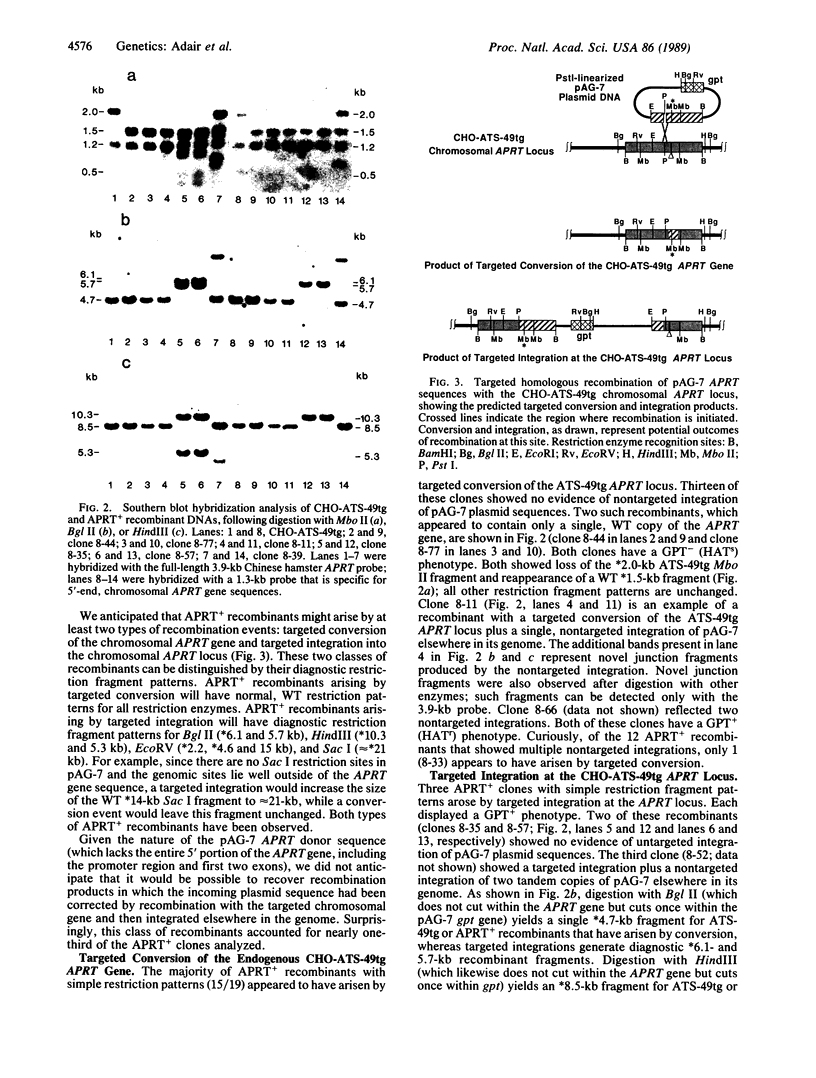
Abstract
We have developed a system that permits analysis of targeted homologous recombination at an endogenous, chromosomal gene locus in cultured mammalian cells. Using a hemizygous, adenine phosphoribosyltransferase (APRT)-deficient, Chinese hamster ovary (CHO) cell mutant as a transfection recipient, we have demonstrated correction of a nonrevertible deletion mutation by targeted homologous recombination. Transfection with a plasmid carrying a fragment of the APRT gene yielded APRT+ recombinants at a frequency of approximately 4.1 x 10(-7). The ratio of targeted recombination to nontargeted integrations of plasmid sequences was approximately 1:4000. Analysis of 31 independent APRT+ recombinants revealed conversions of the endogenous APRT gene, targeted integration at the APRT locus, and a third class of events in which the plasmid donor APRT fragment was converted to a full-length, functional gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair G. M., Carver J. H., Wandres D. L. Mutagenicity testing in mammalian cells. I. Derivation of a Chinese hamster ovary cell line heterozygous for the adenine phosphoribosyltransferase and thymidine kinase loci. Mutat Res. 1980 Sep;72(2):187–205. doi: 10.1016/0027-5107(80)90035-4. [DOI] [PubMed] [Google Scholar]
- Adair G. M., Stallings R. L., Nairn R. S., Siciliano M. J. High-frequency structural gene deletion as the basis for functional hemizygosity of the adenine phosphoribosyltransferase locus in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5961–5964. doi: 10.1073/pnas.80.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., Fink G. R. Yeast: an experimental organism for modern biology. Science. 1988 Jun 10;240(4858):1439–1443. doi: 10.1126/science.3287619. [DOI] [PubMed] [Google Scholar]
- Carroll S. M., DeRose M. L., Gaudray P., Moore C. M., Needham-Vandevanter D. R., Von Hoff D. D., Wahl G. M. Double minute chromosomes can be produced from precursors derived from a chromosomal deletion. Mol Cell Biol. 1988 Apr;8(4):1525–1533. doi: 10.1128/mcb.8.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman T., Gregg R. G., Maeda N., Hooper M. L., Melton D. W., Thompson S., Smithies O. Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature. 1987 Dec 10;330(6148):576–578. doi: 10.1038/330576a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran P., Ashman C. R., Roberts S., Langenberg J. Nucleotide sequence and analysis of deletion mutants of the Escherichia coli gpt gene in plasmid pSV2 gpt. Gene. 1984 Nov;31(1-3):309–313. doi: 10.1016/0378-1119(84)90228-2. [DOI] [PubMed] [Google Scholar]
- Jasin M., de Villiers J., Weber F., Schaffner W. High frequency of homologous recombination in mammalian cells between endogenous and introduced SV40 genomes. Cell. 1985 Dec;43(3 Pt 2):695–703. doi: 10.1016/0092-8674(85)90242-9. [DOI] [PubMed] [Google Scholar]
- Kato S., Anderson R. A., Camerini-Otero R. D. Foreign DNA introduced by calcium phosphate is integrated into repetitive DNA elements of the mouse L cell genome. Mol Cell Biol. 1986 May;6(5):1787–1795. doi: 10.1128/mcb.6.5.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Recombination in mouse L cells between DNA introduced into cells and homologous chromosomal sequences. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1391–1395. doi: 10.1073/pnas.82.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy I., Pellicer A., Jackson J. F., Sim G. K., Silverstein S., Axel R. Isolation of transforming DNA: cloning the hamster aprt gene. Cell. 1980 Dec;22(3):817–823. doi: 10.1016/0092-8674(80)90558-9. [DOI] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G. The essential role of recombination in phage T4 growth. Annu Rev Genet. 1987;21:347–371. doi: 10.1146/annurev.ge.21.120187.002023. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Nairn R. S., Adair G. M., Humphrey R. M. DNA-mediated gene transfer in Chinese hamster ovary cells: clonal variation in transfer efficiency. Mol Gen Genet. 1982;187(3):384–390. doi: 10.1007/BF00332616. [DOI] [PubMed] [Google Scholar]
