Abstract
Purpose
Imexon is an aziridine-derived iminopyrrolidone which has synergy with gemcitabine in pancreatic cancer cell lines. Gemcitabine is a standard therapy for pancreatic cancer. We performed a phase I trial of imexon and gemcitabine to evaluate safety, dose limiting toxicity (DLT), and maximum tolerated dose (MTD) in patients with advanced pancreatic cancer.
Methods
Patients with untreated locally advanced or metastatic pancreatic adenocarcinoma received therapy in sequential cohorts on regimen A (n=19; imexon 200 or 280 mg/m2 intravenously (IV) over 30 minutes days 1–5, 15–19 and gemcitabine 800 or 1,000 mg/m2 IV over 30 minutes on days 1,8,15 every 28 days) or regimen B (n=86; imexon 280–1,300 mg/m2 IV over 30–60 minutes days 1, 8, and 15 and gemcitabine 1,000 mg/m2 IV over 30 minutes on days 1, 8, and 15 every 28 days).
Results
One hundred-five patients received 340 treatment cycles (median 2, range 1–16). Patient characteristics: median age 63, 61% male, ECOG PS 0/1 50%/50%, 93% metastatic. DLT was abdominal cramping and pain, often with transient, acute diarrhea. Best response was confirmed partial response (PR) in 11.4%, 8.9% unconfirmed PR, and 48.1% with stable disease. There was a dose proportional increase in imexon AUC across the doses tested with terminal half-life 69 minutes at the MTD and no alteration of gemcitabine pharmacokinetics.
Conclusions
The recommended phase II dose of imexon is 875 mg/m2 with gemcitabine 1,000 mg/m2. DLT was acute abdominal pain and cramping. Encouraging antitumor responses support further evaluation of this combination in advanced pancreatic cancer.
Keywords: gemcitabine, imexon, pancreatic cancer, phase I clinical trial
Introduction
In 2008, it was estimated that there were 37,680 new cases of pancreatic cancer and 34,290 deaths, making it the fourth leading cause of cancer death in the United States [1]. Systemic treatment options are limited and result in a modest improvement in outcome. For over a decade, gemcitabine has been the standard of care for the initial treatment of advanced pancreatic cancer, with a modest survival advantage over 5-Fluorouracil [2]. More recently, the small molecule inhibitor of the epidermal growth factor receptor, erlotinib, received approval in combination with gemcitabine for the treatment of advanced pancreatic cancer. However, the magnitude of benefit for its addition is quite small [3]. The use of gemcitabine alone or with erlotinib results in a median survival of only 6 months for advanced pancreatic cancer patients. Clearly, new therapies are needed.
Imexon is an aziridine-containing iminopyrrolidone which has demonstrated antitumor activity in vitro.[4] In six pancreatic cancer cell lines, imexon induced apoptosis and cell cycle arrest.[5] Synergy was noted between imexon and gemcitabine in human pancreatic cancer cell lines and in mouse xenograft models. There was no increased toxicity with the combination and the drugs could be combined at full doses. Mechanisms for the antitumor activity of imexon include thiol depletion with generation of reactive oxygen species (ROS) resulting in oxidative stress, mitochondrial damage, leakage of Cytochrome C, and apoptosis. The agent also causes reduction of HIF-1α expression.[6]
Cystine is a critical precursor for the biosynthesis of glutathione, a primary redox molecule that protects cells from internal ROS. Tumor cells have an increased susceptibility to oxidative stress caused by a buildup of ROS. Imexon causes a decrease in intracellular glutathione, either by direct binding and/or other pro-oxidant based pressure on glutathione levels. This initiates the accumulation of ROS, loss of mitochondrial membrane potential and apoptosis. Cystine, the most abundant thiol in the plasma, is imported intracellularly to replenish glutathione levels. Imexon initiates the depletion of cystine in vitro in myeloma cells[7], in vivo in mice[8], and from plasma in humans at doses greater than 750 mg/m2.[9]
A phase I trial of imexon as a single agent has been performed utilizing 5 consecutive daily doses every other week.[9] Forty-nine patients were treated and established an MTD of 875 mg/m2/day. Dose limiting toxicities (DLTs) were grade 3 abdominal pain and fatigue and grade 4 neutropenia. Other common toxicities were nausea, vomiting and constipation. Based on the favorable safety profile of imexon and synergy with gemcitabine in pancreatic cancer cell lines and xenograft models, we initiated this phase I trial to evaluate DLT and the MTD of the combination in patients receiving first line therapy for advanced pancreatic cancer.
