pathway for NAD biosynthesis from nicotinamide, clearly distinguishes NAD biosynthesis in vertebrates from that in yeast and invertebrates.
In mammals, NAMPT has intra- and extracellular forms (iNAMPT and eNAMPT, respectively). iNAMPT is expressed in a wide variety of tissues and organs: highest in the brown adipose tissue (BAT), liver, and kidney, intermediate in the heart, low in the white adipose tissue (WAT), lung, spleen, testis, and skeletal muscle, and under a detectable level in the pancreas and brain [59]. On the other hand, eNAMPT is positively secreted through a non-classical secretory pathway by fully differentiated mouse and human adipocytes [59], as well as rat and human primary hepatocytes [60]. eNAMPT exhibits robust, even higher NAD biosynthetic activity compared to iNAMPT, likely contributing to the extracellular biosynthesis of NMN. Therefore, both iNAMPT and eNAMPT play a critical role in the regulation of NAD biosynthesis at a systemic level in mammals.
Accumulating bodies of evidence have clearly shown that NAMPT-mediated NAD biosynthesis regulates the activity of sirtuins, particularly SIRT1, in a variety of cellular and physiological conditions. Increased dosage of iNAMPT enhances total cellular NAD levels and thereby SIRT1 activity in mouse fibroblasts [57]. Indeed, SIRT1 activity is regulated by NAMPT and NMNAT1, the nuclear NMNAT isoform, at the target gene promoters where both SIRT1 and NMNAT1 are recruited through their interaction [61]. The NAMPT/SIRT1 pathway also plays an important role in the regulation of cellular differentiation. In human vascular smooth muscle cells (SMCs), iNAMPT levels are up-regulated during SMC maturation. Increased iNAMPT promotes their maturation [62] and cellular life span [63,64] by enhancing SIRT1 activity. In skeletal myoblasts, glucose restriction inhibits their differentiation through the AMP-activated protein kinase (AMPK)-dependent induction of Nampt expression and the resultant activation of SIRT1 [65]. The NAMPT/SIRT1 pathway also mediates granulocyte colony-stimulating factor (G-CSF)-triggered granulocyte differentiation in vitro and in vivo [66]. Furthermore, the connection between NAMPT-mediated NAD biosynthesis and sirtuins plays a critical role in regulation of the stress response. Increased iNAMPT protects cardiac myocytes from cell death by a SIRT1-mediated mechanism [67]. NAMPT levels in the heart significantly decrease in several pathophysiological conditions, including ischemia, ischemia/reperfusion, and pressure overload, and heart-specific Nampt transgenic mice prevent this reduction in NAMPT levels and thereby oppose to cardiac damages and apoptosis induced by prolonged ischemia and ischemia/reperfusion [68]. Under genotoxic stress, increased iNAMPT maintains NAD levels in mitochondria and protects cells from cell death through activation of the mitochondrial sirtuins SIRT3 and SIRT4 [69].
NAMPT-mediated NAD biosynthesis also provides a novel layer of metabolic regulation through SIRT1 and other sirtuins. In pancreatic β cells, both SIRT1 and NAMPT-mediated NAD biosynthesis play important roles in the regulation of glucose-stimulated insulin secretion [59,70,71]. More recently, we and others have demonstrated that the NAMPT/SIRT1 pathway modulates circadian transcriptional regulation through a novel circadian clock feedback loop in which NAD functions as a metabolic oscillator and regulates the core clock machinery through SIRT1 [72,73]. NAMPT-mediated NAD biosynthesis also regulates SIRT5 activity in mitochondria in response to low nutritional input [74]. Therefore, the connection between NAD biosynthesis and sirtuins is evolutionarily universal and important, and in mammals, the intimate connection between NAMPT-mediated NAD biosynthesis and sirtuins comprises a critical regulatory network for the regulation of many fundamental biological processes at a systemic level.
