Abstract
The R49C mutation of αA-crystallin (αA-R49C) causes hereditary cataracts in humans; patients in a four-generation Caucasian family were found be heterozygous for this autosomal dominant mutation. We previously generated knock-in mouse models of this mutation and found that by 2 months of age, heterozygous mutant mice exhibited minor lens defects including reduced protein solubility, altered signaling in epithelial and fiber cells, and aberrant interactions between αA-crystallin and other lens proteins. In contrast, homozygous mutant αA-R49C knock-in mice displayed earlier and more extensive lens defects including small eyes and small lenses at birth, death of epithelial and fiber cells, and the formation of posterior, nuclear, and cortical cataracts in the first month of life. We have extended this study to now show that in αA-R49C homozygous mutant mice, epithelial cells failed to form normal equatorial bow regions and fiber cells continued to die as the mice aged, resulting in a complete loss of lenses and overall eye structure in mice older than 4 months. These results demonstrate that expression of the hereditary R49C mutant of αA-crystallin in vivo is sufficient to adversely affect lens growth, lens cell morphology, and eye function. The death of fiber cells caused by this mutation may ultimately lead to loss of retinal integrity and blindness.
Keywords: α-crystallin, cataract, eye, lens, mutation
The normal, healthy ocular lens is a highly organized structure composed of a thin anterior surface covered by a single layer of epithelial cells and an ovoid lens formed by elongated lens fiber cells that contain a high concentration of crystallin proteins. The entire lens mass is enclosed by a basement membrane commonly called the capsule. The human lens grows throughout life by ongoing cell division in the anterior equatorial epithelium. Some of the daughter cells differentiate into lens fiber cells: long, slender cells with ends near either lens pole (Kuszak et al., 2004; Kuszak et al., 2006; Augusteyn, 2007). Murine lens growth occurs most rapidly during early lens development and levels off markedly with age (Kuszak et al., 2006; Augusteyn, 2008). The epithelial layer contains three distinct zones of proliferative activity including 1) the central epithelium, with the lowest mitotic activity; 2) the zone anterior to the lens equator (also known as the germinative zone), with the highest level of cell division; and 3) the transitional zone just posterior to the germinative zone, where there is no cell division and the epithelial cells begin to differentiate into the elongated fiber cells that ultimately form the lens cortical bers (Beebe et al., 2001; Robinson, 2006; Bassnett, 2009).
In many animal models of lens development, the transitional zone has been found to be displaced posterior or anterior of the equator (Shirke et al., 2001; Xie et al., 2007; Rajagopal et al., 2008). For example, transgenic mice overexpressing insulin like growth factor 1 (IGF-1) have an expanded epithelial compartment toward the lens posterior pole (Shirke et al., 2001). This causes expansion of the germinative and transitional zones, suggesting that IGF-1 provides a spatial cue that helps control the normal location of these epithelial zones. Overexpression of insulin in another transgenic mouse model causes a shift of the transitional zone and reduced lens epithelial cell number (Xie et al., 2007). In contrast, mice with a conditional gene deletion of the bone morphogenetic receptor type 1 fail to form a transitional zone at all, suggesting that this receptor is required for cells to withdraw from the cell cycle (Rajagopal et al., 2008). In this study, we describe a method for quantifying the lens proliferation zones from images of histological sections of wild type, αA-R49C heterozygous, and αA-R49C homozygous mutant mice.
Mice were maintained at the Washington University Division of Comparative Medicine by trained veterinary staff. All protocols using animals were approved by the Animal Studies Committee, and followed institutional guidelines for the use and care of animals in research. Mice expressing the αA-R49C mutant protein were generated as described previously (Andley et al., 2008; Xi et al., 2008). The mutation was not lethal in mice, allowing heterozygous mice to be interbred to generate litters containing wild type, heterozygous, and homozygous progeny. Homozygous mice were interbred to maintain homozygous lines. Eyes were freshly dissected, fixed, and stained for histology. Mice were examined by slit lamp biomicroscopy at various ages ranging from the postnatal stage after eye opening to 9 months old. A total of 76 lenses were analyzed by histology. Overall, we analyzed 17 wild type lenses (newborn to 2.5 weeks, n=10; 2–6 months, n=6; 9 months, n=1), 33 heterozygous lenses (newborn to 2.5 weeks, n=10; 2–6 months, n=13; 9–12 months, n=10), and 26 homozygous lenses (newborn to 2.5 weeks, n=21; 2–6 months, n=3; 9 months, n=2).
