Abstract
Understanding the composition and function of the acquired enamel pellicle (AEP) has been a major goal in oral biology. The aim of this study was to test the hypothesis that intact histatins are part of the in vivo AEP and that histatins after adsorption to HA have effects on in vitro enamel demineralization. This is the first study demonstrating the presence of intact histatins in vivo in the AEP. The in vitro experiments show that all naturally occurring histatins in the AEP have the potential to provide some level of protection against acid injury.
Keywords: histatin, proteomics, enamel pellicle, demineralization, saliva
Introduction
Histatins are members of a family of low-molecular-weight salivary proteins secreted by the major and minor salivary glands (Baum et al., 1977; Oppenheim et al., 1986; Siqueira et al., 2008). Like other salivary proteins, histatins appear to be multifunctional, and their major property is their antifungal activity against the opportunistic yeast, Candida albicans (Oppenheim et al., 1988). Besides antifungal properties, antibacterial effects have been attributed to histatins, based on their killing and growth-inhibitory activity against several species of oral bacteria (Pollock et al., 1984; Xu et al., 1991). Based on in vitro hydroxyapatite adsorption studies, histatin 1, histatin 3, and histatin 5 have also been considered to be protein components of the acquired enamel pellicle (AEP). Recent investigations with state-of-the-art proteomics focusing on the protein composition of in vivo AEP have revealed the presence of protein fragments originating from these histatins (Siqueira et al., 2007b; Siqueira and Oppenheim, 2009). These protein fragments are generated due to the high proteolytic activity present in oral fluid (Helmerhorst et al., 2006). Despite the harsh environment of the oral cavity, it is apparent that some of the secreted histatin 1, histatin 3, and histatin 5 could survive and thus could become, after their adsorption to the enamel surface, constituents of the composition and structure of the in vivo-formed AEP. The aim of this study was to investigate whether intact histatins can be detected in in vivo-formed AEP. In addition, we assessed, with in vitro experiments, whether histatins after adsorption to human enamel surfaces could exert a functional effect in protection against demineralization.
Materials & Methods
Human Participants
Saliva and AEP samples were obtained from healthy male and female volunteers, ranging in age from 24 to 40 yrs. Saliva, human teeth, and AEP collection protocols were approved by the Institutional Review Board of Boston University Medical Center, and informed consent was obtained from each person participating in the study.
Collections of Glandular Secretion and Whole Saliva
Stimulated parotid secretion from both glands was collected with the use of 3% citric acid and a Carlson-Crittenden device. Whole-saliva collection was carried out under masticatory stimulation with parafilm. The samples were kept on ice during the collection procedure. The mean flow rates for parotid secretion and whole saliva were 0.9 mL/min and 1.2 mL/min, respectively. Immediately after collection, whole-saliva samples were centrifuged at 14,000 x g for 20 min at 4°C. Whole-saliva supernatants were separated from the pellet and used immediately for electrophoretic analysis. The total protein concentration was measured by the bicinchoninic acid (BCA) assay (Pierce Chemical, Rockford, IL, USA) and bovine serum albumin protein standard. The mean protein concentration in parotid secretion was 0.84 mg/mL, while that in supernatant whole saliva was 1.5 mg/mL.
Harvesting of Human AEP
AEP material was harvested from the enamel surface as described previously (Siqueira and Oppenheim, 2009). Briefly, in vivo AEP formed naturally on the enamel surface over a two-hour period was collected by means of a collection strip with the dimensions of 0.5 x 1.0 cm (Electrode wick filter paper; BIO-RAD, Hercules, CA, USA) pre-soaked in 3% citric acid. After collection, the collection strips containing AEP were placed in a microcentrifuge tube and kept frozen at -20°C until used for elution and electrophoresis.
Cationic PAGE
Cationic PAGE was performed as described previously (Oppenheim et al., 1986). To minimize losses of AEP proteins, we applied a modified sample-handling method as described (Siqueira et al., 2007a). Briefly, a 35-µL quantity of sample buffer was added to a microcentrifuge tube containing 4 collection strips. The tubes were vortexed for 5 sec at maximum speed, and the 4 strips were placed directly in the wells of the stacking gel, thus avoiding any intermediate elution steps. A constant voltage of 120 V was used for the development of electrophoretic separation, and the gel was stained with silver stain.
