Abstract
Background
To identify inflammatory pathways that may contribute to HIV disease pathogenesis, we explored associations between AIDS or death with different inflammatory markers, including selected soluble tumor necrosis factor superfamily receptors and ligands, interleukin (IL)-6, and CD8 T cell activation, in highly active antiretroviral therapy (HAART)-treated individuals.
Methods
Case-control study among subjects in AIDS Clinical Trials Group (ACTG) protocols 384 and 5015, matched by baseline CD4 cell counts and plasma viral load (pVL), using conditional logistic regression.
Results
Higher pre-treatment soluble (s) TNFR-I, sCD27, sCD40L and plasma IL-6 concentrations were associated with a new AIDS-defining illness or death in separate models adjusted for age, sex, hemoglobin and latest CD4 cell counts. In additional models that excluded cases of opportunistic infections, sTNFR-I, sCD27, and sCD40L each was associated with a new AIDS-defining malignancy or death that developed a median of 51 weeks after HAART-initiation, by which time the majority of subjects had CD4 cell counts above 200/cm3 and achieved a pVL<50 copies/mL.
Conclusion
These data are compatible with a model where these soluble inflammatory markers identify pathways that may contribute to the pathogenesis HIV disease progression, pathways that might not be a direct consequence of ongoing HIV-1 replication.
Keywords: Immune activation, TNFR-I, CD27, CD40L, IL-6
INTRODUCTION
Cumulative evidence supports the importance of generalized immune activation in the pathogenesis of HIV disease, although the mechanisms by which this may occur remain incompletely understood [1]. In the absence of highly active antiretroviral therapy (HAART), markers of T cell activation (defined by CD70 or CD38 expression on CD4 or CD8 cells) and diverse plasma markers of immune activation, including soluble tumor necrosis factor receptor (sTNFR)-II, soluble (s) CD27, neopterin, and β2-microglobulin predicted CD4 cell decline, AIDS or death, independently of the contribution of HIV-1 replication to these endpoints [2–10].
HAART is associated with reductions in generalized immune activation and the magnitude of reductions in CD8 T cell activation was associated with the magnitude of CD4 cell increases with HAART [11]. Differences in the magnitude of CD4 cell increases also were associated with single nucleotide haplotype differences in genes that encode TNF-α, TNFR-I, and TNF-related apoptosis-inducing ligand (TRAIL) among others, suggesting a possible role of TNF-related apoptosis pathways in T cell reconstitution with HAART [12]. Finally, higher plasma concentrations of interleukin (IL)-6 predicted a greater risk of all-cause mortality and opportunistic infections in the Strategies for Management of Anti-Retroviral Therapy (SMART) trial, both in subjects who were randomized to interrupt, and to continue antiretroviral therapy [13, 14]. These observations suggest that CD4 cell restoration and improved survival with HAART may be mediated, in part by reductions in generalized immune activation.
To better characterize the activation pathways by which this may occur, we explored the contributions of different immune activation markers, including CD8 T cell activation markers, plasma levels of IL-6, and selected soluble surface receptors and ligands within the tumor necrosis superfamily, to the risk of AIDS or death in a case-control study among subjects who began their first HAART regimen through one of two AIDS Clinical Trials Group studies (ACTG protocols 384 and 5015).
METHODS
HAART naive participants in ACTG protocol 384 were randomized to one of 6 initial HAART regimens that included 2 nucleosides: stavudine and didanosine, or lamivudine and zidovudine, combined with either nelfinavir, efavirenz, or both [15]. HAART naive participants in ACTG protocol 5015 began a uniform regimen of stavudine and emtricitabine with ritonavir-boosted lopinavir [16]. Follow-up visits for both studies were every 4 weeks to week 24, then every 8 or every 12 weeks thereafter, for protocol 384 or 5015, respectively. In both protocols, participants were required to switch to a different HAART regimen if they had pVL concentrations >200 copies/mL on 2 consecutive occasions after week 24. All participants provided written, informed consent to participate in these studies, which were approved by the institutional review board of each participating site.
Virologic and Immunologic Studies
Plasma VL was measured using quantitative HIV-1 RNA polymerase chain reaction (PCR) assays (Ultrasensitive Amplicor HIV-1 MONITOR v1.0; Roche Molecular Systems, Branchburg, NJ, U.S.A.) with a sensitivity of 50 copies/mL. Lymphocyte subsets and expression of CD38 and HLA-DR were examined in freshly obtained whole blood on all subjects at the above time points, using directly labeled murine monoclonal antibodies (PharmMingen, San Diego, CA, U.S.A.) and three-color flow cytometry.
Plasma Cytokines and Soluble Receptors
Plasma specimens were collected in EDTA and stored at ≤−70°C. Cytokines and soluble cellular receptors were measured in thawed plasma aliquots using commercial ELISA kits according to the manufacturer’s specifications for sTNFR-I [R&D Systems Inc., Minneapolis, MN, U.S.A.], for sTNFR-II, sTRAIL, and interleukin (IL)-6 [BioSource, Carlsbad, CA, U.S.A.] and for sCD27 [PeliKine, Amsterdam, The Netherlands]. The lower limits of quantification for these assays were 7.8 pg/mL for sTNFR-I, 1.6 ng/mL for sTNFR-II, 1.56 U/mL for sCD27, 10 ng/mL for CD40L, 46.8 pg/mL for TRAIL, and 0.156 pg/mL for IL-6.