Eyes were fixed immediately in 10% neutral-buffered formalin (Fisher Scientific, Pittsburgh, PA, USA), and paraffin sections were processed for staining or were labeled with a monoclonal antibody to Ki67 (BD Pharmingen, San Jose, CA, USA). To align paraffin-embedded eyes for sectioning, fixed eyes were placed with the cornea on the left side and the optic nerve on the right from the sectioner’s perspective. The optic nerve was in an even plane with the center of the cornea. The knife was perpendicular to the paraffin block, and adjusted so that when it moved towards the center of the eye, the cornea, lens, and optic nerve were in the same plane. Unstained sections were examined under a microscope to determine whether it was necessary to section deeper into the eye. Sequential sections showing the iris opening indicated that we were approaching the center of the eye. In addition, the lens became larger as we approached the eye center. Sections were stained when the lens and iris opening were no longer increasing in size. After this point the iris opening began to get smaller, indicating that we had passed the halfway point corresponding to the center of the eye. For histological staining, 4-μm mid-sagittal sections were cut, mounted on slides, and air-dried overnight. Slides were placed in a 60°C oven for 20 minutes, washed twice with xylene for 10 minutes, washed twice with 100% ethanol for 3 minutes, washed twice with 95% ethanol for 3 minutes, and washed once with 70% ethanol for 3 minutes. Sections were then washed in distilled water for 1 minute, stained with hematoxylin 560 for 5 minutes (Surgipath, Richmond, IL, USA), washed in distilled water, and incubated in Define acid alcohol concentrate (Surgipath) for 1 minute. After washing thoroughly in distilled water, slides were stained with Blue Buffer bluing concentrate (Surgipath) for 1 minute, washed in distilled water, washed with 80% ethanol for 1 minute, stained with alcoholic eosin Y 515 (Surgipath) for 1 minute, washed twice in 95% ethanol for 30 seconds, washed twice in 100% ethanol for 1 minute, and washed three times in xylene for 5 minutes. Coverslips were applied to slides using Cytoseal 60 (Fisher Scientific). Bright eld images of sections were taken with an Olympus uorescence microscope tted with a digital camera (Spot Diagnostic Instruments, Sterling Heights, MI, USA) or a Zeiss 510 confocal microscope as described previously (Andley et al., 1998).
The location and number of proliferating cells were determined by dividing the images of lens histological sections into sectors in 15° increments, with the origin at the intersection of the equatorial plane and the optical axis (Shirke et al., 2001; Rajagopal et al., 2008). The point of origin was identi ed in PhotoShop CS3 (Adobe Systems, Inc.; San Jose, CA, USA), then fed as input into a MATLAB program (v7.0; Mathworks, Inc., Natick, MA, USA) which automatically overlaid the image with radial lines at 15° intervals. The percentage of Ki67-labeled cells within each sector was then easily, reproducibly, and objectively determined. Cell counts for corresponding sectors on the left and right sides were averaged such that the distribution tracked changes from the anterior to the posterior pole. Three to six sections of each genotype were analyzed.
In our previous work, we demonstrated that the R49C mutation in αA-crystallin causes lens opacities in heterozygous and homozygous mutant mice (Andley et al., 2008; Xi et al., 2008). These opacities appear about 2 months after birth of heterozygous mice and at birth in homozygous mutant mice. In the present work, we have extended our previous study of R49C knock-in mouse lenses to examine lens histology and proliferation changes resulting from this mutation. Qualitative observations clearly indicated a severely disrupted, though highly variable, defective lens phenotype in homozygous αA-R49C mice (Fig. 1).
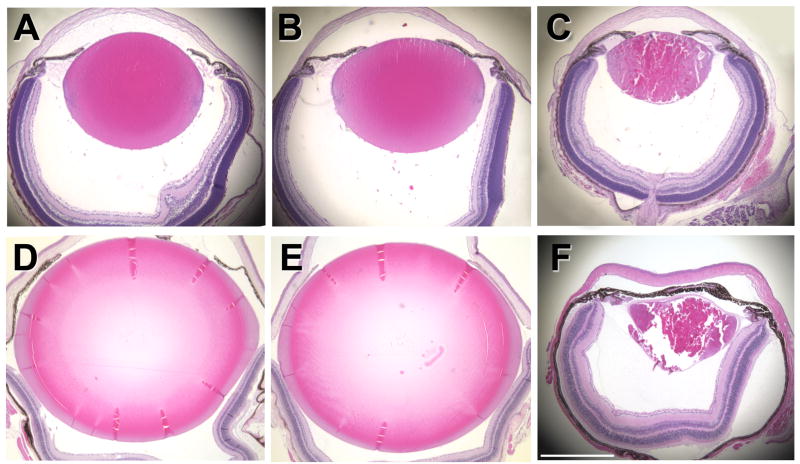
Figure 1.
