Abstract
We examined the sleep EEG in 9- and 10-year old children with (PH+) and without (PH−) a parental history of alcoholism abuse/dependence to determine whether sleep disturbances associated with alcohol precede the onset of alcohol use. Participants slept on a fixed sleep schedule that ensured at least 10-hours time in bed for one week before an adaptation and baseline night. Data were collected in a 4-bed sleep research lab. Thirty healthy boys and girls ages 9 or 10 years were classified as either PH+ or PH− based on DSM-IV criteria applied to structured parental interviews. All-night polysomnography was performed, sleep data were visually scored in 30-second epochs, and EEG power spectra were calculated for each epoch. All-night EEG spectra were calculated for NREM and REM sleep, and cycle-by-cycle spectra were calculated for NREM sleep. The two groups did not differ on any sleep stage variable. All-night analyses revealed normalized power in the delta band and spindle range were lower in PH+ children. Within NREM sleep cycles PH+ children exhibited less normalized power in the delta band and spindle range compared to PH− children. This effect occurred in the first four cycles and was most pronounced in the first sleep cycle of the night. We found no signs of sleep disruption in sleep stages for PH+ children. Sleep EEG spectral differences, however, suggest that certain circuits responsible for “protecting” sleep may be impaired in PH+ children, which may later lead to disrupted sleep later in life.
Introduction
Disrupted sleep is common in adults with alcohol dependence, including such complaints as trouble falling asleep and fragmented sleep. One study for example, found that 18 percent of alcohol-dependent adults in the general population suffer from insomnia versus 10 percent of nonalcoholic adults (Brower, 2001). Objective studies of sleep comparing the sleep of alcoholics to non-alcoholics using polysomnography (PSG) have supported a link between alcoholism and sleep disruption. The most common finding in these studies is that the sleep of alcoholics during acute alcohol consumption, withdrawal, and abstinence is characterized by increased sleep latency (SL) and reduced total sleep time (TST) (for a review, see Brower, 2001).
In addition to SL and TST, other sleep stage variables are also altered in alcoholics and vary based on the specific population studied (e.g., abstinent versus current alcoholics). For example, compared to a baseline night with no alcohol administration, administering alcohol to alcoholics at bedtime results in increased sleep latency, decreased total sleep time, increased slow wave sleep (SWS) percent, decreased rapid eye movement (REM) sleep percent, and increased REM sleep latency (for a review, see Brower, 2001). During acute alcohol withdrawal lasting 1 to 2 weeks, alcohol-dependent adults have difficulty sleeping, as indexed by increased SL, decreased TST, and increased REM duration. Furthermore the percentage of SWS declines during alcohol withdrawal (Drummond et al., 1998, Imatoh et al., 1986, Ishibashi et al., 1987, Williams and Rundell, 1981).
In addition to the sizable literature describing sleep disturbances in alcoholics during prolonged alcohol use, withdrawal, and subsequent abstinence, numerous studies have suggested that insomnia following alcohol abstinence may contribute to relapse. For example, several studies have found a positive correlation between self-report measures of insomnia and relapse (Skoloda et al., 1979, Foster and Peters, 1999, Foster et al., 2000, Brower et al., 1998). The first experimental study of this phenomenon (Skoloda et al., 1979) found that alcoholic men in an inpatient unit given the option to drink after one week of abstinence were more likely to do so if they reported sleep difficulties. A subsequent study found that one item of the five insomnia items from the Sleep Disorders Questionnaire (Douglass et al., 1994) (“I have trouble getting to sleep at night”) was significantly associated with subsequent relapse (Brower et al., 1998). Objective measures of sleep have also provided support for the hypothesis that sleep latency is a predictor of relapse (Drummond et al., 1998, Brower et al., 1998). Other studies have failed to find the association between sleep latency and relapse (Clark et al., 1998, Clark et al., 1999), although in these studies low levels of TST were a significant predictor of relapse.
A role for sleep in predicting subsequent alcohol use in nonalcoholics emerges from other lines of research. Epidemiological studies have shown that insomnia may contribute to drinking initiation (Ford and Kamerow, 1989, Weissman et al., 1997). In the Ford and Kamerow (Ford and Kamerow, 1989) study, a national household survey found that adults who reported insomnia the previous year were 2.4 times more likely to report alcohol abuse the following year. A more detailed analysis of this data set found similar results when respondents with a psychiatric disorder prior to the survey were excluded from the analysis (Weissman et al., 1997), indicating that insomnia might precede the development of alcoholism. Another line of research explores self-medication for sleep problems with alcohol. Estimates of the prevalence of this behavior in the adult general population is estimated to be from 6 to 19 percent, and 15 to 28 percent of people with insomnia have used alcohol to promote sleep (Brower, 2001, Johnson et al., 1998, Foundation, 2000). Further, a laboratory study of the association between alcohol and insomnia by Roehrs et al. (Roehrs et al., 1999) found that insomniacs given a choice between an alcohol or non-alcohol beverage chose the alcohol beverage on 67% of the experimental nights, while non-insomniacs chose the alcohol beverage on only 22% of the experimental nights.
Brower has proposed a reciprocal model for the association between alcoholism and insomnia (Brower, 2001). In this model, sleep problems lead individuals to self-medicate by using alcohol as a sedative; as they build tolerance to the sedative effects of alcohol, they consume greater quantities in order to achieve the same hypnotic effect. While the sedative effects of alcohol may initially help these individuals fall asleep, chronic alcohol use leads to greater sleep disturbances by disrupting the brain systems involved in sleep regulation. These factors form a reciprocal relationship leading to a cycle of greater sleep disruption that in turn leads to more alcohol consumption.