Statistical Methods
For each case, defined as a new AIDS-defining illness (CDC Category C clinical event) or death, at least 2, and up to 3 controls were matched by baseline CD4 cell count, baseline pVL and the duration of study follow-up, based upon the availability of samples. Correlations among continuous baseline variables were assessed by the Spearman rank test. Differences between groups among continuous variables were compared using Wilcoxon rank sum tests; differences among categorical variables used Fisher’s exact tests.
Associations between markers of inflammation and a new AIDS-defining clinical illness or death was assessed using conditional logistic regression models where the exploratory variables included baseline plasma concentrations of: sTNFR-I, sTNFR-II, sCD27, sCD40L, sTRAIL, and IL-6; and CD8 T cell activation levels (defined by the co expression of HLA-DR/CD38 on CD8 cells) that was measured both at baseline and during follow-up. Candidate adjusting variables in these models included: age; race/ethnicity (as white, black, Hispanic, other); sex; baseline plasma hemoglobin concentration; basal metabolic index (BMI); estimated baseline glomerular filtration rate (eGFR) using the simplified Modified Diet and Renal Disease (MDRD) equation; hepatitis C infection (HCV) determined by antibody; and total CD4 cell counts and pVLs that were measured both at baseline, and during follow-up. Time-updated variables for cases used the latest values antecedent to the date of the first AIDS-defining illness or death; these values for control subjects used data from the same study week, or the closest week antecedent to that used for their corresponding case.
Because the association between CD4 cell counts and the risk of an AIDS defining clinical event is not linear, baseline and latest CD4 cell counts were transformed to their cube root values [17]. All other continuous variables were transformed to a standardized score (z score) by subtracting the mean from the original value and then dividing by its standard deviation in order to facilitate comparisons among these variables, which displayed vast differences in their ranges, and to minimize possible collinearity. Each exploratory variable that was identified by a univariate association (P<0.1) was tested in a separate adjusted model that included age, sex, baseline hemoglobin, latest CD4 cell count and latest pVL. Model selection used forward, stepwise selection, based on goodness-of-fit criteria that compared log likelihood ratios by a chi-squared distribution, where the final model included only those variables that contributed significantly to the model (P-values < 0.05). These exploratory variables also were simultaneously examined in a multivariable model that adjusted only for the latest CD4 cell count. The strength of association was expressed as an odds ratio (OR) with 95% confidence intervals (CI) for all variables in the final models. Statistical analyses were performed using R [18] and Stata (Foundation for Statistical Computing; StataCorp, College Station, TX, U.S.A.).
RESULTS
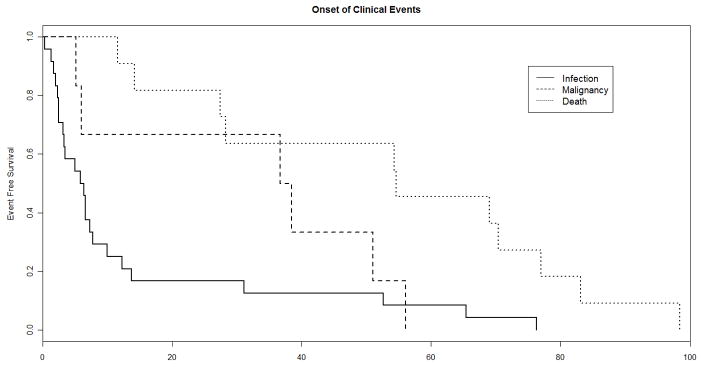
Baseline, pre-treatment plasma samples were available from 42 of 49 subjects who experienced a new AIDS-defining clinical event or death during up to 192 weeks of observation while participating in ACTG protocols 384 and 5015, respectively. One death, due to a motor vehicle accident, was excluded. Baseline and time-updated characteristics among the cases and the 111 matched controls are summarized in Table 1. Included among the clinical events for these cases were 24 opportunistic infections, 6 malignancies, and 11 deaths [Table 2]. Among the listed causes of death were included 2 cardiac-related deaths, and 1 death each from: adenocarcinoma of the lung, gastrointestinal bleeding, disseminated intravascular coagulation, pneumonia, meningitis, and progressive wasting with AIDS dementia complex; a cause of death was not listed in 3 cases. The median onset of a clinical event or death was 12 weeks after HAART-initiation [Table 1, Figure 1]. The median CD4 cell count and pVL at the time of a new AIDS-defining illness or death was 66 cells/mm3 and 2.86 log10 copies/mL, respectively.
Table 1.
Baseline and time-updated characteristics of cases and controls.