A possibility of nutriceuticals as an anti-aging intervention: promoting mammalian sirtuin activity by administering key NAD intermediates
As summarized above, it is now clear that NAMPT-mediated NAD biosynthesis regulates sirtuin activity, particularly SIRT1 activity, in a number of different cellular and physiological conditions. Therefore, it is conceivable that activating SIRT1 by promoting NAD biosynthesis could be another efficient anti-aging intervention (Figure 3). One way to promote NAD biosynthesis and SIRT1 activity in mammals is to use key NAD intermediates, such as NMN and NR, as nutriceuticals. Using NAD itself is probably not a viable idea because NAD administration causes serious hyperglycemia in mice (our unpublished observation) likely due to the stimulation of glycogenolysis in the liver [75]. The idea to supply key NAD intermediates has been brought to the field by a series of our own studies in which we have administered NMN to multiple mouse models of metabolic complications. One of these models was Nampt-heterozygous (Nampt+/−) mice [52,59]. Whereas Nampt-homozygous (Nampt−/−) mice are lethal around embryonic day 10.5, Nampt+/− mice are born and grow normally through adulthood. Interestingly, Nampt+/− females develop moderately impaired glucose tolerance due to a significant defect in glucose-stimulated insulin secretion. Consistent with this phenotype, primary islets isolated from Nampt+/− mice exhibit defects in NAD biosynthesis and insulin secretion. These defects in glucose-stimulated insulin secretion observed in Nampt+/− mice and islets are fully ameliorated by NMN administration. Another model was the BESTO (pancreatic β cell-specific SIRT1-overexpressing) transgenic mouse [71,76]. Young BESTO mice exhibit significantly enhanced glucose-stimulated insulin secretion and improved glucose tolerance. However, these phenotypes are lost completely by 18–24 months of age. Even though SIRT1 protein levels remain overexpressed in old BESTO islets, its activity decreases. The loss of SIRT1 activity in old BESTO islets appears to be due to an age-associated decrease in NAMPT-mediated systemic NAD biosynthesis [76]. Consistent with this notion, NMN administration to aged BESTO mice is able to restore the original phenotypes apparent in young BESTO mice. NMN administration is also able to restore significantly higher levels of glucose-stimulated insulin secretion to aged wild-type mice. Given that a progressive age-associated decline in β cell function has been suggested to be one of the major contributing factors to the pathogenesis of type 2 diabetes [77–79], promoting NAD biosynthesis and SIRT1 activity by NMN could be a novel preventive/therapeutic approach to maintain the functionality of pancreatic β cells.
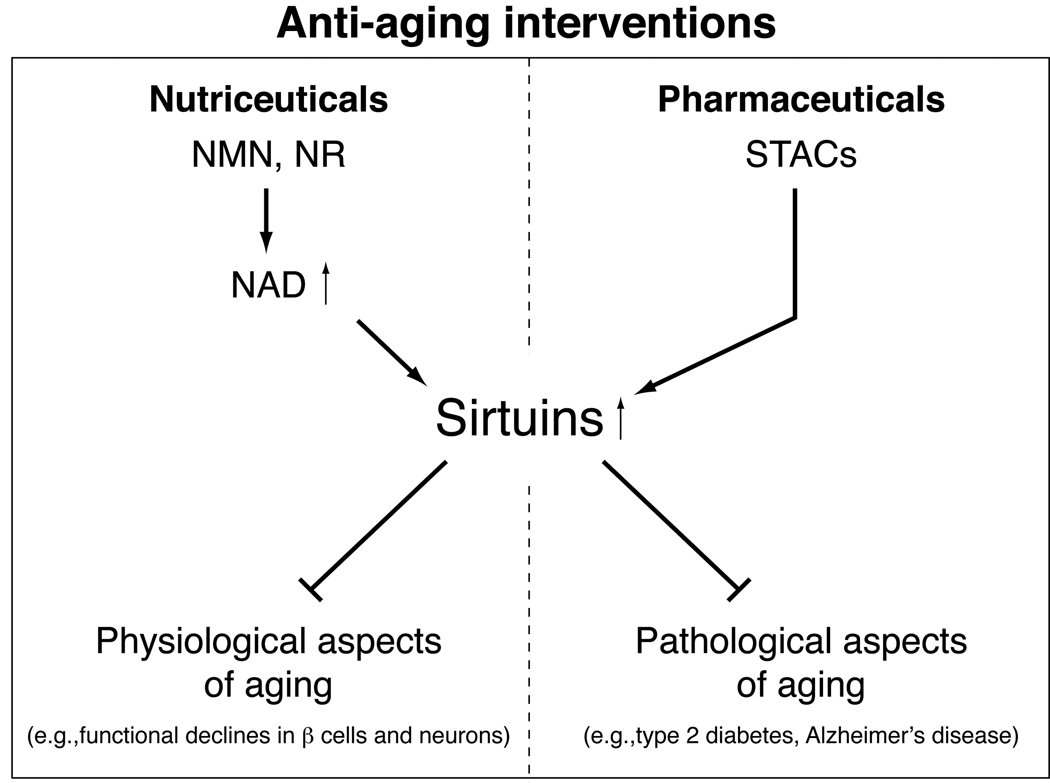
Figure 3.