Histology of eyes from wild type and αA-R49C mutant mice. Above: Eyes from 6-day-old wild type (A), heterozygous (B), and homozygous mutant (C) mice. Below: Eyes from 4-month-old wild type (D), heterozygous (E), and homozygous mutant (F) mice. Mid-sagittal sections were stained with hematoxylin and eosin. At least three sections were analyzed for each age and genotype. Scale bar in (F) = 1 mm for each image (A–F).
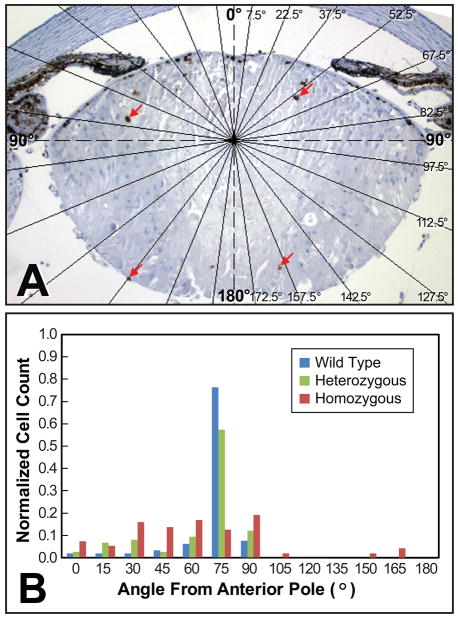
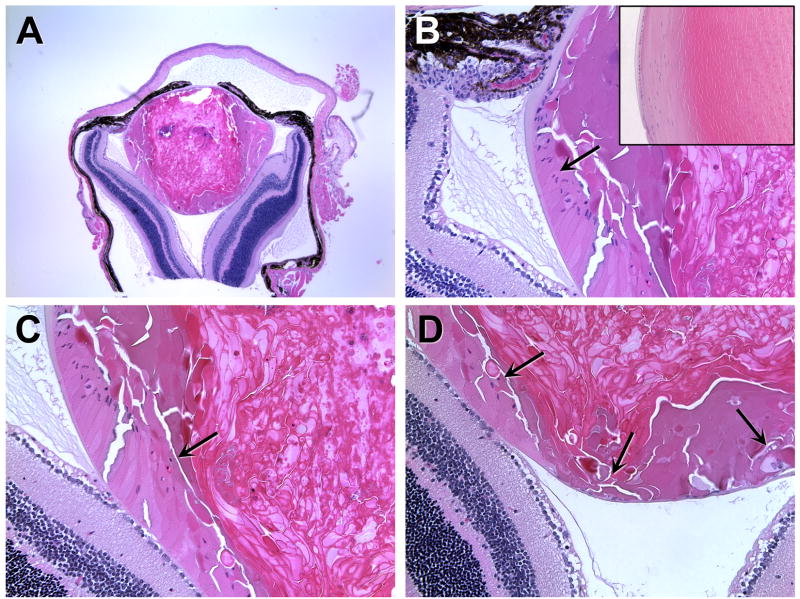
The observed pattern of lens growth suggested that the distinct zones usually found in the lens epithelium might be disrupted by this mutation. To assess changes in the germinative and transitional zones, 6-day-old lens sections were labeled with an antibody specific for Ki67 (Fig. 2). In heterozygous and wild type mice, the proliferation zone was uniform in width, with the majority of labeled cells located slightly anterior of the equator and no labeling of cells posterior to the equator or the cortical cells. The homozygous mutant mice exhibited a drastically different Ki67 labeling distribution, with labeled cells present posterior of the equator and in the lens cortex (Fig. 2B). Additional analysis of older mice indicated that accumulation of nuclei within the ber cell region increased with age (Fig. 3), with a loss of the ability of the homozygous mutant lens to form a normal lens bow in mice older than 4 months. The αA-R49C homozygous mutant mice older than 4 months also demonstrated different growth patterns between the eyes of the same animal, with a complete loss of the eye in some mice.
Figure 2.
Ki67 labeling of interior lens fiber cells in αA-R49C homozygous mutant mice. (A) Lens fiber cells showed significant Ki67 labeling (red arrows) in homozygous mutant mice, but not in wild type or heterozygous mice. (B) The histogram shows the number of Ki67-labeled cells present in each 15° sector normalized against the total number of Ki67-labeled cells in the lens. The data are representative of three mid-sagittal sections per lens, for at least two lenses of each genotype. Overlayed lines in (A) indicate the boundaries of the 15° sectors used for quantifying the angular distribution of labeled cells given in (B). Note that the homozygous mutant lens was labeled in the interior fiber cells (A, B), whereas wild type and heterozygous lenses were both labeled in only in the equatorial region (B).