According to Brower’s model, an individual enters into this sleep/alcohol abuse cycle through a preexisting vulnerability, namely difficulty falling and staying asleep. In line with the notion of a preexisting vulnerability are genetic studies showing that the heritability of alcoholism is anywhere from 40 to 60% (Hesselbrock, 1995). Given a genetic predisposition towards alcoholism, it is not surprising that children of alcoholics (COAs) are at an increased risk to develop alcoholism (Merikangas et al., 1998, Lieb et al., 2002) compared to those with no such family history. For this reason, this population has been widely studied in the hopes of gaining a deeper understanding of the factors that contribute to the onset and maintenance of alcohol use and abuse. COAs are behaviorally and physiologically distinct from non-COAs on a variety of measure. For example, studies of the resting EEG in adult COAs of different ages and drinking histories show enhanced activity in alpha band (8–12 Hz) power when compared to control adults without a parental history of alcoholism (Ehlers and Schuckit, 1988, Ehlers and Schuckit, 1990, Bauer and Hesselbrock, 1993, Ehlers and Phillips, 2003). These findings have been somewhat equivocal, however, with two studies showing lower waking alpha power in COAs than control (Finn and Justus, 1999, Cohen et al., 1993). In addition to the waking EEG alpha band findings, several studies have shown increased resting awake beta power (12 – 28 Hz) in alcoholics and their children (Propping et al., 1981, Rangaswamy et al., 2002, Gabrielli et al., 1982, Rangaswamy et al., 2004). To understand how EEG measures relate to the genetics of alcoholism, a large multi-site family study called the Collaborative Study of the Genetics of Alcoholism (COGA) used EEG markers as endophenotypes and found that the same GABRA2 gene associated with the EEG beta power endophenotype is associated with DSM-IV diagnosis of alcohol dependence (Porjesz and Rangaswamy, 2007). The finding that COAs have altered EEG traits and that these traits are associated with specific genes lends credence to the use of EEG as an endophenotype in this population.
As in the case with the search for waking EEG markers, one can examine sleep EEG in those at risk for alcohol dependence by comparing the sleep of children of alcoholics to that of children without a parental history of alcoholism. Only one pilot study to date has examined the sleep EEG of COAs (Dahl et al., 2003). This study assessed sleep in 32 depressed youth 8 to 12 years old, of whom 18 did and 14 did not have a family history of alcoholism. These investigators found no differences in traditional sleep variables (e.g., sleep latency, total sleep time, stage 1), yet the EEG spectra showed modest power differences in the alpha band. All statistically significant differences were limited to the alpha band (7.50 to 12.25 Hz) and consisted of interactions between family history and sex. Thus, for both NREM and REM sleep EEG, family history-positive-boys showed greater EEG spectral power in the alpha band than family history-negative-boys. Conversely, family history-positive-girls had less power in the alpha band during sleep than family history-negative-girls. The interpretation of these results is confounded because females reach puberty at an earlier age than boys, and the sleep EEG power is influenced by pubertal stage (Jenni and Carskadon, 2004). Another limitation of the Dahl study (Dahl et al., 2003) was that participants were all diagnosed with depression, and others have shown increased EEG spectral power in the alpha band during awake resting in depressed individuals (Bauer and Hesselbrock, 2002). Therefore, these findings may not generalize to COAs who are not depressed.
The aim of the current study is to examine if children with a parental history of alcohol abuse or dependence carry a vulnerability for sleep disturbances that can act as a pathway to the initiation of problems with alcohol. To this end, we compare the sleep EEG of healthy 9 and 10-year-old children with and without a parental history of alcohol abuse or dependence, all with no psychiatric disorder. By studying the sleep EEG in children who are at an elevated risk for alcohol abuse, we can begin to understand whether the sleep anomalies observed in adult alcoholics may precede the onset of alcohol use.
Methods
Participants
Thirty children ages 9 and 10 were recruited using flyers, mailings to previous participants, and radio and newspaper advertisements. Telephone screens, along with child and parent interviews and questionnaires were used to exclude children who had a chronic or current medical illness, evidence of learning disabilities, sleep disorder or personal or family history of psychopathology. Children with a pattern of insufficient sleep or excessive daytime sleepiness were also excluded from the study. In total 13 children (8 boys) with and 17 without (11 boys) a parental history of alcohol abuse and/or dependence took part in this study.
Pubertal stage was determined during a brief physical examination by a physician using the standardized assessment scales developed by Tanner (Tanner, 1962). The Tanner stages of development are based on external primary and secondary sex characteristics. Tanner stage 1 represents pre-pubertal status and the absence of secondary sexual characteristics, while Tanner stage 5 represents sexual maturity. In general, pubic hair ratings are more reliable than genital or breast development (Tanner, 1962); therefore, the Tanner stages reported in this study are from pubic hair ratings.
Family History of Alcohol Abuse or Dependence
Lifetime and current diagnoses of alcohol abuse and/or dependence were made using DSM-IV criteria applied to structured interviews. When possible, both parents were interviewed with the Diagnostic Interview Schedule (Robins et al., 2000) (DIS-IV). In the event that one parent was unavailable for interviewing, however, the parent who was available was interviewed regarding alcohol use in the absent parent, but only if that individual had good recent knowledge about the absent parent. Based on this criterion, three children had a mother and ten children a father who met the DSM-IV criteria for lifetime alcohol abuse or dependence.
Procedures
For one week before the lab session, participants went to bed and woke up on a sleep schedule that provided at least 10-hours of time in bed, slightly longer for children whose typical schedule called for more than 10 hours. Compliance to the sleep schedule was confirmed using sleep diaries, continuous wrist actigraphy, and daily phone calls to the lab’s time-stamped answering machine at rise and bedtimes. On the day of the study, all participants were well slept, healthy, and taking no medications. Participants slept in individual darkened bedrooms while standard sleep recordings were performed on two consecutive nights: an adaptation night and baseline night. Data from the baseline night are reported here.