| Cases (n = 41) | Controls (n = 111) | P-value | |
|---|---|---|---|
| Age (years), med (IQR) | 44 (33, 49) | 37 (30, 43) | 0.04 |
| Female, n (%) | 14 (34) | 13 (12) | 0.003 |
| Race/Ethnicity, n (%) | 0.25 | ||
| White | 21 (51) | 46 (41) | |
| Black | 9 (22) | 42 (38) | |
| Hispanic | 9 (22) | 21 (19) | |
| Other | 2 (5) | 2 (2) | |
| Hepatitis C Seropositive, n (%)§ | 2 (9) | 9 (12) | |
| Study Week (Time to a new AIDS-defining event or death after HAART-initiation), med (IQR) | 12 (5, 53) | – | |
| Baseline values: med (IQR) | |||
| CD4 cells, per mm3 | 42 (17, 176) | 62 (20, 222) | 0.32 |
| HIV-RNA, log10 copies/mL | 5.51 (4.88, 6.02) | 5.40 (4.67, 6.04) | 0.75 |
| Hemoglobin, Gm/dL | 11.9 (10.4, 13.7) | 12.0 (11.9, 13.8) | 0.01 |
| %CD8+/HLA-DR+/CD38+ | 56 (44, 63) | 50 (40, 62) | 0.08 |
| sTNFR-I, pg/mL | 1423(1215, 1789) | 1175 (902, 1487) | 0.001 |
| sTNFR-II, ng/mL | 8.42(6.72, 12.9) | 7.47(4.85, 10.33) | 0.06 |
| sCD27, U/mL | 420 (304, 653) | 304 (230, 448) | 0.007 |
| sCD40L, ng/mL | 0.72 (0.36, 1.13) | 0.52 (0.24, 0.93) | 0.14 |
| sTRAIL, pg/mL | 449 (309, 639) | 504 (349, 715) | 0.31 |
| IL-6, pg/mL | 3.13 (1.77, 4.78) | 1.48 (1.02, 3.10) | 0.002 |
| Time updated values at the onset of an AIDS defining illness or death, med (IQR) | |||
| CD4 cell count, per mm3 | 66 (17, 236) | 145 (25, 366) | 0.07 |
| HIV-RNA, log10 cop/mL | 2.89 (1.69, 5.67) | 2.58 (1.69, 5.37) | 0.39 |
| HIV-RNA < 200 cop/mL, n (%) | 50 (45) | 18 (44) | 1.0 |
| HIV-RNA < 50 cop/mL, n (%) | 12 (29) | 40 (36) | 0.56 |
| %CD8+/HLA-DR+/CD38+ | 44 (36, 57) | 39 (25, 52) | 0.07 |
Time-updated values among cases indicate the latest, antecedent values before a clinical event; for control subjects values from the same study week, or the closest antecedent study week to that of their corresponding case were used. The significance of differences between cases and controls are indicated by:
=p<0.001;
=p<0.05 and p ≥ 0.001;
= p < 0.10 and p ≥ 0.05.
HCV-serostatus was available in 23 cases and 75 controls.
Table 2.
Summary of CDC Category C Clinical Events or Deaths.
| Number | Study Week (IQR) | |
|---|---|---|
| Death* | 11 | 55 (27, 77) |
| Opportunistic Infections | 24 | 6 (2, 11) |
| Mycobacterium avium-complex | 4 | |
| Cryptococcus | 4 | |
| Pneumocystis jiroveci | 4 | |
| Cytomegalovirus | 3 | |
| Tuberculosis | 2 | |
| Toxoplasmosis | 2 | |
| Progressive Multifocal Leukoencephalitis | 2 | |
| Candidal Esophagitis | 2 | |
| Wasting | 1 | |
| Malignancies | 6 | 38 (6, 51) |
| Non-Hodgkin’s Lymphoma | 5 | |
| Kaposi’s Sarcoma | 1 | |
excludes one death due to a motor vehicle accident.
Figure 1.
Kaplan-Meier plot depicting the onset of a CDC Category C clinical event or death among cases, by week after HAART-initiation.
Correlations between the immune activation markers and other variables at baseline among all subjects are summarized in Table 3. Notably, higher levels of sTNFR-I were associated with older age, lower hemoglobin, lower CD4 cell count, lower eGFR, higher pVL, and higher levels of each of the other activation markers except TRAIL and CD8 T cell activation. Neither sex nor HCV antibody status was associated with any baseline inflammatory marker, but women exhibited a trend towards fewer baseline CD4 cells (median 25 vs. 65 cells/mm3 for women vs. men, respectively; P=0.09).
Table 3.
Partial correlation coefficients (r) among baseline variables from both cases and controls, by the Spearman-rank test.
| sTNFR-I | sTNFR-II | sCD27 | sCD40L | sTRAIL | IL-6 | CD8 activation | Age | Hgb | CD4 | pVL | eGFR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sTNFR-I | 1.0 | 0.72*** | 0.26** | 0.13* | 0.02 | 0.51*** | 0.11 | 0.32*** | −0.26** | −0.24** | 0.42*** | −0.14* |
| sTNFR-II | 1.0 | 0.26** | 0.11 | 0.12 | 0.40*** | 0.20** | 0.21** | −0.29*** | −0.17** | 0.42*** | −0.02 | |
| sCD27 | 1.0 | 0.06 | −0.10 | 0.18** | 0.006 | 0.05 | −0.14* | 0.18** | −0.031 | 0.06 | ||
| sCD40L | 1.0 | −0.07 | 0.08 | 0.06 | −0.02 | −0.08 | 0.12 | −0.04 | −0.07 | |||
| sTRAIL | 1.0 | −0.04 | −0.16* | −0.23** | −0.03 | −0.05 | −0.08 | −0.03 | ||||
| IL-6 | 1.0 | 0.02 | 0.10 | −0.32*** | −0.16** | 0.30*** | 0.10 | |||||
| CD8 activation | 1.0 | 0.07 | 0.09 | −0.16** | 0.03 | −0.09 | ||||||
| Age | 1.0 | 0.06 | 0.02 | 0.03 | −0.30*** | |||||||
| Hgb | 1.0 | 0.38*** | −0.28*** | −0.03 | ||||||||
| CD4 | 1.0 | −0.58*** | −0.06 | |||||||||
| pVL | 1.0 | 0.09 | ||||||||||
| eGFR | 1.0 |
Significant associations (P<0.05) are highlighted in bold. CD8 activation=%CD8+/HLA-DR+/CD38+, Hgb=hemoglobin, pVL=plasma viral load;
=p<0.001;
=p<0.05 and p ≥ 0.001;
= p < 0.10 and p ≥ 0.05.