Materials and Methods
Eligibility Criteria
Patients eligible for this clinical trial had locally advanced or metastatic adenocarcinoma of the pancreas with no prior chemotherapy and at least one measurable lesion. Additional eligibility criteria included: ECOG performance status 0–1, age ≥18, ANC ≥ 1,500/mm3, WBC ≥ 3,500/mm3, platelets ≥ 100,000/mm3, hemoglobin > 9.0 gm/dL, serum creatinine ≤2.0 mg/dL, ALT and AST ≤ 3 times the upper limit of normal, and total bilirubin ≤2.0 mg/dL. A negative pregnancy test for women of childbearing potential was required and patients needed a G6PD level ≥ the lower limit of normal. Patients were excluded if they had received prior chemotherapy for advanced pancreatic cancer (adjuvant therapy was permitted), had brain metastases, life expectancy of less than 2 months, congestive heart failure, unstable angina or myocardial infarction during the prior 3 months, or respiratory insufficiency requiring oxygen therapy.
The study was designed and conducted in compliance with good clinical practice guidelines. The study protocol was approved by the institutional review boards of the participating institutions. Written informed consent was obtained from all patients.
Study Treatment
Patients were treated with intravenous gemcitabine over 30 minutes on days 1, 8, and 15 every 28 days with imexon at escalating doses administered over 30–60 minutes for 5 consecutive days every other week on days 1–5 and 15–19 every 28 days (Regimen A). As the time commitment of this regimen was not well accepted and laboratory investigation documented similar synergy with imexon administered weekly with gemcitabine, the protocol was amended after 19 patients were enrolled. The remaining 86 patients were treated with imexon at escalating doses administered over 30–60 minutes followed by gemcitabine administered over 30 minutes on days 1, 8, and 15 every 28 days (Regimen B). Table 1 summarizes dose levels and the numbers of patients evaluated.
Table 1.
| Level | Imexon (mg/m2 IV) | Imexon Infusion (min)a | Gemcitabine (mg/m2 IV) | Regimen | N/Expanded | DLTs |
|---|---|---|---|---|---|---|
| 1 | 200 | 30 | 800 | A | 3 | None |
| 2 | 280 | 30 | 800 | A | 3 | None |
| 3 | 200 | 30 | 1,000 | A | 6 | None |
| 4 | 280 | 30 | 1,000 | A | 7 | Febrile neutropenia (1) |
| 5 | 390 | 30–45 | 1,000 | B | 9 | Hyperbilirubinemia (1) |
| 6 | 280 | 30 | 1,000 | B | 6 | Acute renal failure (1) |
| 7 | 335 | 30 | 1,000 | B | 3 | None |
| 8 | 540 | 30 | 1,000 | B | 4 | None |
| 9 | 750 | 30 | 1,000 | B | 3 | None |
| 10 | 1,000 | 30 | 1,000 | B | 6 | Alkaline phosphatase (1) |
| 11 | 1,300 | 30–60 | 1,000 | B | 5 | Abdominal pain (2) Abdominal pain/diarrhea (1) |
| 12 | 1,150 | 30–60 | 1,000 | B | 6/3 | Abdominal pain (1) Fatigue (1) Allergic reaction (1) |
| 13 | 1,000 | 30–60 | 1,000 | B | 6/7 | Abdominal pain (1) Dyspnea (1) Fatigue (1) Syncope (1) Transaminase elevation (1) |
| 14 | 875 | 60 | 1,000 | B | 6/22 | None |
CT scans of the chest, abdomen, and pelvis were obtained at baseline, after 2 cycles, and every 3 cycles thereafter. Partial or complete responses had confirmatory imaging 4 weeks after a response was first documented. Responses without confirmatory imaging were classified as “unconfirmed”. Patients continued on treatment until evidence of progressive disease, unacceptable toxicity, or patient/physician preference.