Polysomnography (PSG) Recording
EEG was recorded from referential central (C3/A2 and C4/A1) and occipital (O2/A1 and O1/A2) electrodes placed according to the international 10–20 system (Jasper, 1958). Right and left electrooculgram (EOG), electromyogram (EMG; mentalis, submentalis), and electrocardiogram (ECG) were also recorded. On the adaptation night, respiration (oral/nasal thermocouple) and leg EMG were recorded to detect breathing abnormalities or periodic leg movements. No subjects were excluded for breathing or limb movement disorders. The PSG data were collected on two separate EEG systems. The first 24 subjects were recorded using the Albert Grass Heritage System (Astromed, Grass, West Warwick, RI) using GAMMA software. The signals were collected and stored at a sampling frequency of 100 Hz, filtered with Grass Model 8 amplifiers (high-pass EEG filter, 0.3 Hz; low-pass EEG filter, 35 Hz; notch filter 60 Hz) and digitized online (12 bit AD converter; Butterworth filter, −12 dB/octave; low pass-filter, −6dB at 35 Hz, time constant 1.0). The remaining six subjects’ studies were recorded on the TWin system (Astromed, Grass, West Warwick, RI) using TWin AS40 bedside amplifiers, from which the signals were collected digitally with a sampling frequency of 400 Hz and filtered offline (high-pass EEG filter 0.3 Hz; low-pass filter 35 Hz; notch filter 60 Hz).
In order to determine whether the outputs from the two systems were comparable, a known signal was input into both systems simultaneously. Signals from the two systems were in good agreement from 1 to 16 Hz; however, small discrepancies emerged at higher frequencies. Therefore, spectral power above 16 Hz is not reported in this paper. The impedance value at each channel for both systems was below 10 KΩ.
Sleep Analysis
Definition of the Sleep Cycle and the First NREM Episode
NREM sleep cycles were defined according to the criteria of Feinberg and Floyd (Feinberg and Floyd, 1979). A NREM sleep episode was required to last 15 minutes and defined as beginning after the first occurrence of stage 2 and ending with the beginning of REM sleep. Children often ‘skip’ the first REM sleep episode, which impacts the calculation of sleep cycles. In order to account for ‘skipped’ REM episodes, the criteria of Jenni and Carskadon (Jenni and Carskadon, 2004) were applied: the first NREM episode was divided into 2 separate cycles if a continuous episode lasting 12 minutes or longer of combined stages 1, 2, awake, or movement time occurred and was followed by stage 3 or 4. The second NREM episode began with stage 3 or 4 and ended with the beginning of REM sleep. According to these criteria, 22 children (11 PH− Males, 2 PH− Females, 6 PH+ Males, 3 PH+ Females) skipped the first REM sleep episode. Because a large number of participants skipped the first REM sleep episode, REM sleep is not included in the cycle-by-cycle analyses.
Power Spectral Analysis
Sleep data were visually scored in 30-second epochs from central and occipital EEG, right and left outer canthus EOG (ROC/A1, LOC/A2), and chin EMG using standard criteria (Rechtschaffen and Kales, 1968). Intrarater and interrater reliability was at least 86%. Epochs with artifacts were rejected based on visual inspection. The power spectrum of each 30-second epoch at each EEG channel was calculated using a fast Fourier transform (Matlab, The MathWorks Inc., Natick, MA). A Hanning window was applied to the data, and averages of six 5-second epochs were calculated, resulting in a frequency resolution of 0.2 Hz. The lowest 2 frequency bins, 0.2 and 0.4 Hz, were discarded due to the sensitivity of these bins to artifact.
Spectral data are presented below in two ways: averaged over the entire night and averaged for NREM sleep over four sleep cycles, which was the highest number of cycles common to all participants. For all-night averages, NREM (stage 2, 3, and 4) and REM episodes were averaged separately. In the cycle-by-cycle analysis, the EEG data were averaged for only NREM sleep within each sleep cycle.
Normalization
Because these children exhibited large intersubject variability in the amplitude of the EEG signal, each subject’s power spectrum was normalized to the maximum value of the all-night averaged spectrum. For all subjects, this maximum value appeared at 1 Hz. This procedure controls for individual variability in the amplitude of the EEG signal; however, one limitation in the procedure is its insensitivity to power differences between the two groups that occur at every frequency. In order to determine if one group had significantly greater power at all frequencies, a t-test was performed on the maximum power value used for the normalization. There were no significant differences between the two groups at any electrode position (p >.05). Therefore, the results are unaffected by the normalization procedure, and the results derived from the normalized data are identical to those derived from the absolute data.
Statistics
Sleep Stage Variables
The distribution of most sleep stage variables approximates a normal distribution. Therefore, sleep stage variables were examined using a 2 (parental history) by 2 (sex) ANOVA. All analyses are presented for main effects and sex by parental history interactions. P-values less than 0.05 are considered significant.
EEG Power Spectrum
Group differences of the spectral data (cycle-by-cycle and all-night) were assessed using a bootstrap analysis (Efron and Tibshirani, 1993). The principle underlying the bootstrap is to generate a random sample, called the bootstrap distribution, by randomly sampling (with replacement) from the original pool of data. In this study, the pool of data comprised the normalized power spectra of all subjects, regardless of parental history group (i.e., PH+, PH−). This data pool was randomly divided into two sets, S1 and S2. The number of participants assigned to S1 and S2 was based on the number of children in each experimental group (PH+ and PH−), thus 17 versus 13. The power value at each frequency and electrode was then averaged across subjects in sets S1 and S2. The data were randomly assigned and averaged in this manner 5000 times resulting in two bootstrap distributions; one for S1 and the other for S2. At each frequency and electrode, the 5000 randomly generated values of S1 were subtracted from the 5000 randomly generated values of S2, resulting in a distribution of bootstrap differences.
An example of a difference distribution at 13 Hz from electrode C3/A2 can be found in the appendix. Power differences computed from the actual groups were compared to the appropriate bootstrap difference distributions, and the differences between PH+ and PH− children at a given frequency f and electrode e were considered statistically significant if the value was greater or less than 97.5% of the difference distribution at that frequency and electrode. The bootstrap test was performed for each frequency and electrode for all-night (NREM and REM) and cycle-by-cycle (NREM) data to test for parental history (PH+ versus PH−) and sex (Male versus Female) differences.