Sirtuin-targeted nutriceutical and pharmaceutical anti-aging interventions. Whereas small molecule sirtuin activators (STACs) aim to treat pathological aspects of aging as pharmaceutical drugs, key NAD intermediates, such as nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR), can be used to manage physiological aspects of aging and prevent functional declines in particular cell types, including pancreatic β cells and neurons, as nutriceuticals. See text for details.
An idea similar to that just proposed for pancreatic β cells might be applicable to neurons as both cell types have such low levels of iNAMPT that they must rely on an extracellular source of NAD intermediates to maintain sufficient NAD biosynthesis [21]. Considering that one of the triad symptoms in pellagra, vitamin B3 deficiency, is dementia [80], it is plausible that the age-associated decline in NAMPT-mediated systemic NAD biosynthesis causes neurological problems. If this is the case, it will be of great interest to examine whether NMN administration is also able to improve age-associated neurological complications in mammals.
Taken together, our findings have provided evidence for the notion that applying endogenous NAD intermediates can be an effective anti-aging nutriceutical approach. The intimate connection between NAD biosynthesis and sirtuin activity suggests that promoting NAD biosynthesis by nutriceutical NMN application could effectively enhance sirtuin activity at a systemic level and maintain better body functionality, particularly the functionality of key cell types, such as pancreatic β cells and neurons, throughout our entire lives [21] (Figure 3). Such an approach is well-suited to deal with the natural, physiological aspects of aging, as well as the pathophyiological aspects of aging. The efficacy of NMN is currently being assessed for age-associated metabolic alterations in mice, which will hopefully lead to testing this key NAD intermediate in humans in the near future.
Concluding remarks
Societies in most advanced countries are now experiencing serious issues to accommodate their large populations of aging baby boomers. Considering a frightening increase in medical expenses required for the elderly, it is indeed urgent to develop effective, affordable anti-aging interventions to maximize the duration of high quality of life. The biology of sirtuins and NAD biosynthesis provides a substantial opportunity to think about how to achieve this challenging goal. In this regard, the development of effective, scientifically sound nutriceutical interventions provides a reasonable option to deal with physiological, as well as pathological, aspects of aging in this new arena of anti-aging medicine. More rigorous investigation on key NAD intermediates and sirtuin-related biology will hopefully make this dream a reality.
Acknowledgments
I apologize to those whose work is not cited due to the focus of this review and space limitations. I thank Liana Roberts, Cynthia Brace, and Jun Yoshino for their helpful discussions and comments. This work was supported by grants from the National Institute on Aging (AG024150), Ellison Medical Foundation, and Longer Life Foundation to S. I. S.I. serves as a scientific advisory board member for Sirtris Pharmaceuticals, a GSK company.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stagnitti MN. Quality AfHRa ed. 2008. Trends in statins utilization and expenditures for the U.S. Civilian noninstitutionalized population, 2000 and 2005. [PubMed] [Google Scholar]
- 2.Blagosklonny MV. An anti-aging drug today: From senescence-promoting genes to anti-aging pill. Drug Discov Today. 2007;12:218–224. doi: 10.1016/j.drudis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Wick G. 'anti-aging' medicine: Does it exist? A critical discussion of 'anti-aging health products'. Exp Gerontol. 2002;37:1137–1140. doi: 10.1016/s0531-5565(02)00081-5. [DOI] [PubMed] [Google Scholar]
- 4.Ho YS, So KF, Chang RC. Anti-aging herbal medicine-how and why can they be used in aging-associated neurodegenerative diseases? Ageing Res Rev. 2009 doi: 10.1016/j.arr.2009.10.001. Epub on Oct 13. [DOI] [PubMed] [Google Scholar]