Dose escalation and modification, dose limiting toxicity (DLT) and maximum tolerated dose (MTD)
Patients were enrolled at each dose level in cohorts of 3. If evidence of a DLT was encountered in one patient in the first cycle, the cohort was expanded to at least 6 patients. DLT was defined as one of the following occurring during the first cycle and at least possibly related to drug: ≥grade 3 non-hematologic toxicity, grade 4 thrombocytopenia or grade 3 thrombocytopenia associated with bleeding or requiring transfusion, ≥grade 3 neutropenia lasting at least 7 days, neutropenic fever, or hospitalization related to neutropenia. The MTD was defined as the highest dose of the combination for which, at most, 1 patient experienced a DLT and at least 6 patients were treated and evaluable for toxicity. Up to 30 additional patients could be enrolled at the MTD.
For the first episode of DLT, treatment was held until resolution to ≤grade 1 and resumed with reduction of gemcitabine by one dose level. For a second occurrence, treatment was held until resolution to ≤grade 1 and resumed with one dose level reduction of both drugs. A third occurrence resulted in removal of the patient from study.
Pharmacokinetics and correlative studies
Peripheral blood samples were obtained on day 1 of study treatment for pharmacokinetics at the following time points: baseline (pre-imexon infusion), at the end of the imexon infusion, at 15, 30, 60, 90, 150, 210, and 270 minutes following the imexon infusion. PK parameters were determined via a non-compartmental approach. Imexon plasma levels were measured by high performance liquid chromatography with tandem mass spectrometric detection (HPLC/MS) using a previously disclosed assay.[9] Gemcitabine was analyzed using HPLC/MS. The HPLC conditions included a gradient from Mobile phase A (100% formic acid in water) and 0% Mobile phase B (0.1% formic acid in acetonitrile) at the start to 50% A/50% B over 8 minutes, then changing back to the original mixture for another 4 minutes (12 minute run time). Both assays were developed and performed by Aptuit Laboratories, Edinburgh, Scotland. [The effects of dose on the pharmacokinetics of imexon were assessed by comparing Cmax/dose, AUC/dose, CLs, Vss, and t1/2 among different dose levels using one-way analysis of variance. A P .05 was considered statistically significant.
Plasma Cystine Methods
Blood samples were obtained at baseline and 8 hours after dosing on day 1, cycle 1 in patients at the 875 mg/m2 and 1,000 mg/m2 dose levels. The plasma fraction was separated by centrifugation and stored at −80° C prior to analysis by a reverse-phase HPLC technique. The cystine concentration was quantified by MS analysis under GLP conditions at ABC Laboratories, Columbus, MO. The difference between baseline and 8 hour cystine levels was compared by imexon dose level and by the best clinical response using analysis of variance (ANOVA). Statistical significance was inferred at the p< 0.05 level for a two-tailed analysis.
Results
Patient Characteristics
Between February 2005 and April 2008, 105 patients were enrolled onto this clinical trial and received a total of 340 cycles of therapy (median of 2, range 1–16). Patient characteristics are summarized in Table 2. Ninety-three percent of patients had metastatic disease with an even split between ECOG PS 0 and 1.
Table 2.