The bootstrap method has several advantages over traditional parametric analyses when evaluating EEG data. For example, the bootstrap makes no assumptions about the distribution of the data and accounts for uneven sample sizes, which is an important consideration in EEG data analysis, due to the inverse relationship between “noise level” and number of samples. With these reasons in mind, we chose the bootstrap test for this study and set alpha to 0.05 as noted above. We note, too, that traditional corrections for multiple comparisons are too conservative for time series data, since such multiple comparison corrections as the Bonferroni assume that each sample is statistically independent. Here, we report as statistically significant only instances in which a frequency bin manifested significant differences at more than one channel or where adjacent frequency bins show statistically significant differences. This approach reduces the number of “false positive” findings.
Results
Participant Characteristics
Ages did not differ for the four sex by parental history groups: PH+ Boys (mean = 10.15, SD = 0.52), PH+ Girls (mean = 10.25, SD = 0.53), PH− Boys (mean = 10.06, SD = 0.49), PH− Girls (mean = 10.20, SD = 0.47). With the exception of three girls (2 PH−, 1 PH+), two of whom were Tanner 3 and one of whom was Tanner 4, all participants were either pre (Tanner 1) or early (Tanner 2) pubertal. Our sample included 22 White, 3 Hispanic/Latino, 3 Black/African, 1 Asian/Asian American, and 1 multiracial child.
All night sleep stage variables
A summary of the sleep stage variables is provided in Table 1. No significant main effects or interactions were observed for minutes of stage 1, 2, slow wave sleep, REM latency, sleep latency, total sleep time or time in bed. Wake after sleep onset (WASO) showed a significant main effect of sex (p < 0.05), with boys (11 ± 10 minutes) manifesting less WASO than girls (25 ± 25 minutes). The data was reanalyzed after removing the three girls who were Tanner 3/4 because maturational factors might be driving this effect. After removing these participants, this effect was no longer statistically significant (p >.05). [Insert Table 1 here]
Table 1.
All-night sleep stage variables derived from visual scoring
| Sleep Variable | PH+ Boys | PH+ Girls | PH− Boys | PH− Girls |
|---|---|---|---|---|
| Stage 1 | 38 (± 8) | 33 (± 14) | 31 (± 11) | 38 (± 12) |
| Stage 2 | 186 (± 57) | 197 (± 69) | 215 (± 41) | 180 (± 42) |
| SWS | 241 (± 62) | 241 (± 76) | 219 (± 36) | 231 (± 48) |
| REM | 112 (± 18) | 93 (± 10) | 105 (± 23) | 107 (± 26) |
| WASO | 12 (± 12) | 26 (± 28) | 11 (± 9) | 24 (± 22) |
| TST | 577 (± 17) | 564 (± 35) | 570 (± 16) | 556 (± 21) |
| REM Latency | 245 (± 137) | 183 (± 67) | 201 (± 85) | 118 (± 43) |
| Sleep Latency | 9 (± 5) | 16 (± 16) | 13 (± 9) | 16 (± 7) |
Mean and standard deviation in minutes of sleep variables based on visual scoring. Stage 1 = minutes of stage 1; Stage 2 = minutes of stage 2; SWS = minutes of stage 3 and 4; REM = minutes of REM sleep; WASO = wake after sleep onset measured from first occurrence of 1.5 minutes of stage 1 or first stage 2 to final awakening; TST = total sleep time from lights off to lights on; REM Latency = Number of minutes to first REM episode; Sleep Latency = time in minutes to first 1.5 consecutive minutes of stage 1 or first stage 2. Significance values are based on a 2 (parental history) by 2 (sex) ANOVA.
All night Spectral EEG NREM and REM sleep
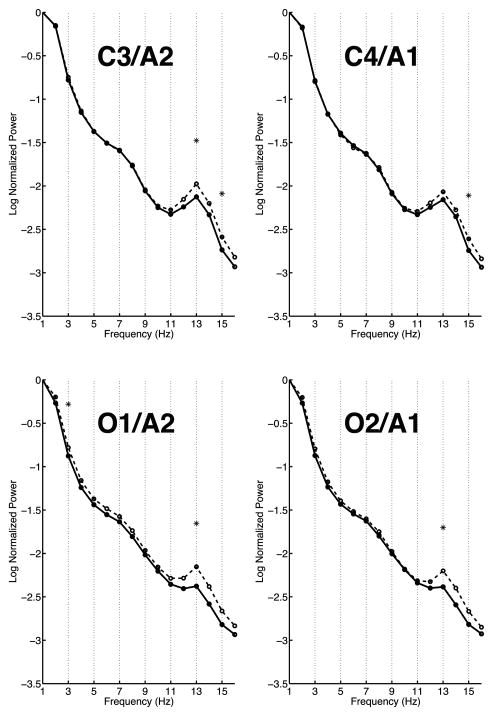
PH+ children showed lower normalized power during NREM sleep in the spindle frequency range (11 – 16 Hz) than the PH− children (see Figure 1). This effect was statistically significant for 13 Hz at three electrode sites (C3/A2, O1/A2, and O2/A1) and significant for 15 Hz at both central leads (C3/A2 and C4/A1). In the delta band, PH+ children showed less normalized power at both occipital leads for 2 and 3 Hz. No significant spectral EEG differences were seen between the two parental history groups for REM sleep. Furthermore, no significant differences were found in the EEG spectra at any lead or frequency comparing males and females during either NREM or REM sleep. [Insert Figure 1 here]
Figure 1. All-night spectra.