Protein Extraction
Protein zones of interest were removed by means of a razor blade, and each gel band was further cut into small pieces. Proteins were extracted from the gel pieces as described (Cohen and Chait, 1997), with a solution containing formic acid/water/2-propanol in a 1:3:2 v/v/v ratio. An aliquot of 200 µL of this elution medium was added to the microcentrifuge tube containing the gel fragments, and the tube was subjected to shaking for 20 hrs at 4°C. Gel pieces and supernatant were separated by centrifugation, and the pellet was extracted twice after one-hour periods of shaking. The resulting 600 µL of supernatant were dried in a speed vac, and this residue was utilized for mass spectrometric analysis.
Mass Spectrometric Analysis
To determine the molecular weight of the protein bands harvested from Cationic PAGE, we used a Voyager DE-Pro MALDI-TOF mass spectrometer (Applied Biosystems, Foster City, CA, USA) in the reflector mode, 25 kV accelerating voltage, delayed extraction, position ion mode, and close external calibration. The matrix used consisted of alpha-cyano-4-hydroxy-cinnamic acid.
Enamel Demineralization Assay
To evaluate the effects of full-length histatins on the in vitro demineralization of human enamel, we isolated native histatin 1, histatin 3, and histatin 5 from human glandular secretions and obtained synthetic histatin 1, lacking phosphate, and synthetic histatin 3, phosphorylated at residue 2, commercially (American Peptide Company, Sunnyvale, CA, USA). Protein solutions were prepared in 50 mM NaCl, pH 7.0, at a protein concentration of 1 mg/mL.
Human permanent first molars were selected and cleaned with a 5-micron alumina suspension with the use of a prophy cup and rinsed with distilled water. The crowns were sectioned into 2 parts, each of which was cut perpendicular to the surface with a diamond-coated band saw, yielding 4 sections of approximately 300 µm thickness. Subsequently, these sections were ground to a uniform thickness of 180 µm. All surfaces of each enamel section, except a 2-mm-long zone of the natural surface enamel (lingual or buccal surface), were then coated with nail varnish. A gold finder grid used for electron microscopy was buried in the nail varnish close to the uncoated area to help orient the microradiographic images on the computer before and after the specific treatment.
The demineralization was carried out with a total of 30 enamel sections, which were randomly divided into 6 groups (n = 5 per group). Each specimen was exposed to 100 µL of protein solution (100 µg specific protein/100 µL) for a period of 2 hrs at 37°C with gentle agitation, and the control group specimens were exposed to 100 µL of 50 mM NaCl containing no protein. After this period, the specimens were washed in distilled water. Subsequently, the specimens were immersed in individual tubes containing 2 mL of demineralization solution (2.2 mM CaCl2; 2.2 mM NaH2PO4; 0.05 M acetic acid; pH 4.5) for a period of 12 days at 37ºC. Immediately after the demineralization period, the specimens were extensively washed in distilled water and dried with filter paper. We used 2 mL of demineralization solution to determine the calcium and phosphate concentrations released from the enamel specimens during the demineralization procedure, and we further used the enamel specimens to evaluate mineral loss and lesion depth by comparing the microradiographs before and after exposure to acidic conditions.
Calcium and Phosphate Analyses
The calcium content of the samples was analyzed by atomic absorption spectrophotometry (Model 3300, Perkin Elmer Analytical Instruments, Norwalk, CT, USA) at a wavelength of 422.7 nm, with the addition of 1% LaCl3 to prevent phosphate interference. The phosphate concentration was measured spectrophotometrically as described previously (Margolis and Moreno, 1985), with a UV-visible spectrophotometer (Cary 50 Scan, Varian, Lake Forest, CA, USA) for determination of the O.D. at a wavelength of 390 nm. All samples were analyzed in triplicate.
Mineral Loss Determination
The loss of mineral content was assessed by quantitative transverse microradiography (Margolis et al., 1999). Enamel section microradiographs were obtained with the use of a Faxitron x-ray system (Hewlett Packard, McMinnville, OR, USA) and high-speed holographic film before and after demineralization treatment. Images were analyzed by means of a video image system and software developed by Ed Marcus Laboratories (Newton, MA, USA). The gold grid attached to the cut surface of each section and the outer enamel edge was used to align the microradiographs obtained for each section prior to and after exposure to demineralization solution. The area density between the mineral content profile acquired before demineralization and the corresponding density obtained after demineralization were integrated, and the differences were established, yielding the numerical value of ΔZ.