| Parameter | N=105 |
|---|---|
| Median Age (range) | 63 (38–86) |
| % Male | 61 |
| % ECOG 0/1 | 50/50 |
| % Metastatic /Locally Advanced | 93/7 |
| Race % (White/AA/Asian/Other) | 84/11/2/3 |
Toxicity
Table 3 summarizes all toxicities regardless of causality. The most common was fatigue, reaching grade 3/4 in 17% of patients. In regimen A, one DLT was noted (grade 4 neutropenia). Through dose levels 5–10 in regimen B, one DLT was noted at 3 of these levels (grade 3 hyperbilirubinemia, grade 4 renal failure, and grade 3 alkaline phosphatase elevation). Dose de-escalation occurred at dose level 6 due to an initial report of a second hyperbilirubinemia at dose level 5 that was later determined not to be a DLT. Dose escalation proceeded to dose level 11 (1,300 mg/m2 imexon weekly with 1,000 mg/m2 gemcitabine) where 3 of 5 patients experienced DLT, three with grade 3 acute abdominal pain and one with associated grade 3 diarrhea. Thus, dose level 11 was thought to be above the MTD, and an intermediate dose level 12 was opened at 1,150 mg/m2 imexon. Of 9 patients enrolled, 3 experienced DLT (acute abdominal pain, fatigue, and allergic reaction). As acute abdominal pain again occurred, cohort expansion was halted and the dose de-escalated to imexon 1,000 mg/m2 (dose level 13, identical to dose level 10). However, at dose level 13, five of 13 treated patients experienced DLT (abdominal pain, dyspnea, fatigue, syncope, and transaminase elevation). Thus, the imexon dose was decreased to 875 mg/m2 (dose level 14). Of 28 patients at this level, there was 1 patient who experienced a grade 3 allergic reaction at cycle 2 day 1, and one patient with a grade 2 allergic reaction (not considered dose limiting) at cycle 3 day 1 without any dose limiting abdominal pain or other gastrointestinal symptoms. Thus, imexon 875 mg/m2 weekly with gemcitabine 1,000 mg/m2 weekly was considered the MTD of this combination and the recommended phase II dose for pancreatic cancer.
Table 3.
| Adverse Event (CTC Term) | # of Patients Experiencing Event (%) | # of Patients Experiencing Grade 3 Events | # of Patients Experiencing Grade 4 Events |
|---|---|---|---|
| Fatigue | 77 (76.2%) | 16 (15.8%) | 1 (1.0%) |
| Hemoglobin | 71 (70.3%) | 17 (16.8%) | 1 (1.0%) |
| Abdominal Pain | 55 (54.5%) | 11 (10.9%) | 0 (0.0%) |
| Leukocytes | 51 (50.5%) | 20 (19.8%) | 2 (2.0%) |
| Platelets | 45 (44.6%) | 11 (10.9%) | 1 (1.0%) |
| Neutrophils | 42 (41.6%) | 23 (22.8%) | 5 (5.0%) |
| ALT | 41 (40.6%) | 11 (10.9%) | 0 (0.0%) |
| AST | 39 (38.6%) | 11 (10.9%) | 0 (0.0%) |
| Dyspnea | 33 (32.7%) | 12 (11.9%) | 0 (0.0%) |
| Lymphopenia | 29 (28.7%) | 19 (18.8%) | 14 (13.9%) |
| Bilirubin | 25 (24.8%) | 12 (11.9%) | 1 (1.0%) |
| Thrombosis/Thrombus/Embolism | 21 (20.8%) | 15 (14.9%) | 6 (5.9%) |
Efficacy
Of 105 enrolled patients, 79 are evaluable for response. There was confirmed partial response in 9 (11.4%), unconfirmed partial response in 7 (8.9%), stable disease in 38 (48.1%), and progressive disease in 25 (31.6%). The median duration of confirmed partial responses was 8.7 months (range 3.0–15.7). The median duration of disease stabilization for all patients who had stable disease or better on study was 4.3 months (range 1.3–14.5). This includes patients who were stable or responding but came off study for reasons other than disease progression, including toxicity, withdrawal of consent, or declining performance status.
Imexon pharmacokinetics
Pharmacokinetic data are summarized for treatment regimen B at 9 dose levels of imexon in Table 4 and Figure 1A. Maximum plasma concentrations of imexon were at the end of infusion with Cmax at the MTD (875 mg/m2 imexon, 1,000 mg/m2 gemcitabine administered on days 1, 8, and 15) of 47.9 μg/mL and the mean AUC at the MTD of 84.5 μg*hr/mL. Over the range of 280 mg to 1,300 mg/m2 of imexon there is a dose proportional increase in systemic exposure (AUC) to imexon (Figure 1B). The imexon concentrations declined in a mono-or bi-phasic manner with the geometric mean apparent terminal half-lives ranging from 1.12 to 1.89 hours. The plasma half-life at the MTD was 69 minutes and was similar to than that observed in the prior studies [9].