Average spectra of the log of the normalized power at two central (C3/A2 and C4/A1) and two occipital (O1/A2 and O2/A1) electrodes. The spectra of children with a parental history of alcoholism (PH+) are shown with a solid line while the spectra of children with no parental history of alcoholism (PH−) are shown with a dashed line. Frequency bins that were significantly different between the two groups are indicated with an asterisk. As apparent in the plots, there were significant differences between the two groups at all channels in the spindle range (11 – 16 Hz) and at occipital channels in the delta band (2 – 3 Hz).
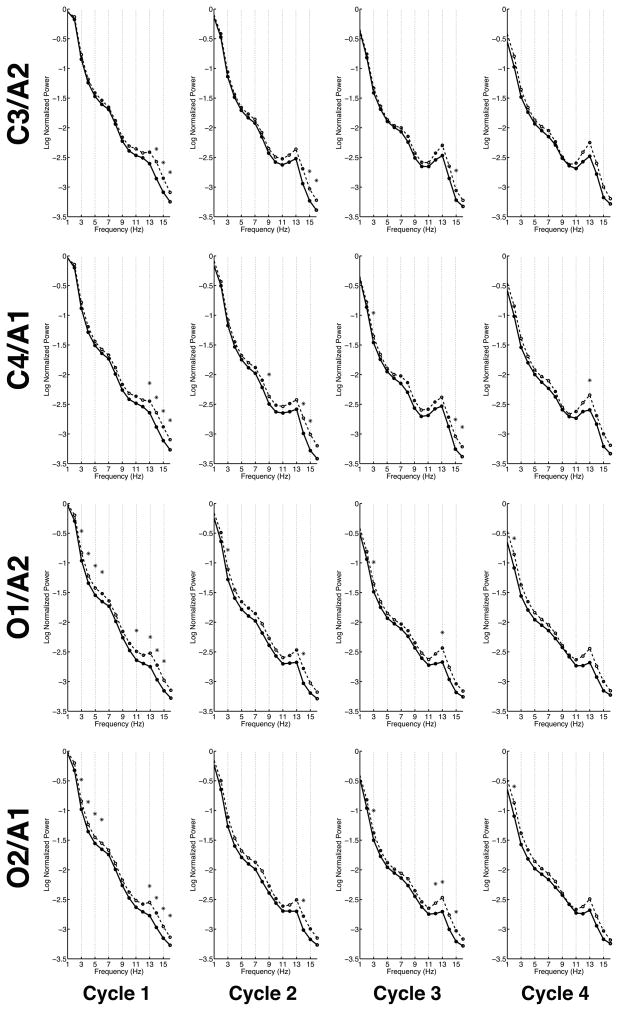
Cycle-by-Cycle EEG NREM sleep
Nine of the thirteen PH+ children (8 boys) and thirteen of the seventeen PH− children (11 boys) skipped the first REM cycle. Analysis of the first sleep cycle showed significantly less normalized power in PH+ children for the high spindle frequency range (13 – 16 Hz) at all electrode positions. Furthermore, PH+ children had significantly less normalized power than PH− children in the delta and theta bands (3 – 6 Hz) for both occipital derivations (O1/A2 and O2/A1). The only frequency where significant sex difference appeared was for 2 Hz, where females showed significantly greater normalized power than males at three electrode positions (C3/A2, C4/A1, and O2/A1). The frequency at which this significant sex difference was found did not overlap with the family history frequency differences for the first NREM cycle.
Fewer significant differences occurred between the two parental-alcohol-history groups in the second sleep cycle. The only consistent statistically significant differences between the groups occurred in the spindle range (11 – 16 Hz). As in the first cycle, the PH+ children had significantly less normalized power than the PH− children for 14 Hz at both occipital and one central electrode and for central electrodes at 15 and 16 Hz as well. Significant sex differences occurred from 14 to 16 Hz, with females exhibiting greater normalized power than males in both central electrodes.
In the third cycle of the night, the PH+ children showed less normalized power for 3 Hz at three electrodes sites (C4/A1, O1/A2, and O2/A1) in contrast to cycle one where only the occipital channels were significantly different. On the other hand – as with previous cycles – the PH+ group showed less normalized power compared to the PH− group in the spindle range (11 – 16 Hz) at all electrode locations. The third cycle also showed the emergence of a sex difference for 4 and 5 Hz (girls greater than boys at C4/A1) and a continuation of the spindle frequency difference as in cycle two, with females exhibiting greater normalized power than males at 15 and 16 Hz at a single electrode site (C4/A1).
The fourth cycle manifested fewer significant differences between the parental alcohol groups, though those that occurred were at the same frequencies and with the same direction of effects as in the first three cycles. These significant differences occurred at the occipital channels in the 2 – 3 Hz range and at 13 Hz at C4/A1 and O1/A2. No sex differences emerged in the fourth cycle.
In summary, consistent findings from NREM sleep across the cycles of the night were that PH+ children displayed less normalized power in the delta/theta frequency ranges and in the spindle range than the PH− group over central and occipital regions. Furthermore, in the first three cycles, females exhibited greater normalized power than males primarily over central leads at 2 Hz and in the spindle range. [Insert figure 2 here]
Figure 2. Cycle-by-cycle Spectra.
Average spectra of the log of the normalized power at two central (C3/A2 and C4/A1) and two occipital (O1/A2 and O2/A1) electrodes across the cycles of the night. The spectra of children with a parental history of alcoholism (PH+) are shown with a solid line while the spectra of children with no parental history of alcoholism (PH−) are shown with a dashed line. Frequencies that were significantly different between the two groups are indicated with an asterisk. Significant differences were most pronounced in the first cycle of the night. The two parental-alcohol groups were consistently different in the delta (1 – 4 Hz) and theta band (4 – 7 Hz) and spindle range (11 – 16 Hz) across cycles.
Discussion
In this study, the sleep EEG of thirty healthy 9- and 10-year old children with and without a parental history of alcohol abuse/dependence was examined. Although no significant differences in traditional sleep stage variables were found between PH+ and PH− children, spectral analyses revealed significant differences in EEG power between the groups.