Older age, female sex, lower baseline hemoglobin concentration, and higher baseline levels of sTNFR-I, sCD27, sCD40L and IL-6 were each associated with the composite endpoint of a new AIDS-defining illness or death on univariate analyses [Table 4]. Neither race/ethnicity, baseline CD8 T cell activation levels nor baseline: sTNFR-II, sTRAIL, eGFR, BMI, HCV-serostatus was associated with this endpoint. Consistent with the matching strategy, neither was baseline CD4 cell count nor baseline pVL. Lower latest CD4 cell count and higher latest pVL each was associated with this endpoint, however, as was higher most recent CD8 T cell activation level.
Table 4.
Summary of univariate, adjusted and multivariable conditional logistic regression models of a new AIDS-defining illness or death, among baseline and time-updated variables. Separate models for each inflammatory marker were adjusted for age, sex, baseline hemoglobin and the latest CD4 cell count and the latest pVL, where only those adjusting variables that contributed significantly remained in the final model. The multivariable model simultaneously tested all baseline and time-updated inflammatory markers and was adjusted only for the latest CD4 cell count. Among continuous variables, odds ratios (OR) and their 95% confidence intervals (CI) indicate incremental differences by one standard deviation in the distribution of each variable, except for the latest CD4 cell count, in which the OR represents incremental differences in the cube root of the value.
| Univariate Associations OR (95%CI) | Adjusted Models OR (95%CI) | Multivariable Model OR (95%CI) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-value | P-value | P-value | P-value | P-value | P-value | P-value | ||||||||
| Baseline sTNFR-I per 615 pg/mL | 1.79 (1.15, 2.77) | 0.01 | 1.97 (1.13, 3.43) | 0.02 | 3.10 (1.44, 6.67) | 0.004 | ||||||||
| Baseline sCD27 per 248 U/mL | 1.66 (1.09, 2.52) | 0.02 | 1.82 (1.01, 3.29) | 0.05 | 1.87(0.97, 3.63) | 0.06 | ||||||||
| Baseline sCD40L per 0.67 ng/mL | 1.67 (1.04, 2.67) | 0.02 | 1.83 (1.08, 3.09) | 0.03 | 2.46 (1.20,5.04) | 0.01 | ||||||||
| Baseline IL6 per 2.89 pg/mL | 1.52 (1.05, 2.21) | 0.03 | 1.54 (1.06, 2.26) | 0.02 | ||||||||||
| Latest %CD8+/HLA-DR+/CD38+ per 17% more | 1.85 (1.06, 3.20) | 0.03 | 1.94 (1.04, 3.60) | 0.04 | ||||||||||
| Age per 10 yrs more | 1.53 (1.04, 2.25) | 0.03 | 2.13 (1.12, 4.07) | 0.02 | 1.83 (1.11, 3.02) | 0.02 | 1.89 (1.13, 3.17) | 0.02 | ||||||
| Sex women vs. men | 1.36 (1.51,10.1) | 0.005 | 4.91 (1.57, 15.3) | 0.006 | 7.27 (1.36, 38.8) | 0.02 | 7.60 (1.99, 29.1) | 0.02 | 7.98 (2.14, 29.7) | 0.002 | ||||
| Baseline Hemoglobin per 1.6 Gm/dL less | 1.97 (1.24, 3.16) | 0.004 | 1.98 (1.02, 3.82) | 0.04 | 2.32 (1.26, 4.29) | 0.007 | ||||||||
| Latest CD4 cellvb per cube root (cells/mm3) less | 2.00 (1.16, 3.45) | 0.01 | 2.16 (1.25, 3.72) | 0.006 | 2.65 (1.25, 5.65) | 0.009 | 2.24 (1.22,4.10) | 0.009 | 2.13 (1.18, 3.86) | 0.01 | 1.92 (1.03, 3.57) | 0.04 | 3.12 (1.47, 6.41) | 0.003 |
| Latest pVL per 1.88 log10 cop/mL more | 1.77 (0.92, 3.41) | 0.09 | ||||||||||||
Baseline sTNFR-I, sCD27, sCD40L, and IL-6 levels, and the latest CD8 T cell activation level remained significantly associated with this composite endpoint in separate adjusted models in which the latest CD4 cell count also contributed significantly to each of these models, as did age (in models testing sCD27, sCD40L, and IL-6), sex (in models testing sTNFR-I, sCD27, sCD40L, and IL6), and pretreatment hemoglobin (in models testing sCD27 and latest T cell activation levels) [Table 4]; latest pVL did not contribute to any of these adjusted models. Pretreatment sTNFR-I, sCD27, sCD40L also remained significantly associated with this composite endpoint in a multivariable model that simultaneously tested all of these inflammatory markers, adjusting only for the latest CD4 cell count.