Table 4.
| Imexon | B | 750 | 30 | 3 | 41.3 (20.8) | 0.583 (0.567–0.633) | 62.6 (18.4) | 1.32 (18.3) | 11000 (21.0) | 19600 (15.2) |
| B | 875 | 60 | 6 | 47.9 (23.8) | 1.22 (1.07–1.38) | 84.5 (27.1) | 1.15 (24.0) | 9680 (28.2) | 15800 (21.1) | |
| B | 1000 | 30 | 6 | 52.6 (39.7) | 0.567 (0.500–0.600) | 71.9 (36.6) | 1.35 (11.9) | 12600 (39.8) | 21300 (37.6) | |
| B | 1150 | 30 | 2 | 93.1 (9.36) | 0.525 (0.500–0.550) | 140 (7.79) | 1.70 (71.8) | 7010 (26.6) | 15200 (36.1) | |
| B | 1150 | 45 | 5 | 76.0 (22.8) | 0.750 (0.733–0.850) | 157 (24.7) | 1.52 (9.65) | 6430 (25.7) | 13900 (19.8) | |
| B | 1150 | 60 | 1 | 105 - | 1.00 - | 105 - | 1.33 - | 5210 - | 10000 - | |
| B | 1300 | 30 | 1 | 59.4 - | 0.867 - | 133 - | 1.67 - | 8540 - | 22100 - | |
| B | 1300 | 45 | 2 | 47.5 (20.1) | 0.758 (0.683–0.833) | 110 (1.43) | 1.89 (27.8) | 8700 (25.2) | 24400 (6.83) | |
| B | 1300 | 60 | 1 | 90.5 - | 1.00 - | 167 - | 1.24 - | 7330 - | 12900 - | |
| Gemcitabine | 1000 | 30 | 36 | 10700 (41.6) | 0.425b (0.133–0.667) | 7640 (36.7) | 0.435[16]a (47.9) | 144000[16]a (41.2) | 45600[16]a (90.6) | |
| 1000 | 45 | 5 | 19400 (91.0) | 0.333b (0.300–0.550) | 12500 (73.2) | 0.661[4]a (130) | 75800[4]a (85.8) | 20100[4]a (104) |
Fig 1.
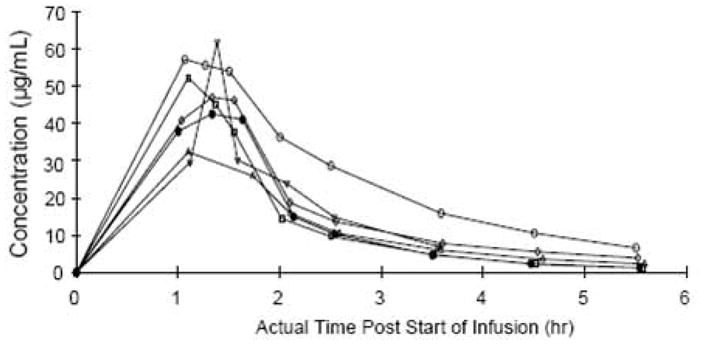
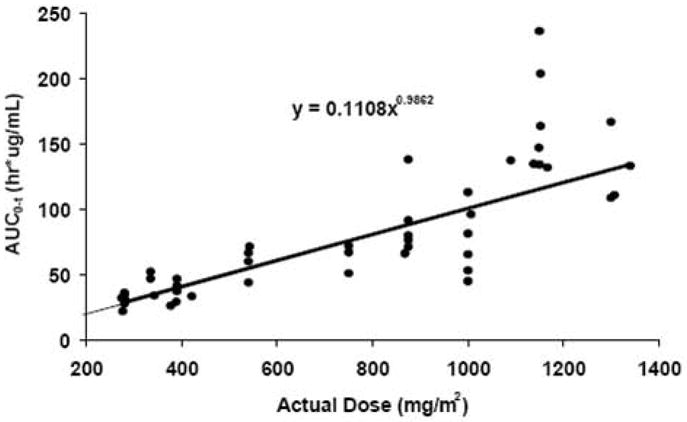
(A) Mean plasma concentration (μg/mL) of imexon following a single 60 minute intravenous infusion at 875 mg/m2. B) Relationship between AUC of imexon and dose following a single 30, 45, or 60 minute intravenous infusion of imexon
Gemcitabine pharmacokinetics
Typical retention times for gemcitabine and the internal standard, cimetidine, were 5.4 and 5.6 minutes, respectively. The validated concentration range was 5.00 to 1,000 ng/mL and batch accuracy (coefficient of variation) varied from 6.3% to 10.2 % intraday and 6.8% to 11.8% inter-day. Initial experiments showed no analytical interference between gemcitabine and imexon.