One of the primary and consistent findings of this study was lower NREM delta power in PH+ children compared to PH− children. Several sleep studies of alcohol dependent and abstinent alcoholics have found significantly reduced spectral power in the delta and theta bands during NREM sleep compared to non-alcoholics (Gillin et al., 1990, Kurella et al., 1990, Irwin et al., 2000). One such study performed in male African-American and Euro-American primary alcoholics and age-matched controls reported decreased NREM delta/theta power in both Euro-American and African-American alcoholics as compared to the control groups (Irwin et al., 2000). Further, two studies of abstinent alcoholics (Gillin et al., 1990, Kurella et al., 1990) also reported diminished NREM delta power compared to non-alcoholics. The COGA project found that lower delta and theta event related oscillations are significantly linked with the CHRM2 gene, which is also associated with a diagnosis of alcohol dependence (Porjesz and Rangaswamy, 2007). In light of these studies, one interpretation of the delta/theta differences reported in this paper is that the diminished delta/theta observed in alcoholics may be a “trait” marker that precedes the onset of alcoholism. A number of studies have shown that the EEG spectrum (awake and asleep) is among the most highly heritable traits (Buckelmuller et al., 2006, van Beijsterveldt et al., 1996); it therefore follows that those genetically predisposed for alcoholism may carry a marker in the EEG spectrum. An important caveat is that it is unknown which (if any) of the children in this study will develop alcohol problems.
Although we describe spectral differences between PH+ and PH− children concentrated in the delta/theta and sigma frequency bands, the only other study of sleep in this young COAs population found no significant main effects of family history in these frequency ranges (Dahl et al., 2003). In their study, however, Dahl et al. (Dahl et al., 2003) reported significant interactions between family history and sex in the alpha frequency band. The disparate findings between our study and that of Dahl and colleagues could arise from a number of factors. For example, in the current study, we examined sleep within a narrow age range (9- and 10- year olds), while in the Dahl study the ages of the participants ranged from 8 to 12 years. Significant and large changes in the sleep EEG, such as a steep decline in overall power, have been well documented from pre- to post-puberty (Jenni and Carskadon, 2004, Feinberg et al., 2006) and limit the interpretation of the Dahl study. Furthermore, the participants in our study were healthy children, whereas those in the Dahl study met DSM-III-R criteria for depression.
In addition to the findings in the delta and theta frequency bands, we find that PH+ children exhibit less power in the spindle range compared to PH− children. To our knowledge, no studies in alcoholics or their children have reported alterations of the sleep EEG in the spindle range. Spindles are generated in the thalamus by thalamic reticular neurons and transmitted to the cortex via thalamocortical pathways. Due to the prominent role of the thalamus in generating spindles, some have suggested that spindles play a role in protecting sleep by blocking the flow of sensory information from the thalamus to the cortex (Yamadori, 1971, De Gennaro and Ferrara, 2003). In fact, there is some experimental evidence in support of this hypothesis. For example, one study by Ehrhart and colleagues (Ehrhart et al., 1981) found that sleep spindles were rare in the 10-second interval preceding a transient activation during sleep suggesting that sleep spindles may inhibit the occurrence of a transient activation. If sleep spindles do in fact play a role in protecting sleep, the reduced power in the spindle range of PH+ children as compared to PH− children may reflect a deficit in one of the mechanisms responsible for protecting sleep. One caveat, however, is that we did not directly measure sleep spindles, but rather spectral power in the spindle range.
Further, others have hypothesized that slow wave activity (SWA) is a measure of the homeostatic sleep process (process S) and as the amount of time awake increases, the amount of SWA also increases in the subsequent sleep episode (Borbely, 1982). Moreover, SWA activity is greatest early on in the evening and declines over the course of the night. Besset and colleagues (Besset et al., 1998) have hypothesized that insomniacs have a low level of processes S at bedtime leading to increased awakenings over the course of the night. We propose that the reduced power in the delta, theta and sigma bands found in PH+ children compared to controls may also reflect a failure of the structures responsible for protecting sleep and may result in a vulnerability in PH+ children.
We acknowledge several limitations of this study. In the first place, the use of normalized power instead of absolute power may limit the interpretation. Because we found no significant group differences in the values used to normalize, we feel that our results identify EEG power differences between the groups of interest. Another limitation is that our sample consisted primarily of children with a father who met DSM-IV criteria for alcohol abuse/dependence; however, three of the children had a mother who met these criteria. We examined the data from these three participants separately due to concerns that the mothers were drinking while pregnant. While we cannot rule out this possibility, the spectral EEG data of these children appeared no different from the overall group of children with a parental history of alcohol abuse/dependence. Furthermore, many of the parents of the PH+ children despite meeting DSM-IV criteria for a lifetime history of alcohol abuse/dependence did not meet these criteria at the time of enrollment. Additionally, we do not know if any of the parents of these children experienced subjective or objective sleep disturbances.
Despite these limitations, we feel this study makes an important contribution to comparisons of PH+ versus PH− children who are naive to alcohol by assessing the sleep EEG in healthy children. Although we did not find any of the traditional signs of sleep disruption, such as increased stage 1 sleep or WASO or reduced TST, we found a pattern in the sleep EEG that we suggest may indicate deficits in circuits responsible for protecting sleep in children with a parental history of alcohol abuse/dependence. Spectral analysis of the sleep EEG may be more sensitive to subtle sleep disruption, and as they mature, these children may manifest more traditional signs of sleep disruption. Future longitudinal studies are necessary to determine whether such sleep anomalies predict future alcohol abuse.