Figure 3.
Failure to form the normal epithelial bow region in 160-day-old αA-R49C homozygous mutant mice. Homozygous mutant lenses lacked the normal equatorial bow region seen in the wild type lenses. Posterior migration of epithelial cell nuclei in R49C homozygous mutant lenses: (A) low magnification image of the homozygous mutant eye, (B) epithelial cell nuclei at the equator, (C) epithelial cell nuclei migrating along the posterior capsule, and (D) epithelial cells at the posterior pole. (B, inset) The inset shows the lens bow region of an age-matched wild-type lens. Arrows indicate migration of nuclei from the equator towards the posterior of the lens.
Patients heterozygous for the αA-crystallin R49C mutation develop lens opacities in infancy or at birth (Mackay et al., 2003). We have demonstrated that this mutation causes similar lens opacities in gene knock-in mice (Andley et al., 2008; Xi et al., 2008). While the heterozygous knock-in mice have one wild type and one mutant R49C αA-crystallin gene and the homozygous knock-in mice have two mutant and no wild type R49C αA-crystallin genes, both heterozygous and homozygous knock-in mice possess two copies of wild type αB-crystallin, the aggregation partner of αA-crystallin in the lens. Here we expand our previous work to examine the mechanisms whereby the αA-crystallin R49C mutation disrupts lens function using a new method based on detailed histology to assess changes in the distribution of proliferating lens cells.
Although early fiber cell differentiation was not significantly impacted, the deeper cortical fibers exhibited a dramatically disrupted morphology in homozygous mutant lenses, and the proliferation zone was shifted towards the posterior of the lens. As shown in Fig. 2, Ki67 labeling extended into the fiber cell compartment in homozygous lenses, suggesting that the αA-crystallin mutation disrupts cell cycle exit, impacting both the size and shape of the lens. These changes presumably interact with variable environmental factors to cause loss of the lens, loss of the entire eye, or other serious tissue disruptions. The posterior shift of the proliferation zone (Fig. 3) suggests that these dividing cells are dysplastic and no longer have the normal fiber-cell phenotype (Kuszak and Costello, 2004). Dysplastic fiber cells and posterior shifting of the proliferating cells are also common features of human posterior subcapsular cataracts (Streeten and Eshaghian, 1978; Eshaghian and Streeten, 1980).
The absence of any noticeable differences in the size and shape of wild type and heterozygous mutant mouse lenses indicates that a single copy of the wild type αA-crystallin (Cryaa) gene is sufficient to prevent cell death in mutant lenses at the ages examined. In contrast, mice homozygous for the R49C mutant Cryaa gene exhibited disrupted lens development even in young postnatal mice. While the αA−/− null lenses also develop opacities, the phenotype and loss of αA-crystallin function are not nearly as severe as in the αA-crystallin mutants. This shows that the αA-R49C mutant protein dominantly interferes with lens function, an important observation that requires further clarification to fully understand the pathogenesis cataract development, and reflects more than the loss of normal αA-crystallin in vivo function. The variability of the phenotype in the knock-in mice, observed even between eyes of the same animal, presents problems for understanding the mechanism by which αA-crystallin might govern lens growth and development. Such variability has been reported with another αA-crystallin mutation that causes hereditary human cataracts (Richter et al., 2008). It is interesting to note that microphthalmia and hyperplastic irises have recently been reported to occur in the lenses of mice with conditional deletion of the adhesion molecules N- or E-cadherin (Pontoriero et al., 2009). These observations support a link between cell adhesion signaling pathways and αA-crystallin, as previously proposed (Menko et al., 2005; Xi et al., 2008).