Table 4 summarizes gemcitabine pharmacokinetic data for 41 patients who received gemcitabine over 30 or 45 minutes at the MTD. Cmax was 10,700 ng/mL for a 30 minute infusion and 19,400 ng/mL for a 45 minute infusion. The geometric mean plasma T1/2 was 0.44 hours and 0.66 hours for the 30 minute and 45 minute groups, respectively. Plasma concentrations declined in a biphasic mode. Tmax was 0.43 hours and 0.33 hours for the 30 minute and 45 minute groups, respectively. As there was no end of infusion sample for gemcitabine, Cmax and Tmax must be considered approximations. Systemic clearance of gemcitabine was 144.000 mL/hr/m2 for the 30 minute group and 75,800 mL/hr/m2 for the 45 minute group. Systemic clearance of gemcitabine was high relative to hepatic blood flow in man. The volume distribution was greater than total body water volume suggesting extensive distribution in tissue or binding in tissue.
Overall these pharmacokinetic results were similar to previously reported gemcitabine disposition patterns [10].
Thiol depletion/pharmacodynamics
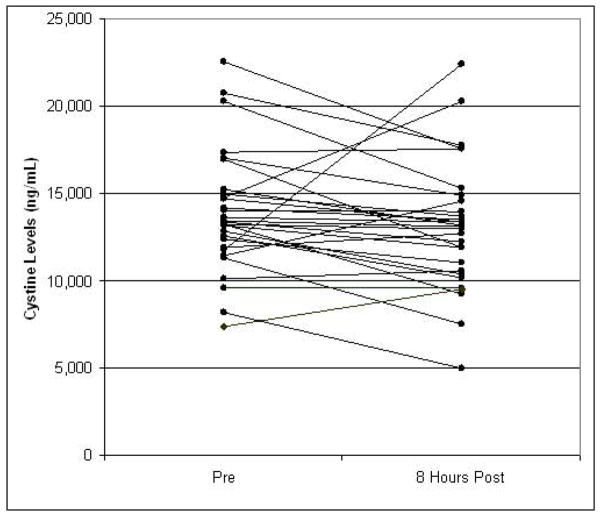
There were 29 patients who had paired baseline and 8 hour plasma samples analyzed for cystine content: 18 at the 875 mg/m2 dose level, and 11 at the 1,000 mg/m2 dose level (Figure 2). The baseline mean (SD) cystine concentration was 13,870 (3,459) ng/mL for all 29 patients. The 8hour cystine level decreased in 23 patients and increased in 6. There was no difference in baseline values for the two imexon dose levels evaluated. Similarly, the mean decrease in cystine levels was 5.2 % for all 29 patients, 4.8 % for the 875 mg/m2 dose level and 5.8% for the 1,000 mg/m2 dose levels (not significant by ANOVA). No difference in percent change in cystine levels by clinical response was evident (data not shown).
Fig 2.
Thiol levels (cystine) pre and 8 hours post imexon infusion, N=29
Discussion
This phase 1 study demonstrates that imexon can be safely combined with gemcitabine in patients with advanced pancreatic cancer. The primary DLT is acute abdominal pain and cramping associated with sudden bowel movement(s) or diarrhea occurring at the end of the infusion of imexon. This toxicity is rarely seen at the recommended phase II dose of imexon 875 mg/m2 with gemcitabine 1,000 mg/m2.