Statistical Analyses
Statistical procedures were performed with the software package Minitab 13.1. After checking for normal distribution, we subjected the data to analysis of variance (ANOVA) followed by Tukey’s test. The level of significance was set at a value of p < 0.05.
Results
The silver-stained cationic PAGE of AEP formed over 2 hrs showed 3 major bands in the middle to lower regions of the AEP lane (Fig. 1, lane 3), while the lane corresponding to whole saliva showed the absence of these 3 major bands (Fig. 1, lane 2). The bands from lane 3 exhibited electrophoretic mobilities similar to those of standard native histatin 1, histatin 3, and histatin 5 (Fig. 1, lane 4), as well as those of native histatin 1, histatin 3, and histatin 5 present in parotid secretion (Fig. 1, lane 1). For further exploration of the identity of these proteins, the 3 major bands from lane 3 were excised from the gel, and the contained proteins were eluted and subjected to MALDI-TOF analysis. The mass spectrometry results obtained are shown in Fig. 2. The band with the slowest electrophoretic mobility contained a protein with an m/z of 4933.60, consistent with the molecular-weight (Mr) value of histatin 1. The middle band showed a predominant protein with an m/z of 4062.21, consistent with the Mr value of 4062.5 Da of histatin 3, and the fastest-moving band contained a protein with an m/z of 3036.45, consistent with the Mr value of 3036.7 Da of histatin 5.
Figure 1.
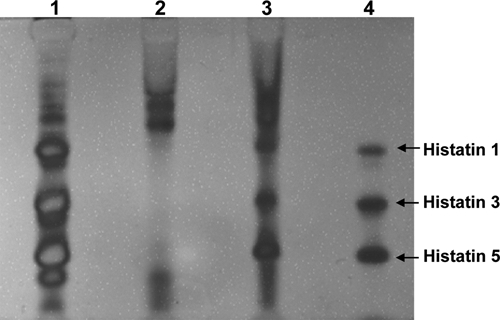
Cationic PAGE with samples applied to the stacking gel trough after the strip was dipped into the protein solution. Lane 1, parotid secretion; Lane 2, whole saliva; Lane 3, AEP material from 8 collection strips; Lane 4, 0.125 µg each of native histatins 1, 3, and 5.
Figure 2.
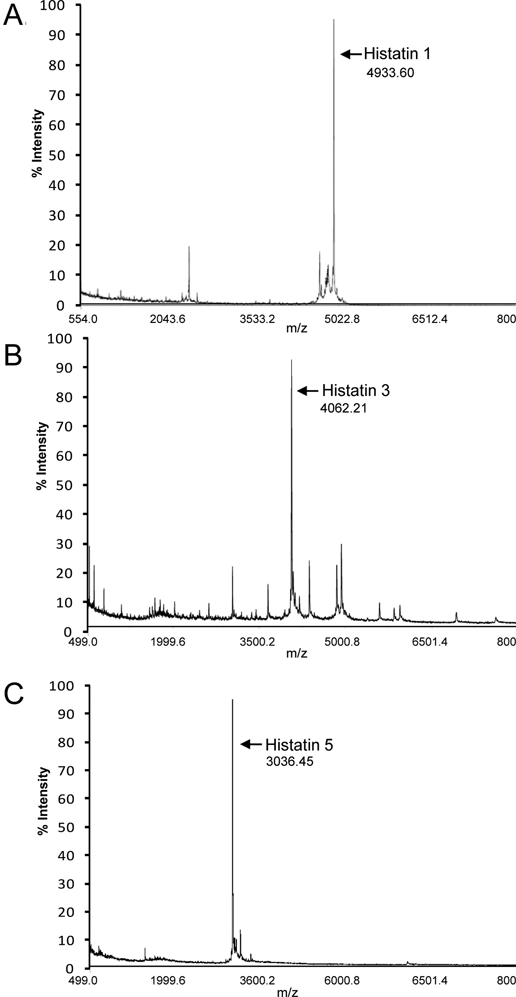
Mass spectrum of protein eluted from an excised gel piece containing the protein band of in vivo AEP exhibiting the same electrophoretic mobility as histatin 1 standard (A). Mass spectrum of protein eluted from an excised gel piece containing the protein band of in vivo AEP exhibiting the same electrophoretic mobility as histatin 3 standard (B). Mass spectrum of protein eluted from an excised gel piece containing the protein band of in vivo AEP exhibiting the same electrophoretic mobility as histatin 5 standard (C).