Acknowledgments
The authors thank Belinda McMahan for help with histology, staining, and immunolabeling. This work was supported by NIH grants EY05681 and EY02687 (Core Grant for Vision Research), an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andley UP, Hamilton PD, Ravi N. Mechanism of insolubilization by a single-point mutation in alphaA-crystallin linked with hereditary human cataracts. Biochemistry (Mosc) 2008;47:9697–9706. doi: 10.1021/bi800594t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andley UP, Song Z, Wawrousek EF, Bassnett S. The molecular chaperone alphaA-crystallin enhances lens epithelial cell growth and resistance to UVA stress. J Biol Chem. 1998;273:31252–31261. doi: 10.1074/jbc.273.47.31252. [DOI] [PubMed] [Google Scholar]
- Augusteyn RC. Growth of the human eye lens. Mol Vis. 2007;13:252–257. [PMC free article] [PubMed] [Google Scholar]
- Augusteyn RC. Growth of the lens: in vitro observations. Clin Exp Optom. 2008;91:226–239. doi: 10.1111/j.1444-0938.2008.00255.x. [DOI] [PubMed] [Google Scholar]
- Bassnett S. On the mechanism of organelle degradation in the vertebrate lens. Exp Eye Res. 2009;88:133–139. doi: 10.1016/j.exer.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DC, Vasiliev O, Guo J, Shui YB, Bassnett S. Changes in adhesion complexes define stages in the differentiation of lens fiber cells. Invest Ophthalmol Vis Sci. 2001;42:727–734. [PubMed] [Google Scholar]
- Eshaghian J, Streeten BW. Human posterior subcapsular cataract. An ultrastructural study of the posteriorly migrating cells. Arch Ophthalmol. 1980;98:134–143. doi: 10.1001/archopht.1980.01020030136016. [DOI] [PubMed] [Google Scholar]
- Kuszak JR, Costello MJ. The structure of the vertebrate lens. In: Lovicu F, Robinson M, editors. Development of the Ocular Lens. Cambridge University Press; Cambridge, UK: 2004. pp. 71–118. [Google Scholar]
- Kuszak JR, Mazurkiewicz M, Zoltoski R. Computer modeling of secondary fiber development and growth: I. Nonprimate lenses. Mol Vis. 2006;12:251–270. [PubMed] [Google Scholar]
- Kuszak JR, Zoltoski RK, Sivertson C. Fibre cell organization in crystalline lenses. Exp Eye Res. 2004;78:673–687. doi: 10.1016/j.exer.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Mackay DS, Andley UP, Shiels A. Cell death triggered by a novel mutation in the alphaA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet. 2003;11:784–793. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- Menko AS, Zhang L, Weber G, Orlova I, Bai F, Xi J, Andley UP. Regulation of signaling pathways by αA-crystallin: A possible role for αA-crystallin association with α6 integrin complexes. Invest Ophthalmol Vis Sci Supplement, Program number 3873 2005 [Google Scholar]
- Pontoriero GF, Smith AN, Miller LA, Radice GL, West-Mays JA, Lang RA. Co-operative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival. Dev Biol. 2009;326:403–417. doi: 10.1016/j.ydbio.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal R, Dattilo LK, Kaartinen V, Deng CX, Umans L, Zwijsen A, Roberts AB, Bottinger EP, Beebe DC. Functions of the type 1 BMP receptor Acvr1 (Alk2) in lens development: cell proliferation, terminal differentiation, and survival. Invest Ophthalmol Vis Sci. 2008;49:4953–4960. doi: 10.1167/iovs.08-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter L, Flodman P, Barria Von-Bischhoffshausen F, Burch D, Brown S, Nguyen L, Turner J, Spence MA, Bateman JB. Clinical variability of autosomal dominant cataract, microcornea and corneal opacity and novel mutation in the alpha A crystallin gene (CRYAA) Am J Med Genet A. 2008;146:833–842. doi: 10.1002/ajmg.a.32236. [DOI] [PubMed] [Google Scholar]
- Robinson ML. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006;17:726–740. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirke S, Faber SC, Hallem E, Makarenkova HP, Robinson ML, Overbeek PA, Lang RA. Misexpression of IGF-I in the mouse lens expands the transitional zone and perturbs lens polarization. Mech Dev. 2001;101:167–174. doi: 10.1016/s0925-4773(00)00584-0. [DOI] [PubMed] [Google Scholar]
- Streeten BW, Eshaghian J. Human posterior subcapsular cataract. A gross and flat preparation study. Arch Ophthalmol. 1978;96:1653–1658. doi: 10.1001/archopht.1978.03910060279020. [DOI] [PubMed] [Google Scholar]
- Xi JH, Bai F, Gross J, Townsend RR, Menko AS, Andley UP. Mechanism of small heat shock protein function in vivo: a knock-in mouse model demonstrates that the R49C mutation in alpha A-crystallin enhances protein insolubility and cell death. J Biol Chem. 2008;283:5801–5814. doi: 10.1074/jbc.M708704200. [DOI] [PubMed] [Google Scholar]
- Xie L, Chen H, Overbeek PA, Reneker LW. Elevated insulin signaling disrupts the growth and differentiation pattern of the mouse lens. Mol Vis. 2007;13:397–407. [PMC free article] [PubMed] [Google Scholar]