Importantly, encouraging evidence of antitumor activity was noted in this large, single arm phase 1 experience. Pharmacokinetic data demonstrate that levels of imexon consistent with preclinical activity can be achieved in patients with pancreatic cancer. There also appears to be no PK interaction between imexon and gemcitabine as the results of this study were similar to those reported for the administration of each drug alone as well as in gemcitabine combinations [9, 10].
A phase 1 study of imexon monotherapy administered on days 1–5 and 15–19 every 28 days was recently reported [9]. The recommended dose on this schedule was 875 mg/m2 with one of two DLTs at 1000 mg/m2 being abdominal pain similar to that seen in this study and occurring in a patient with pancreatic cancer. In another phase 1 study of imexon given daily for 5 days every 3 weeks, the MTD was 1150 mg/m2. Moulder et al. treated patients with breast, lung, or prostate cancer with imexon days 1–5 combined with docetaxel on day 1 every 3 weeks [11]. The MTD was imexon 1,300 mg/m2 with docetaxel 75 mg/m2. The DLT was non-cardiac chest pain. Samlowski et al. treated 68 patients with metastatic melanoma with imexon combined with DTIC both given days 1–5 every 21 days.[12] The MTD was 1,000 mg/m2 for both imexon and DTIC. The DLT of this regimen was hepatorenal failure in one patient presumably due to the DTIC and myelosuppression, while the most common non-DLT toxicities in the phase 2 portion included nausea, fatigue, and vomiting.
It is noteworthy that abdominal cramping and pain were not major toxicities of these other trials, they were clearly dose limiting in the current trial. One hypothesis is that patients with pancreatic cancer are more prone to the cholinergic effects of imexon, either through compromised pancreatic function or greater incidence of intra-abdominal metastases. Given the discovery of abdominal pain and cramping as DLT, additional laboratory investigation was undertaken in the guinea pig ileum. Imexon resulted in a cholinergic effect similar to that of acetylcholine. We hypothesized that administration of atropine would ameliorate this effect and this was achieved in vivo in the guinea pig ileum model (Dorr et al. unpublished). Based upon this evaluation, atropine was subsequently utilized at physician discretion for patients experiencing this toxicity with success. Additionally, we have demonstrated in the laboratory that radioactive imexon localizes in the intestine and pancreas in the mouse (Data on file, AmpliMed Corporation). Thus, pancreatic cancer patients may be particularly susceptible to the cholinergic effects of imexon. Alternatively, the weekly schedule evaluated through the majority of this clinical trial may predispose to cholinergic abdominal cramping affects to a greater extent than daily X 5 periodic dosing. The topoisomerase-I inhibitor irinotecan has cholinergic and gastrointestinal promotility effects which are more pronounced with weekly dosing than every 3 week administration.[13]
The objective response and stable disease rates are encouraging compared to historic controls. Gemcitabine alone or with erlotinib results in objective response rates of less than 10%.[2, 3] Studies combining two cytotoxic chemotherapy agents in pancreatic cancer have also rarely resulted in response rates above 10–15%.[14–16] Of increasing interest in pancreatic clinical trials is the rate of disease control, typically defined as response plus stable disease. Our rate of disease control of approximately 70% compares favorably to recent large randomized trials,[3] particularly when recognizing that our study was comprised nearly entirely of metastatic patients while other trials have included 20–25% locally advanced patients with more favourable prognosis. In addition, only 28 of the patients in the current trial received the combination at the MTD. However, our study is a single arm trial and these encouraging results need to be evaluated in subsequent randomized trials.