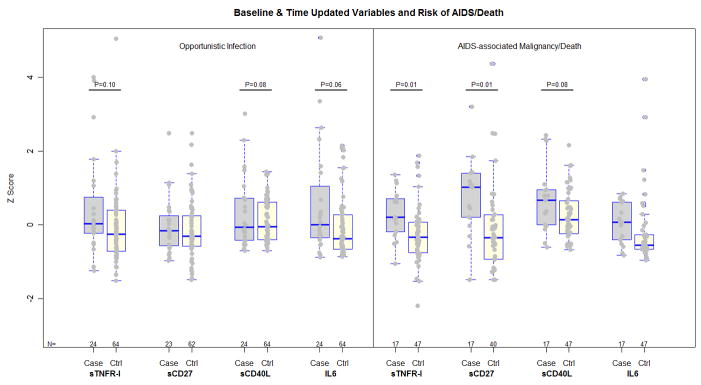
The associations between these immune activation markers and clinical outcomes are graphically depicted in Figure 2. In additional models, fitted on data that excluded opportunistic infections, higher baseline levels of sTNFR-I and sCD27, and higher latest CD8 T cell activation levels were significantly associated with an AIDS-defining malignancy or death after adjusting for the latest CD4 cell count (OR for sTNFRI: 5.03, 95% CI [1.42, 17.7], P=0.01; OR for sCD27: 3.37 [1.31, 8.64], P= 0.01, OR for the latest CD8 T cell activation level 2.62 [1.03, 6.67], P=0.04), and a trend was evident in association with higher sCD40L (OR 1.90 [0.92, 3.91], P=0.08). In this subset, the median onset of an AIDS-defining malignancy or death was 51 weeks after HAART-initiation (IQR 27, 69 weeks), with 59%, and 70% of these individuals achieving pVL<50 and <200 copies/mL, respectively by the latest antecedent pVL measurement. The median, latest CD4 cell count was 232 cells/mm3 (IQR 103, 378 cells/mm3) among these cases, and 303 cells/mm3 (IQR 198, 423 cells/mm3) among corresponding controls.
Figure 2.
Scatter/box plots of baseline immune activation markers among cases of opportunistic infection, and AIDS-associated malignancy or death, and their corresponding matched controls. The boxes encompass the upper and lower quartiles of the distribution of each variable, where the median value is represented by the line within each box. To facilitate comparisons between these markers, values of each variable were subtracted by their mean then divided by their standard deviation (z score). Trends and significant differences between cases and controls that were identified by multivariable models are indicated by the P-value for these differences.
In the subset of cases who developed a new opportunistic infection, a trend was evident in the association between higher infection risk with higher pre-treatment plasma sTNFR-I, sCD40L and IL-6 levels in separate models that also were adjusted for the latest CD4 cell count (OR for sTNFR-I 1.50 [0.93, 2.41] P=0.10; OR for sCD40L 1.87 [0.92, 3.79], P=0.08; OR for IL-6 1.60 [0.99, 2.58], P=0.06.
DISCUSSION
In this study of treatment-naive subjects who initiated HAART as part of two clinical trials, higher pre-treatment baseline plasma concentrations of sTNFR-I, sCD27, sCD40L, and IL-6, and the latest CD8 T cell activation level were each associated with a new AIDS-defining illness or death in models that adjusted for other important predictors and correlates of HIV disease progression that were identified from previous studies including age, sex, hemoglobin, and the latest CD4 cell count [19–22]. Although many of the clinical events in this study included opportunistic infections that presented soon after HAART-initiation, pre-treatment sTNFR-I, sCD27, and sCD40L levels remained associated with an AIDS-defining malignancy or death, clinical events that presented a median of 51 weeks after HAART-initiation, by which time the majority of subjects had more than 200 circulating CD4 cells/mm3 and hadsuppressed plasma viremia to <50 copies/mL.
TNFR-I is expressed on virtually all somatic cell types and mediates most of the known cellular responses that are attributed to TNF, including cellular proliferation via NF-κB activation, and apoptosis via the activation of caspases [23]. Receptor activation by various agonists initiates proteolytic cleavage of its extracellular domain, with plasma shedding as sTNFR-I, and down regulation of cell surface receptor expression [24]. Soluble TNFR-I modulates TNF activity by competing for circulating TNF, but it also may activate caspase-mediated apoptosis by interacting with cell membrane associated TNF [25].
CD27 is induced on B cells following antigenic stimulation, and is constitutively expressed on T cells as a costimulatory molecule for T cell activation through binding to its ligand CD70 that is found on activated T cells, B cells and other antigen-presenting cells (APC). Plasma shedding of sCD27 on T cells follows T cell receptor-mediated activation [26]. While essential for effector T cell maturation, CD27–CD70 costimulation also may impair B cell activation and function by inducing interferon (IFN)-γ and TNF-α mediated disruption of germinal centers [27]. Elevated plasma sCD27 predicted an increased risk of AIDS-associated non-Hodgkin’s lymphoma in the Multicenter AIDS Cohort [28], and CD4 cell decline in HIV-1 infected Ethiopians in the absence of HAART [7].
CD40L is predominately expressed by activated CD4 cells and induces B cell proliferation, while promoting B cell survival and APC maturation upon interacting with its receptor CD40 [29]. HIV infection is associated with reduced cell associated CD40L, but increased sCD40L in plasma [30], which in one study did not normalize with HAART [31].