- 5.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 6.Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R. The silencing protein sir2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci USA. 2000;97:5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, Avalos JL, Escalante-Semerena JC, Grubmeyer C, Wolberger C, Boeke JD. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the sir2 protein family. Proc Natl Acad Sci USA. 2000;97:6658–6663. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blander G, Guarente L. The sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 9.Imai S, Guarente L. Sirtuins: A universal link between NAD, metabolism, and aging. In: Guarente L, Partridge L, Wallace D, editors. The molecular biology of aging. New York: Cold Spring Habor Laboratory Press; 2007. pp. 39–72. [Google Scholar]
- 10.Astrom SU, Cline TW, Rine J. The drosophila melanogaster sir2+ gene is nonessential and has only minor effects on position-effect variegation. Genetics. 2003;163:931–937. doi: 10.1093/genetics/163.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 12.Kaeberlein M, McVey M, Guarente L. The sir2/3/4 complex and sir2 alone promote longevity in saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 15.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 16.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and pnc1 govern lifespan extension by calorie restriction in saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin S-J, Defossez P-A, Guarente L. Life span extension by calorie restriction in s. Cerevisiae requires NAD and sir2. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 18.Lin S-J, Kaeberlein M, Andalis AA, Sturtz LA, Defossez P-A, Culotta VC, Fink GR, Guarente L. Calorie restriction extends saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Tissenbaum HA. Overlapping and distinct functions for a caenorhabditis elegans sir2 and daf-16/foxo. Mech Ageing Dev. 2006;127:48–56. doi: 10.1016/j.mad.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: The 'magnificent seven, function, metabolism and longevity. Ann Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- 21.Imai S. The NAD world: A new systemic regulatory network for metabolism and aging - SIRT1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys. 2009;53:65–74. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michan S, Sinclair D. Sirtuins in mammals: Insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008;7:104–112. doi: 10.1016/j.cmet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Imai S, Kiess W. Therapeutic potential of SIRT1 and nampt-mediated NAD biosynthesis in type 2 diabetes. Front Biosci. 2009;14:2983–2995. doi: 10.2741/3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milne JC, Denu JM. The sirtuin family: Therapeutic targets to treat diseases of aging. Curr Opin Chem Biol. 2008;12:11–17. doi: 10.1016/j.cbpa.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 27.Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell S, Napper A, Curtis R, Distefano PS, Fields S, Bedalov A, Kennedy BK. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 28.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and pgc-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 32.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, Israelian K, Song J, Razvadauskaite G, Lynch AV, Carney DP, Johnson RJ, Lavu S, Iffland A, Elliott PJ, Lambert PD, Elliston KO, Jirousek MR, Milne JC, Boss O. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamazaki Y, Usui I, Kanatani Y, Matsuya Y, Tsuneyama K, Fujisaka S, Bukhari A, Suzuki H, Senda S, Imanishi S, Hirata K, Ishiki M, Hayashi R, Urakaze M, Nemoto H, Kobayashi M, Tobe K. Treatment with srt1720, a SIRT1 activator, ameliorates fatty liver with reduced expression of lipogenic enzymes in msg mice. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.90997.2008. Epub on Sept 1. [DOI] [PubMed] [Google Scholar]
- 36.Westphal CH, Dipp MA, Guarente L. A therapeutic role for sirtuins in diseases of aging? Trends Biochem Sci. 2007;32:555–560. doi: 10.1016/j.tibs.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Holub DJ, Holub BJ. Omega-3 fatty acids from fish oils and cardiovascular disease. Mol Cell Biochem. 2004;263:217–225. doi: 10.1023/B:MCBI.0000041863.11248.8d. [DOI] [PubMed] [Google Scholar]