- Nairn R. S., Humphrey R. M., Adair G. M. Transformation of UV-hypersensitive Chinese hamster ovary cell mutants with UV-irradiated plasmids. Int J Radiat Biol Relat Stud Phys Chem Med. 1988 Feb;53(2):249–260. doi: 10.1080/09553008814550601. [DOI] [PubMed] [Google Scholar]
- Nalbantoglu J., Meuth M. DNA amplification--deletion in a spontaneous mutation of the hamster aprt locus: structure and sequence of the novel joint. Nucleic Acids Res. 1986 Nov 11;14(21):8361–8371. doi: 10.1093/nar/14.21.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Genetic applications of yeast transformation with linear and gapped plasmids. Methods Enzymol. 1983;101:228–245. doi: 10.1016/0076-6879(83)01017-4. [DOI] [PubMed] [Google Scholar]
- Rommerskirch W., Graeber I., Grässmann M., Grässmann A. Homologous recombination of SV40 DNA in COS7 cells occurs with high frequency in a gene dose independent fashion. Nucleic Acids Res. 1988 Feb 11;16(3):941–952. doi: 10.1093/nar/16.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Porter T. N., Wilson J. H. Mechanisms of nonhomologous recombination in mammalian cells. Mol Cell Biol. 1985 Oct;5(10):2599–2607. doi: 10.1128/mcb.5.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Sherwood S. W., Hill A. B., Johnston R. N. Overreplication and recombination of DNA in higher eukaryotes: potential consequences and biological implications. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2157–2161. doi: 10.1073/pnas.83.7.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul Y., Laub O., Walker M. D., Rutter W. J. Homologous recombination between a defective virus and a chromosomal sequence in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3781–3784. doi: 10.1073/pnas.82.11.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., Berg P. Homologous recombination between defective neo genes in mouse 3T6 cells. Cold Spring Harb Symp Quant Biol. 1984;49:171–181. doi: 10.1101/sqb.1984.049.01.020. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Song K. Y., Schwartz F., Maeda N., Smithies O., Kucherlapati R. Accurate modification of a chromosomal plasmid by homologous recombination in human cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6820–6824. doi: 10.1073/pnas.84.19.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield S. W., Helinski D. R. Cloning and characterization of small circular DNA from Chinese hamster ovary cells. Mol Cell Biol. 1984 Jan;4(1):173–180. doi: 10.1128/mcb.4.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S. Rescue of chromosomal T-antigen sequences onto extrachromosomally replicating, defective simian virus 40 DNA by homologous recombination. Mol Cell Biol. 1986 Apr;6(4):1320–1325. doi: 10.1128/mcb.6.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunnerhagen P., Sjöberg R. M., Karlsson A. L., Lundh L., Bjursell G. Molecular cloning and characterization of small polydisperse circular DNA from mouse 3T6 cells. Nucleic Acids Res. 1986 Oct 24;14(20):7823–7838. doi: 10.1093/nar/14.20.7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Introduction of homologous DNA sequences into mammalian cells induces mutations in the cognate gene. Nature. 1986 Nov 6;324(6092):34–38. doi: 10.1038/324034a0. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Folger K. R., Capecchi M. R. High frequency targeting of genes to specific sites in the mammalian genome. Cell. 1986 Feb 14;44(3):419–428. doi: 10.1016/0092-8674(86)90463-0. [DOI] [PubMed] [Google Scholar]
- Wiberg F. C., Sunnerhagen P., Bjursell G. New, small circular DNA in transfected mammalian cells. Mol Cell Biol. 1986 Feb;6(2):653–662. doi: 10.1128/mcb.6.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F., Chumley F., Fink G. R. Eviction and transplacement of mutant genes in yeast. Methods Enzymol. 1983;101:211–228. doi: 10.1016/0076-6879(83)01016-2. [DOI] [PubMed] [Google Scholar]