IL-6 is a major mediator of the acute-phase response that is expressed by antigen presenting cells, and non-hematopoietic cells. It is an important growth factor for B cells while also promoting CD4 T cell proliferation and survival [32]. Elevated IL-6 plasma levels preceded the onset of Burkitt’s and small non-cleaved cell lymphoma in HIV infected subjects, and non Hodgkin’s lymphomatous cells from both HIV-infected and HIV- uninfected patients expressed heightened levels of IL-6 in situ, suggesting that IL-6 may act as a paracrine growth factor in these malignancies.[33, 34]. Although IL-6 was not associated with the risk of a malignancy or death in the present study, it is likely that the small sample size may have precluded our ability to detect any differences should they exist. IL-6 expression was substantially reduced in TNFR-I deficient mice [35], and elevated IL-6 levels were associated with an increased risk of death or an opportunistic infection in the SMART study, and with a greater risk of coronary heart disease among HIV-uninfected participants in a large prospective cohort study [13, 14, 36].
sTNFR-I, and sCD27and CD40 belong to the TNF superfamily of receptors that regulate diverse pathways involving mechanisms of B and T cell proliferation, differentiation and survival. Among the possible sources of the heightened immune activation that these markers represent included are toll-like receptor (TLR)-7/8 activation, via single stranded HIV-1 RNA or activation mediated by HIV proteins [37, 38], or TLR-4 activation, via lipopolysaccharide or other bacterial products arising from translocation through the disrupted gut mucosa [39]. HIV-1 regulatory proteins Nef, Tat and Vpr also can activate TNFR-I and TNFR-II signaling pathways [40].
In contrast to previous pre-HAART-era studies, we did not observe an association between baseline sTNFR-II and HIV disease progression [3, 6]. TNFR-II enhances TNFR-I induced apoptosis, and plays an important role in TNF-induced cellular activation [41]. TNFR-II has a significantly lower affinity for circulating TNF than TNFR-I [42], and is a less potent inducer of NF-κB activation [43]. It is possible that sTNFR-II may identify inflammatory processes that are more readily reversed by HAART than are those that are associated with sTNFR-I and the other activation markers that we identify, to account for this discrepancy. This also might explain similar discrepancies involving CD8 T cell activation, which predicted CD4 cell decline and clinical outcomes in previous studies of patients who did not receive HAART [2, 5], but was associated with CD4 cell changes and clinical outcomes (in the present study) only when using time-updated levels in HAART-treated individuals [11, 44].
Anemia and older age are long standing predictors of accelerated HIV disease progression that were demonstrated both among antiretroviral naive, and HAART-treated individuals [19, 22, 45]. In the present study, baseline hemoglobin concentration was correlated with both sTNFR-I and IL-6, and hemoglobin did not contribute to adjusted models that examined either of these two inflammatory markers, or sCD40L, suggesting that heightened immune activation may underlie the association between anemia and accelerated HIV disease progression. An important role of immune activation in age-associated accelerated HIV disease progression also is suggested by the correlations between older age and baseline sTNFR-I in the present study, and by the lack of significant contributions by age to adjusted models that included sTNFR-I or latest CD8 T cell activation levels. This is consistent with the observations of elevated sTNFR-I levels in association with normal aging [46, 47], and the recent evidence of an important role of TNF-α and TNFR-I in CD8 T cell replicative senescence [48], a process of CD8 T cell terminal differentiation that follows prolonged, or enhanced cellular proliferation that is a characteristic of both aging and HIV disease.
Consistent with some previous studies among pre-HAART and HAART-era cohorts, women exhibited a greater risk of HIV disease progression [19, 49]. In a recent study, plasmacytoid dendritic cells from women exhibited higher IFN-α production following in vitro TLR 7/8 activation by HIV RNAs, and higher CD8 T cell activation levels also were observed in HIV-infected women when compared to HIV-infected men for the same level of pVL [50]. Consistent with this observation, sex did not significantly contribute to the adjusted model that examined latest CD8 T cell activation levels in the present study, but women had a higher risk of AIDS or death in models that examined each of the other pretreatment inflammatory markers implying that additional activation pathways may be important in HIV disease pathogenesis to those which may be accentuated among women.
This study is limited by a small number of clinical events and the relatively short delay between HAART initiation and these events, which often developed before the maximal effects on HIV-1 viral suppression might have been achieved. Although we examined soluble plasma immune activation markers only at baseline, it would also be of interest to examine the impact of HAART on time-updated levels of these markers and to assess the variability of these markers. We also cannot exclude the possibility that these associations merely reflected the host immune response to infections or malignancies that were clinically latent at the start of theray. While both baseline and latest plasma IL-6 levels were associated with an increased risk of opportunistic infection in the SMART study, only the baseline levels remained significant when both were included in the same model, supporting a true predictive association with this cytokine.. Finally, although HCV-serostatus was available in only 98 of our subjects, HCV infection did not appear to influence our results.
Despite the small number of cases, however, our models identified many consistent associations among previously described predictors and correlates of HIV disease progression in HAART-treated subjects, while also identifying sTNFR-I, and sCD40L as possible new correlates of disease progression. Soluble TNFR-I, sCD27, sCD40L and the latest CD8 T cell activation level were associated with disease progression in the subset of subjects who developed an AIDS defining malignancy or death, complications that developed later, among subjects whose CD4 cell counts were often above 200 cells/cm3 and who had achieved excellent suppression of viral replication. These inflammatory markers may help to identify cellular pathways that are important in the pathogenesis of HIV disease, but may not depend on ongoing viral replication and might represent future targets of immune-based therapies.
Acknowledgments
The authors gratefully acknowledge the study volunteers, and the study site staff who participated in ACTG protocols 384 and 5015, and also thank the members of the Cleveland Immunopathogenesis Consortium for helpful discussions.
Funding: The National Institutes of Allergy and Infectious Diseases, the National Institutes of Aging, and the National Institutes of Health (AI 69501, AI 36219, AI 68636, AI069472, 5 RO1 AI066992-04, AI U01 68635, AI062435). Abbott Laboratories, Agouron/Pfizer, Brystol-Myers Squibb, GlaxoSmithKline, and Triangle Pharmaceuticals.