- 38.Martin D, Song J, Mark C, Eyster K. Understanding the cardiovascular actions of soy isoflavones: Potential novel targets for antihypertensive drug development. Cardiovasc Hematol Disord Drug Targets. 2008;8:297–312. doi: 10.2174/187152908786786214. [DOI] [PubMed] [Google Scholar]
- 39.Mann GE, Bonacasa B, Ishii T, Siow RC. Targeting the redox sensitive nrf2-keap1 defense pathway in cardiovascular disease: Protection afforded by dietary isoflavones. Curr Opin Pharmacol. 2009;9:139–145. doi: 10.1016/j.coph.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Frye RA. Phylogenetic classification of prokaryotic and eukaryotic sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- 41.Penfound T, Foster JW. Biosynthesis and recycling of NAD. In: Neidhardt FC, editor. Escherichia coli and salmonella: Cellular and molecular biology. Washington, D.C: ASM Press; 1996. pp. 721–730. [Google Scholar]
- 42.Denu JM. Linking chromatin function with metabolic networks: Sir2 family of NAD(+)-dependent deacetylases. Trends Biochem Sci. 2003;28:41–48. doi: 10.1016/s0968-0004(02)00005-1. [DOI] [PubMed] [Google Scholar]
- 43.Lin S-J, Guarente L. Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr Opin Cell Biol. 2003;15:241–246. doi: 10.1016/s0955-0674(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 44.Anderson R, Bitterman K, Wood J, Medvedik O, Cohen H, Lin S, Manchester J, Gordon J, Sinclair D. Manipulation of a nuclear NAD+ salvage pathway delays aging without altering steady-state NAD+ levels. J Biol Chem. 2002;277:18881–18890. doi: 10.1074/jbc.M111773200. [DOI] [PubMed] [Google Scholar]
- 45.Sandmeier J, Celic I, Boeke J, Smith J. Telomeric and rdna silencing in saccharomyces cerevisiae are dependent on a nuclear NAD(+) salvage pathway. Genetics. 2002;160:877–889. doi: 10.1093/genetics/160.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang H, Lavu S, Sinclair DA. Nampt/pbef/visfatin: A regulator of mammalian health and longevity? Exp Gerontol. 2006;41:718–726. doi: 10.1016/j.exger.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide riboside promotes sir2 silencing and extends lifespan via nrk and urh1/pnp1/meu1 pathways to NAD+ Cell. 2007;129:473–484. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 48.Lu SP, Kato M, Lin SJ. Assimilation of endogenous nicotinamide riboside is essential for calorie restriction-mediated life span extension in saccharomyces cerevisiae. J Biol Chem. 2009;284:17110–17119. doi: 10.1074/jbc.M109.004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magni G, Amici A, Emanuelli M, Raffaelli N, Ruggieri S. Enzymology of NAD+ synthesis. Adv Enzymol Relat Areas Mol Biol. 1999;73:135–182. doi: 10.1002/9780470123195.ch5. [DOI] [PubMed] [Google Scholar]
- 50.Rongvaux A, Andris F, Van Gool F, Leo O. Reconstructing eukaryotic NAD metabolism. Bioessays. 2003;25:683–690. doi: 10.1002/bies.10297. [DOI] [PubMed] [Google Scholar]
- 51.Garten A, Petzold S, Korner A, Imai S, Kiess W. Nampt: Linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20:130–138. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imai S. Nicotinamide phosphoribosyltransferase (nampt): A link between NAD biology, metabolism, and diseases. Curr Pharm Des. 2009;15:20–28. doi: 10.2174/138161209787185814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lau C, Niere M, Ziegler M. The nmn/namn adenylyltransferase (nmnat) protein family. Front Biosci. 2009;14:410–431. doi: 10.2741/3252. [DOI] [PubMed] [Google Scholar]
- 54.Preiss J, Handler P. Enzymatic synthesis of nicotinamide mononucleotide. J Biol Chem. 1957;225:759–770. [PubMed] [Google Scholar]
- 55.Martin P, Shea R, Mulks M. Identification of a plasmid-encoded gene from haemophilus ducreyi which confers NAD independence. J Bacteriol. 2001;183:1168–1174. doi: 10.1128/JB.183.4.1168-1174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller ES, Heidelberg JF, Eisen JA, Nelson WC, Durkin AS, Ciecko A, Feldblyum TV, White O, Paulsen IT, Nierman WC, Lee J, Szczypinski B, Fraser CM. Complete genome sequence of the broad-host-range vibriophage kvp40: Comparative genomics of a t4-related bacteriophage. J Bacteriol. 2003;185:5220–5233. doi: 10.1128/JB.185.17.5220-5233.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 58.Ghislain M, Talla E, Francois JM. Identification and functional analysis of the saccharomyces cerevisiae nicotinamidase gene, pnc1. Yeast. 2002;19:215–324. doi: 10.1002/yea.810. [DOI] [PubMed] [Google Scholar]