To gain insight into the biological functions of these histatins when adsorbed onto the enamel surface, we evaluated their effects on the demineralization of enamel in vitro. To evaluate the role of covalently linked phosphate, we included 2 synthetic proteins (histatin 1 lacking phosphate and synthetic histatin 3 phosphorylated at residue 2) in the analyses. The amounts of phosphate dissolved after demineralization at pH 4.5 are shown in Fig. 3A. The control group, not treated with any protein, showed the most drastic phosphate loss. All specimens treated with histatins demonstrated reduction in phosphate loss. Histatin 1 and phosphorylated histatin 3 treated specimens both demonstrated the highest inhibition of phosphate loss. Unphosphorylated histatins showed an intermediate degree of phosphate loss when compared with control and phosphorylated histatins.
Figure 3.
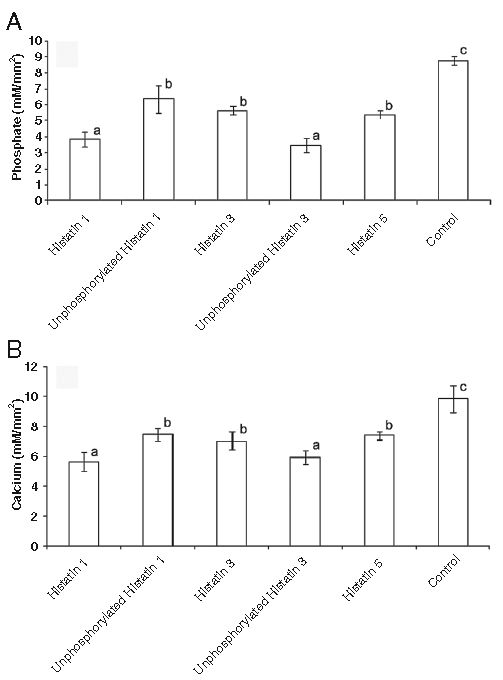
Phosphate (A) and calcium (B) released from human enamel sections first exposed to specific histatin preparations or control buffer followed by exposure to demineralizing solution. Ordinates: Ion composition of demineralizing solution after 12 days’ exposure. Abscissae: Columns indicating protein preparations and controls used in enamel window treatment for 2 hrs (n = 5 per group). Different superscripts indicate statistical difference, and the same superscripts indicate no statistical difference, according to Tukey’s test among proteins and control. Bars represent standard deviation of the mean.
Similar results were obtained in relation to the release of calcium. Histatin 1 and phosphorylated histatin 3 treated specimens showed the lowest calcium loss. Sections exposed to unphosphorylated histatins (synthetic histatin 1, histatin 3, and histatin 5) demonstrated more calcium release, but their calcium loss was significantly lower than that observed with the control group (Fig. 3B).
The average values of densitometric mineral loss and lesion depth after acidic treatment with demineralization solution are presented in the Table. As observed with ionic calcium and phosphate release measurements, enamel sections exposed to histatin 1 and synthetic phosphorylated histatin 3 showed a level of protection significantly higher than that observed for the other groups. Mineral losses with specimens treated with unphosphorylated histatin 1, histatin 3, and histatin 5 were significant lower than those of the control group (p < 0.05).
Table.
Micrographic Density Measurements of Enamel Sections after Exposure to Demineralization Conditions with Prior Coating with Specific Histatins and Control Solution (n = 5 per group)
| ΔZ (vol % x µm)1,2 | Lesion Depth (µm)2 | |
|---|---|---|
| Histatin 1 | 1520 ± 530a | 55 ± 11a |
| Unphosphorylated histatin 1 | 3326 ± 646b | 130 ± 23c |
| Histatin 3 | 3737 ± 931b | 86 ± 13b |
| Phosphorylated histatin 3 | 1627 ± 555a | 69 ± 20b |
| Histatin 5 | 4128 ± 718b | 94 ± 12b |
| Control | 7354 ±1258c | 150 ± 14c |
ΔZ = numerical value for mineral loss due to demineralization.