Footnotes
Protocol Registry Numbers: NCT00006144
Conflicts of Interest:
R.T.G. received grant funding from Tibotec and Gilead and an honorarium from GlaxoSmith Kline.
R.B.P. is on the Speaker’s bureau for Bristol Meyers Squibb and Gilead.
G.K.R. received grant funding from Gilead, Schering-Plough, and consulting fees from Abbott Laboratories, Boehringer Ingelheim Pharmaceuticals and Tibotec.
A.L. received honoraria from Abbott Laboratories and Bristol-Myers Squibb.
References
- 1.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–41. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 2.Eggena MP, Barugahare B, Okello M, et al. T cell activation in HIV-seropositive Ugandans: differential associations with viral load, CD4+ T cell depletion, and coinfection. J Infect Dis. 2005;191:694–701. doi: 10.1086/427516. [DOI] [PubMed] [Google Scholar]
- 3.Erikstrup C, Kallestrup P, Zinyama-Gutsire RB, et al. Reduced mortality and CD4 cell loss among carriers of the interleukin-10 −1082G allele in a Zimbabwean cohort of HIV-1-infected adults. Aids. 2007;21:2283–91. doi: 10.1097/QAD.0b013e3282f153ed. [DOI] [PubMed] [Google Scholar]
- 4.Fahey JL. Cytokines, plasma immune activation markers, and clinically relevant surrogate markers in human immunodeficiency virus infection. Clin Diagn Lab Immunol. 1998;5:597–603. doi: 10.1128/cdli.5.5.597-603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giorgi JV, Lyles RH, Matud JL, et al. Predictive value of immunologic and virologic markers after long or short duration of HIV-1 infection. J Acquir Immune Defic Syndr. 2002;29:346–55. doi: 10.1097/00126334-200204010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Godfried MH, van der Poll T, Weverling GJ, et al. Soluble receptors for tumor necrosis factor as predictors of progression to AIDS in asymptomatic human immunodeficiency virus type 1 infection. J Infect Dis. 1994;169:739–45. doi: 10.1093/infdis/169.4.739. [DOI] [PubMed] [Google Scholar]
- 7.Messele T, Brouwer M, Girma M, et al. Plasma levels of viro-immunological markers in HIV-infected and non-infected Ethiopians: correlation with cell surface activation markers. Clin Immunol. 2001;98:212–9. doi: 10.1006/clim.2000.4958. [DOI] [PubMed] [Google Scholar]
- 8.Mildvan D, Spritzler J, Grossberg SE, et al. Serum neopterin, an immune activation marker, independently predicts disease progression in advanced HIV-1 infection. Clin Infect Dis. 2005;40:853–8. doi: 10.1086/427877. [DOI] [PubMed] [Google Scholar]
- 9.Stein DS, Lyles RH, Graham NM, et al. Predicting clinical progression or death in subjects with early-stage human immunodeficiency virus (HIV) infection: a comparative analysis of quantification of HIV RNA, soluble tumor necrosis factor type II receptors, neopterin, and beta2-microglobulin. Multicenter AIDS Cohort Study. J Infect Dis. 1997;176:1161–7. doi: 10.1086/514108. [DOI] [PubMed] [Google Scholar]
- 10.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. Aids. 2003;17:1881–8. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi RT, Spritzler J, Chan E, et al. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. J Acquir Immune Defic Syndr. 2006;42:426–34. doi: 10.1097/01.qai.0000226789.51992.3f. [DOI] [PubMed] [Google Scholar]
- 12.Haas DW, Geraghty DE, Andersen J, et al. Immunogenetics of CD4 lymphocyte count recovery during antiretroviral therapy: An AIDS Clinical Trials Group study. J Infect Dis. 2006;194:1098–107. doi: 10.1086/507313. [DOI] [PubMed] [Google Scholar]
- 13.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodger AJ, Fox Z, Lundgren JD, et al. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis. 2009;200:973–83. doi: 10.1086/605447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shafer RW, Smeaton LM, Robbins GK, et al. Comparison of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349:2304–15. doi: 10.1056/NEJMoa030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalayjian RC, Landay A, Pollard RB, et al. Age-related immune dysfunction in health and in human immunodeficiency virus (HIV) disease: association of age and HIV infection with naive CD8+ cell depletion, reduced expression of CD28 on CD8+ cells, and reduced thymic volumes. J Infect Dis. 2003;187:1924–33. doi: 10.1086/375372. [DOI] [PubMed] [Google Scholar]
- 17.Geskus RB, Prins M, Hubert JB, et al. The HIV RNA setpoint theory revisited. Retrovirology. 2007;4:65. doi: 10.1186/1742-4690-4-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Available at:http://cran.r-project.org/doc/manuals/R-FAQ.html. Accessed 8/19/09.