- 59.Revollo JR, Körner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S. Nampt/pbef/visfatin regulates insulin secretion in β cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garten A, Petzold S, Barnikol-Oettler A, Korner A, Thasler WE, Kratzsch J, Kiess W, Gebhardt R. Nicotinamide phosphoribosyltransferase (nampt/pbef/visfatin) is constitutively released from human hepatocytes. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.11.066. Epub on Nov 11. [DOI] [PubMed] [Google Scholar]
- 61.Zhang T, Berrocal JG, Frizzell KM, Gamble MJ, DuMond ME, Krishnakumar R, Yang T, Sauve AA, Kraus WL. Enzymes in the NAD+ salvage pathway regulate SIRT1 activity at target gene promoters. J Biol Chem. 2009;284:20408–20417. doi: 10.1074/jbc.M109.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Veer E, Nong Z, O'Neil C, Urquhart B, Freeman D, Pickering JG. Pre-b-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res. 2005;97:25–34. doi: 10.1161/01.RES.0000173298.38808.27. [DOI] [PubMed] [Google Scholar]
- 63.van der Veer E, Ho C, O'Neil C, Barbosa N, Scott R, Cregan SP, Pickering JG. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem. 2007;282:10841–10845. doi: 10.1074/jbc.C700018200. [DOI] [PubMed] [Google Scholar]
- 64.Ho C, van der Veer E, Akiwa O, Pickering JG. SIRT1 markedly extends replicative lifespan if NAD(+) salvage is enhanced. FEBS Lett. 2009 doi: 10.1016/j.febslet.2009.08.031. Epub on Aug 28. [DOI] [PubMed] [Google Scholar]
- 65.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through ampk-mediated regulation of nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skokowa J, Lan D, Thakur BK, Wang F, Gupta K, Cario G, Brechlin AM, Schambach A, Hinrichsen L, Meyer G, Gaestel M, Stanulla M, Tong Q, Welte K. Nampt is essential for the g-csf-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway. Nat Med. 2009;15:151–158. doi: 10.1038/nm.1913. [DOI] [PubMed] [Google Scholar]
- 67.Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(adp-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced sir2α deacetylase activity. J Biol Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- 68.Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD(+) levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, Easlon EJ, Lin SJ, Guarente L. SIRT1 regulates insulin secretion by repressing ucp2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S. Increased dosage of mammalian sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by clock-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. Circadian clock feedback cycle through nampt-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakagawa T, Lomb DJ, Haigis MC, Guarente L. Sirt5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Broetto-Biazon AC, Bracht A, Ishii-Iwamoto EL, de Moraes Silva V, Kelmer-Bracht AM. The action of extracellular NAD+ on ca2+ efflux, hemodynamics and some metabolic parameters in the isolated perfused rat liver. Eur J Pharmacol. 2004;484:291–301. doi: 10.1016/j.ejphar.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 76.Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of SIRT1-mediated enhancement of glucose-stimulated insulin secretion in β cell-specific SIRT1-overexpressing (besto) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. Mechanisms of the age-associated deterioration in glucose tolerance: Contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–1748. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- 78.Iozzo P, Beck-Nielsen H, Laakso M, Smith U, Yki-Jarvinen H, Ferrannini E. Independent influence of age on basal insulin secretion in nondiabetic humans. European group for the study of insulin resistance. J Clin Endocrinol Metab. 1999;84:863–868. doi: 10.1210/jcem.84.3.5542. [DOI] [PubMed] [Google Scholar]
- 79.Muzumdar R, Ma X, Atzmon G, Vuguin P, Yang X, Barzilai N. Decrease in glucose-stimulated insulin secretion with aging is independent of insulin action. Diabetes. 2004;53:441–446. doi: 10.2337/diabetes.53.2.441. [DOI] [PubMed] [Google Scholar]
- 80.Williams AC, Ramsden DB. Pellagra: A clue as to why energy failure causes diseases? Med Hypotheses. 2007;69:618–628. doi: 10.1016/j.mehy.2007.01.029. [DOI] [PubMed] [Google Scholar]