Different superscripts indicate statistical difference, and the same superscripts indicate no statistical difference, according to Tukey’s test among proteins and control.
Discussion
This is the first study demonstrating the presence of intact histatins in in vivo-formed AEP. This observation is of interest in view of the fact that the life-span of full-length histatins in the whole-saliva environment is very short (Helmerhorst et al., 2006). This is due to the high sensitivity of these proteins to whole-saliva-associated proteases (Castagnola et al., 2004; Hardt et al., 2005). It thus appears that binding of intact histatins to the enamel surface occurs prior to their proteolytic degradation, and that binding to the mineral exerts a protective effect against further enzymatic proteolytic degradation.
From a functional point of view, the identification of intact histatin 1, histatin 3, and histatin 5 in the in vivo AEP is of considerable interest, considering the various biological properties of histatins described so far. Histatins are components of the non-immune or innate oral host defense system, able to kill pathogenic yeasts (Helmerhorst et al., 2005) and exhibit microbicidal activity against a variety of oral bacteria (Gusman et al., 2001). Their presence in an intact form in the AEP would suggest that they could exhibit at least some of their known biological functions and could provide additional biological properties specific for the role AEP plays in vivo. The in vitro results obtained in this study showed that all 3 naturally occurring histatins in AEP have the potential to provide some level of protection against acid injury.
It should be pointed out that the adsorption behavior of a single protein to hydroxyapatite may show differences when compared with its adsorption in the presence of more than one polypeptide, as must be the case in the in vivo formation of AEP. Studies focusing on such differences with histatins have been carried out in the presence of other phosphoproteins, and the use of binary and ternary systems has indeed shown differences in the adsorption behavior of histatins (Yin et al., 2003, 2006). No studies have as yet been carried out to address the functional effect of histatin in such multi-component protein films. The single-component functional consequences after adsorption represent the first step in gaining insights into the possible role of the 3 major histatins in enamel protection. A clear functional difference was found in this study between phosphorylated and unphosphorylated histatins. Analysis of the data provides evidence that the presence of phosphoserine at position 2, whether present in native histatin or the synthetic histatin 3, showed the highest degree of protection against acid demineralization. This is consistent with previous adsorption data which demonstrated that native histatin 1 shows a higher hydroxyapatite adsorption affinity than recombinant histatin 1, lacking covalently linked phosphate at position 2 (Driscoll et al., 1995). The protection with phosphorylated histatins was higher than that observed with the protection gained with the unphosphorylated histatins. The latter group still reduced demineralization when compared with the control. These findings indicate that the phosphorylation of serine residue 2 is not the only determining factor in enamel/hydroxyapatite binding, and other residues must be contributing to the selective adsorption of histatins to hydroxyapatite. The precise mechanisms of how phosphorylated and unphosphorylated histatins reduce mineral dissolution remain to be elucidated. The ultimate goal is to understand how the entire population of specific polypeptides constituting the in vivo-formed AEP affects the mineral homeostasis of enamel surfaces.
Footnotes
This study is supported by NIH/NIDCR Grants DE05672, DE07652, DE15163, DE17788, and DE18132.