- 19.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawn SD, Little F, Bekker LG, et al. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. Aids. 2009;23:335–42. doi: 10.1097/QAD.0b013e328321823f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundgren JD, Babiker A, El-Sadr W, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008;197:1145–55. doi: 10.1086/529523. [DOI] [PubMed] [Google Scholar]
- 22.Moore RD, Keruly JC, Chaisson RE. Anemia and survival in HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:29–33. doi: 10.1097/00042560-199809010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Muppidi JR, Tschopp J, Siegel RM. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity. 2004;21:461–5. doi: 10.1016/j.immuni.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Porteu F, Hieblot C. Tumor necrosis factor induces a selective shedding of its p75 receptor from human neutrophils. J Biol Chem. 1994;269:2834–40. [PubMed] [Google Scholar]
- 25.Grell M, Douni E, Wajant H, et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 26.Hintzen RQ, de Jong R, Hack CE, et al. A soluble form of the human T cell differentiation antigen CD27 is released after triggering of the TCR/CD3 complex. J Immunol. 1991;147:29–35. [PubMed] [Google Scholar]
- 27.Matter M, Odermatt B, Yagita H, Nuoffer JM, Ochsenbein AF. Elimination of chronic viral infection by blocking CD27 signaling. J Exp Med. 2006;203:2145–55. doi: 10.1084/jem.20060651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widney D, Gundapp G, Said JW, et al. Aberrant expression of CD27 and soluble CD27 (sCD27) in HIV infection and in AIDS-associated lymphoma. Clin Immunol. 1999;93:114–23. doi: 10.1006/clim.1999.4782. [DOI] [PubMed] [Google Scholar]
- 29.Bachmann MF, Wong BR, Josien R, Steinman RM, Oxenius A, Choi Y. TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation. J Exp Med. 1999;189:1025–31. doi: 10.1084/jem.189.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sipsas NV, Sfikakis PP, Kontos A, Kordossis T. Levels of soluble CD40 ligand (CD154) in serum are increased in human immunodeficiency virus type 1-infected patients and correlate with CD4(+) T-cell counts. Clin Diagn Lab Immunol. 2002;9:558–61. doi: 10.1128/CDLI.9.3.558-561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf K, Tsakiris DA, Weber R, Erb P, Battegay M. Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J Infect Dis. 2002;185:456–62. doi: 10.1086/338572. [DOI] [PubMed] [Google Scholar]
- 32.Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clin Immunol. 2009;130:27–33. doi: 10.1016/j.clim.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breen EC, van der Meijden M, Cumberland W, Kishimoto T, Detels R, Martinez-Maza O. The development of AIDS-associated Burkitt’s/small noncleaved cell lymphoma is preceded by elevated serum levels of interleukin 6. Clin Immunol. 1999;92:293–9. doi: 10.1006/clim.1999.4760. [DOI] [PubMed] [Google Scholar]
- 34.Emilie D, Coumbaras J, Raphael M, et al. Interleukin-6 production in high-grade B lymphomas: correlation with the presence of malignant immunoblasts in acquired immunodeficiency syndrome and in human immunodeficiency virus-seronegative patients. Blood. 1992;80:498–504. [PubMed] [Google Scholar]
- 35.Wang M, Markel T, Crisostomo P, et al. Deficiency of TNFR1 protects myocardium through SOCS3 and IL-6 but not p38 MAPK or IL-1beta. Am J Physiol Heart Circ Physiol. 2007;292:H1694–9. doi: 10.1152/ajpheart.01063.2006. [DOI] [PubMed] [Google Scholar]
- 36.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 37.Baenziger S, Heikenwalder M, Johansen P, et al. Triggering TLR7 in mice induces immune activation and lymphoid system disruption, resembling HIV-mediated pathology. Blood. 2009;113:377–88. doi: 10.1182/blood-2008-04-151712. [DOI] [PubMed] [Google Scholar]
- 38.Chang JJ, Altfeld M. TLR-mediated immune activation in HIV. Blood. 2009;113:269–70. doi: 10.1182/blood-2008-10-184598. [DOI] [PubMed] [Google Scholar]
- 39.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 40.Herbein G, Khan KA. Is HIV infection a TNF receptor signalling-driven disease? Trends Immunol. 2008;29:61–7. doi: 10.1016/j.it.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Carpentier I, Coornaert B, Beyaert R. Function and regulation of tumor necrosis factor type 2. Curr Med Chem. 2004;11:2205–12. doi: 10.2174/0929867043364694. [DOI] [PubMed] [Google Scholar]
- 42.Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998;95:570–5. doi: 10.1073/pnas.95.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McFarlane SM, Pashmi G, Connell MC, et al. Differential activation of nuclear factor-kappaB by tumour necrosis factor receptor subtypes. TNFR1 predominates whereas TNFR2 activates transcription poorly. FEBS Lett. 2002;515:119–26. doi: 10.1016/s0014-5793(02)02450-x. [DOI] [PubMed] [Google Scholar]
- 44.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–85. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 46.Aggarwal S, Gollapudi S, Gupta S. Increased TNF-alpha-induced apoptosis in lymphocytes from aged humans: changes in TNF-alpha receptor expression and activation of caspases. J Immunol. 1999;162:2154–61. [PubMed] [Google Scholar]
- 47.Catania A, Airaghi L, Motta P, et al. Cytokine antagonists in aged subjects and their relation with cellular immunity. J Gerontol A Biol Sci Med Sci. 1997;52:B93–7. doi: 10.1093/gerona/52a.2.b93. [DOI] [PubMed] [Google Scholar]
- 48.Parish ST, Wu JE, Effros RB. Modulation of T lymphocyte replicative senescence via TNF-{alpha} inhibition: role of caspase-3. J Immunol. 2009;182:4237–43. doi: 10.4049/jimmunol.0803449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farzadegan H, Hoover DR, Astemborski J, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet. 1998;352:1510–4. doi: 10.1016/S0140-6736(98)02372-1. [DOI] [PubMed] [Google Scholar]
- 50.Meier A, Chang JJ, Chan ES, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–9. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]