Supplementary Material
Appendix FigureThe top two plots are histograms of the bootstrap distribution obtained by random shuffling for groups S1 and S2 at a single exemplary electrode (C3/A2) and frequency (13 Hz). The bottom plot depicts the histogram of the distribution of bootstrap differences between S1 and S2 at electrode C3/A2 at 13 Hz. In the bottom plot, lines mark the upper and lower 2.5 %. A star shows the actual power difference between PH+ and PH− children at C3/A2 at 13 Hz. In this instance, the difference between the two groups is considered significant since it is larger than 2.5 % of the data. The figures presented here demonstrate this methodology.
Acknowledgments
The authors would like to thank Drs. Ronald Seifer, Christine Acebo, Eliza Van Reen, Oskar Jenni, Monique LeBourgeois, Gahan Fallone, and Margaret Borkowski for technical assistance. The authors are grateful to Drs. Elizabeth Forbes and Judith Owens for performing the Tanner staging. Drs. Peter Monti, Timothy Roehrs, Todd Arnedt, and Robert Swift served as consultants on this project, and we thank them for their contributions. We also thank our research staff and laboratory technicians. This research was supported by the National Institute on Alcohol Abuse and Alcoholism, grants AA13252 (to MAC) and AA07459–21 (to LT).
Appendix
The bootstrap procedure described in the methods section consists of creating two power distributions at each frequency and electrode by randomly shuffling the data. Subtracting one distribution from the other creates a difference distribution. The power difference between PH+ and PH− children at a given frequency and electrode can then be compared to the difference distribution at that electrode and frequency in order to determine significance. If the power difference between PH+ and PH− children is greater than or less than 2.5 % of the values in the difference distribution, then the difference between PH+ and PH− children is deemed significant at that frequency and electrode.
Footnotes
Conflict of Interest: None
Contributor Information
Leila Tarokh, EP Bradley Hospital Sleep and Chronobiology Research Laboratory Center for Alcohol and Addiction Studies, Brown University.
Mary A. Carskadon, EP Bradley Hospital Sleep and Chronobiology Research Laboratory, Department of Psychiatry and Human Behavior, Warren Alpert Medical School of Brown University, Department of Psychology, Brown University.
References
- Bauer LO, Hesselbrock VM. EEG, autonomic and subjective correlates of the risk for alcoholism. J Stud Alcohol. 1993;54:577–89. doi: 10.15288/jsa.1993.54.577. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Lateral asymmetries in the frontal brain: effects of depression and a family history of alcoholism in female adolescents. Alcohol Clin Exp Res. 2002;26:1662–8. doi: 10.1097/01.ALC.0000036283.60525.B3. [DOI] [PubMed] [Google Scholar]
- Besset A, Villemin E, Tafti M, Billiard M. Homeostatic process and sleep spindles in patients with sleep-maintenance insomnia: effect of partial (21 h) sleep deprivation. Electroencephalogr Clin Neurophysiol. 1998;107:122–32. doi: 10.1016/s0013-4694(98)00048-0. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Res Health. 2001;25:110–25. [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcohol Clin Exp Res. 1998;22:1864–71. [PubMed] [Google Scholar]
- Buckelmuller J, Landolt HP, Stassen HH, Achermann P. Trait-like individual differences in the human sleep electroencephalogram. Neuroscience. 2006;138:351–6. doi: 10.1016/j.neuroscience.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Clark CP, Gillin JC, Golshan S, et al. Increased REM sleep density at admission predicts relapse by three months in primary alcoholics with a lifetime diagnosis of secondary depression. Biol Psychiatry. 1998;43:601–7. doi: 10.1016/s0006-3223(97)00457-5. [DOI] [PubMed] [Google Scholar]
- Clark CP, Gillin JC, Golshan S, et al. Polysomnography and depressive symptoms in primary alcoholics with and without a lifetime diagnosis of secondary depression and in patients with primary major depression. J Affect Disord. 1999;52:177–85. doi: 10.1016/s0165-0327(98)00078-0. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H. The effects of ethanol on EEG activity in males at risk for alcoholism. Electroencephalogr Clin Neurophysiol. 1993;86:368–76. doi: 10.1016/0013-4694(93)90132-f. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Williamson DE, Bertocci MA, et al. Spectral analyses of sleep EEG in depressed offspring of fathers with or without a positive history of alcohol abuse or dependence: a pilot study. Alcohol. 2003;30:193–200. doi: 10.1016/j.alcohol.2003.06.001. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–40. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- Douglass AB, Bornstein R, Nino-Murcia G, et al. The Sleep Disorders Questionnaire. I: Creation and multivariate structure of SDQ. Sleep. 1994;17:160–7. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Smith TL, Demodena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22:1796–802. [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the bootstrap. 1993 [Google Scholar]
- Ehlers CL, Phillips E. EEG low-voltage alpha and alpha power in African American young adults: relation to family history of alcoholism. Alcohol Clin Exp Res. 2003;27:765–72. doi: 10.1097/01.ALC.0000065439.09492.67. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Schuckit MA. EEG response to ethanol in sons of alcoholics. Psychopharmacol Bull. 1988;24:434–7. [PubMed] [Google Scholar]
- Ehlers CL, Schuckit MA. EEG fast frequency activity in the sons of alcoholics. Biol Psychiatry. 1990;27:631–41. doi: 10.1016/0006-3223(90)90531-6. [DOI] [PubMed] [Google Scholar]
- Ehrhart J, Ehrhart M, Muzet A, Schieber JP, Naitoh P. K-complexes and sleep spindles before transient activation during sleep. Sleep. 1981;4:400–7. [PubMed] [Google Scholar]
- Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16:283–91. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Higgins LM, Khaw WY, Campbell IG. The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1724–9. doi: 10.1152/ajpregu.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Justus A. Reduced EEG alpha power in the male and female offspring of alcoholics. Alcohol Clin Exp Res. 1999;23:256–62. [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? Jama. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Foster JH, Marshall EJ, Peters TJ. Application of a quality of life measure, the life situation survey (LSS), to alcohol-dependent subjects in relapse and remission. Alcohol Clin Exp Res. 2000;24:1687–92. [PubMed] [Google Scholar]
- Foster JH, Peters TJ. Impaired sleep in alcohol misusers and dependent alcoholics and the impact upon outcome. Alcohol Clin Exp Res. 1999;23:1044–51. [PubMed] [Google Scholar]
- Foundation NS. 2000 Omnibus Sleep in America Poll 2000 [Google Scholar]
- Gabrielli WF, Jr, Mednick SA, Volavka J, et al. Psychophysiology. 1982;19:404–7. doi: 10.1111/j.1469-8986.1982.tb02495.x. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Smith TL, Irwin M, Kripke DF, Schuckit M. EEG sleep studies in “pure” primary alcoholism during subacute withdrawal: relationships to normal controls, age, and other clinical variables. Biol Psychiatry. 1990;27:477–88. doi: 10.1016/0006-3223(90)90439-9. [DOI] [PubMed] [Google Scholar]
- Hesselbrock VM. The genetic epidemiology of alcoholism. In: BEGLEITER H, KISSIN B, editors. Alcohol and Alcoholism. The Genetics of Alcoholism. Oxford University Press; New York: 1995. [Google Scholar]
- Imatoh N, Nakazawa Y, Ohshima H, Ishibashi M, Yokoyama T. Circadian rhythm of REM sleep of chronic alcoholics during alcohol withdrawal. Drug Alcohol Depend. 1986;18:77–85. doi: 10.1016/0376-8716(86)90116-x. [DOI] [PubMed] [Google Scholar]
- Irwin M, Miller C, Gillin JC, Demodena A, Ehlers CL. Polysomnographic and spectral sleep EEG in primary alcoholics: an interaction between alcohol dependence and African-American ethnicity. Alcohol Clin Exp Res. 2000;24:1376–84. [PubMed] [Google Scholar]
- Ishibashi M, Nakazawa Y, Yokoyama T, et al. Cerebral atrophy and slow wave sleep of abstinent chronic alcoholics. Drug Alcohol Depend. 1987;19:325–32. doi: 10.1016/0376-8716(87)90019-6. [DOI] [PubMed] [Google Scholar]
- Jasper H. Report of the Committee on Methods of Clinical Examination in Electroencephalography. Electroenceph Clin Neurophysiol. 1958;10:370–1. [Google Scholar]
- Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–83. [PubMed] [Google Scholar]
- Johnson EO, Roehrs T, Roth T, Breslau N. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep. 1998;21:178–86. doi: 10.1093/sleep/21.2.178. [DOI] [PubMed] [Google Scholar]
- Kurella B, Heitmann A, Dormann S, Meister K. Characteristics of sleep in abstinent alcoholics. A comparison of alcohol- and age-induced reduction of deep sleep. EEG EMG Z Elektroenzephalogr Elektromyogr Verwandte Geb. 1990;21:157–60. [PubMed] [Google Scholar]
- Lieb R, Merikangas KR, Hofler M, et al. Parental alcohol use disorders and alcohol use and disorders in offspring: a community study. Psychol Med. 2002;32:63–78. doi: 10.1017/s0033291701004883. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973–9. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. ScientificWorldJournal. 2007;7:131–41. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propping P, Kruger J, Mark N. Genetic disposition to alcoholism. An EEG study in alcoholics and their relatives. Hum Genet. 1981;59:51–9. doi: 10.1007/BF00278854. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, et al. Beta power in the EEG of alcoholics. Biol Psychiatry. 2002;52:831–42. doi: 10.1016/s0006-3223(02)01362-8. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, et al. Resting EEG in offspring of male alcoholics: beta frequencies. Int J Psychophysiol. 2004;51:239–51. doi: 10.1016/j.ijpsycho.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. UCLA Brain Information Service/Brain Research Institute; Los Angeles: 1968. [Google Scholar]
- Robins L, Cottler L, Bucholz K, et al. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) 2000. [Google Scholar]
- Roehrs T, Papineau K, Rosenthal L, Roth T. Ethanol as a hypnotic in insomniacs: self administration and effects on sleep and mood. Neuropsychopharmacology. 1999;20:279–86. doi: 10.1016/S0893-133X(98)00068-2. [DOI] [PubMed] [Google Scholar]
- Skoloda T, Alterman A, Gottheil E. Sleep quality reported by drinking and non-drinking alcoholics. In: GOTTHEIL EL, editor. Addiction Research and Treatment: Converging Trends. Pergamon; Elmsford, NY: 1979. [Google Scholar]
- Tanner J. Growth at Adolescence. Blackwell; Oxford: 1962. [Google Scholar]
- Van Beijsterveldt CE, Molenaar PC, De Geus EJ, Boomsma DI. Heritability of human brain functioning as assessed by electroencephalography. Am J Hum Genet. 1996;58:562–73. [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Greenwald S, Nino-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry. 1997;19:245–50. doi: 10.1016/s0163-8343(97)00056-x. [DOI] [PubMed] [Google Scholar]
- Williams HL, Rundell OH., Jr Altered sleep physiology in chronic alcoholics: reversal with abstinence. Alcohol Clin Exp Res. 1981;5:318–25. doi: 10.1111/j.1530-0277.1981.tb04905.x. [DOI] [PubMed] [Google Scholar]
- Yamadori A. Role of the spindles in the onset of sleep. Kobe J Med Sci. 1971;17:97–111. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix FigureThe top two plots are histograms of the bootstrap distribution obtained by random shuffling for groups S1 and S2 at a single exemplary electrode (C3/A2) and frequency (13 Hz). The bottom plot depicts the histogram of the distribution of bootstrap differences between S1 and S2 at electrode C3/A2 at 13 Hz. In the bottom plot, lines mark the upper and lower 2.5 %. A star shows the actual power difference between PH+ and PH− children at C3/A2 at 13 Hz. In this instance, the difference between the two groups is considered significant since it is larger than 2.5 % of the data. The figures presented here demonstrate this methodology.