References
- Baum BJ, Bird JL, Millar DB, Longton RW. (1977). Isolation and partial characterization of a histidine-rich polypeptide from parotid saliva of the monkey, Macaca nemestrina. Comp Biochem Physiol A Comp Physiol 56:115-120 [DOI] [PubMed] [Google Scholar]
- Castagnola M, Inzitari R, Rossetti DV, Olmi C, Cabras T, Piras V, et al. (2004). A cascade of 24 histatins (histatin 3 fragments) in human saliva. Suggestions for a pre-secretory sequential cleavage pathway. J Biol Chem 279:41436-41443 [DOI] [PubMed] [Google Scholar]
- Cohen SL, Chait BT. (1997). Mass spectrometry of whole proteins eluted from sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Anal Biochem 247:257-267 [DOI] [PubMed] [Google Scholar]
- Driscoll J, Zuo Y, Xu T, Choi JR, Troxler RF, Oppenheim FG. (1995). Functional comparison of native and recombinant human salivary histatin 1. J Dent Res 74:1837-1844 [DOI] [PubMed] [Google Scholar]
- Gusman H, Travis J, Helmerhorst EJ, Potempa J, Troxler RF, Oppenheim FG. (2001). Salivary histatin 5 is an inhibitor of both host and bacterial enzymes implicated in periodontal disease. Infect Immun 69:1402-1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt M, Thomas LR, Dixon SE, Newport G, Agabian N, Prakobphol A, et al. (2005). Toward defining the human parotid gland salivary proteome and peptidome: identification and characterization using 2D SDS-PAGE, ultrafiltration, HPLC, and mass spectrometry. Biochemistry 44:2885-2899 [DOI] [PubMed] [Google Scholar]
- Helmerhorst EJ, Venuleo C, Beri A, Oppenheim FG. (2005). Candida glabrata is unusual with respect to its resistance to cationic antifungal proteins. Yeast 22:705-714 [DOI] [PubMed] [Google Scholar]
- Helmerhorst EJ, Alagl AS, Siqueira WL, Oppenheim FG. (2006). Oral fluid proteolytic effects on histatin 5 structure and function. Arch Oral Biol 51:1061-1070 [DOI] [PubMed] [Google Scholar]
- Margolis HC, Moreno EC. (1985). Kinetic and thermodynamic aspects of enamel demineralization. Caries Res 19:22-35 [DOI] [PubMed] [Google Scholar]
- Margolis HC, Zhang YP, Lee CY, Kent RL, Jr, Moreno EC. (1999). Kinetics of enamel demineralization in vitro. J Dent Res 78:1326-1335 [DOI] [PubMed] [Google Scholar]
- Oppenheim FG, Yang YC, Diamond RD, Hyslop D, Offner GD, Troxler RF. (1986). The primary structure and functional characterization of the neutral histidine-rich polypeptide from human parotid secretion. J Biol Chem 261:1177-1182 [PubMed] [Google Scholar]
- Oppenheim FG, Xu T, McMillian FM, Levitz SM, Diamond RD, Offner GD, et al. (1988). Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem 263:7472-7477 [PubMed] [Google Scholar]
- Pollock JJ, Denepitiya L, MacKay BJ, Iacono VJ. (1984). Fungistatic and fungicidal activity of human parotid salivary histidine-rich polypeptides on Candida albicans. Infect Immun 44:702-707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira WL, Oppenheim FG. (2009). Small molecular weight proteins/peptides present in the in vivo formed human acquired enamel pellicle. Arch Oral Biol 54:437-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira WL, Helmerhorst EJ, Zhang W, Salih E, Oppenheim FG. (2007a). Acquired enamel pellicle and its potential role in oral diagnostics. Ann NY Acad Sci 1098:504-509 [DOI] [PubMed] [Google Scholar]
- Siqueira WL, Zhang W, Helmerhorst EJ, Gygi SP, Oppenheim FG. (2007b). Identification of protein components in in vivo human acquired enamel pellicle using LC-ESI-MS/MS. J Proteome Res 6:2152-2160 [DOI] [PubMed] [Google Scholar]
- Siqueira WL, Salih E, Wan DL, Helmerhorst EJ, Oppenheim FG. (2008). Proteome of human minor salivary gland secretion. J Dent Res 87:445-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Levitz SM, Diamond RD, Oppenheim FG. (1991). Anticandidal activity of major human salivary histatins. Infect Immun 59:2549-2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin A, Margolis HC, Grogan J, Yao Y, Troxler RF, Oppenheim FG. (2003). Physical parameters of hydroxyapatite adsorption and effect on candidacidal activity of histatins. Arch Oral Biol 48:361-368 [DOI] [PubMed] [Google Scholar]
- Yin A, Margolis HC, Yao Y, Grogan J, Oppenheim FG. (2006). Multi-component adsorption model for pellicle formation: the influence of salivary proteins and non-salivary phospho proteins on the binding of histatin 5 onto hydroxyapatite. Arch Oral Biol 51:102-110 [DOI] [PubMed] [Google Scholar]