The pharmacokinetic analysis reveals plasma imexon levels consistent with preclinical antitumor activity.[8] Gemcitabine pharmacokinetics were in line with prior single agent reports without negative interaction with imexon.[10, 17] The degree of plasma cystine decrease was slightly less than that seen in the prior single agent study of imexon,[9] and may reflect a mediating effect of the gemcitabine infusion. Furthermore, while the majority (23/29) of patients evaluated for plasma cystine levels experienced a decrease 8 hours after imexon, there was substantial variability in the degree of change, and six patients (four with SD, two not evaluable), had plasma cystine levels increase. There did not appear to be any impact of dose or clinical outcome on change in cystine levels. However, the patient number was small, and this may have influenced the results.
In summary, this large phase 1 trial demonstrates that imexon and gemcitabine can be safely combined with encouraging clinical activity in advanced pancreatic cancer. The MTD is imexon 875 mg/m2 and gemcitabine 1000 mg/m2 weekly. A randomized phase II trial of gemcitabine with or without imexon is ongoing.
Acknowledgments
This work was funded and conducted by AmpliMed Corporation.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:24032413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 4.Hersh EM, Gschwind CR, Taylor CW, Dorr RT, Taetle R, Salmon SE. Antiproliferative and antitumor activity of the 2-cyanoaziridine compound imexon on tumor cell lines and fresh tumor cells in vitro. J Natl Cancer Inst. 1992;84:1238–1244. doi: 10.1093/jnci/84.16.1238. [DOI] [PubMed] [Google Scholar]
- 5.Dorr RT, Raymond MA, Landowski TH, Roman NO, Fukushima S. Induction of apoptosis and cell cycle arrest by imexon in human pancreatic cancer cell lines. Int J Gastrointest Cancer. 2005;36:15–28. doi: 10.1385/IJGC:36:1:015. [DOI] [PubMed] [Google Scholar]
- 6.Samulitis BK, Landowski TH, Dorr RT. Inhibition of protein synthesis by imexon reduces HIF-1alpha expression in normoxic and hypoxic pancreatic cancer cells. Invest New Drugs. 2008 doi: 10.1007/s10637-008-9149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dvorakova K, Payne CM, Tome ME, Briehl MM, McClure T, Dorr RT. Induction of oxidative stress and apoptosis in myeloma cells by the aziridine-containing agent imexon. Biochem Pharmacol. 2000;60:749–758. doi: 10.1016/s0006-2952(00)00380-4. [DOI] [PubMed] [Google Scholar]
- 8.Pourpak A, Meyers RO, Samulitis BK, Sherry Chow HH, Kepler CY, Raymond MA, Hersh E, Dorr RT. Preclinical antitumor activity, pharmacokinetics and pharmacodynamics of imexon in mice. Anticancer Drugs. 2006;17:1179–1184. doi: 10.1097/01.cad.0000236305.43209.f0. [DOI] [PubMed] [Google Scholar]
- 9.Dragovich T, Gordon M, Mendelson D, Wong L, Modiano M, Chow HH, Samulitis B, O’Day S, Grenier K, Hersh E, Dorr R. Phase I trial of imexon in patients with advanced malignancy. J Clin Oncol. 2007;25:1779–1784. doi: 10.1200/JCO.2006.08.9672. [DOI] [PubMed] [Google Scholar]
- 10.Storniolo AM, Allerheiligen SR, Pearce HL. Preclinical, pharmacologic, and phase I studies of gemcitabine. Semin Oncol. 1997;24:S7-2–S7-7. [PubMed] [Google Scholar]
- 11.Moulder S, Dhillon N, Ng C, Hong D, Wheler J, Naing A, Tse S, La Paglia A, Dorr R, Hersh E, Boytim M, Kurzrock R. A phase I trial of imexon, a pro-oxidant, in combination with docetaxel for the treatment of patients with advanced breast, non-small cell lung and prostate cancer. Invest New Drugs. 2009 doi: 10.1007/s10637-009-9273-1. (e-published ahead of print) [DOI] [PubMed] [Google Scholar]
- 12.Samlowski WE, Weber JS, Gonzalez R, et al. Phase I/II study of imexon (AMP) plus dacarbazine (DTIC) in patients (Pts) with metastatic malignant melanoma. J Clin Oncol. 2008;26 (May 20 suppl; abstr 9066) [Google Scholar]
- 13.Fuchs CS, Moore MR, Harker G, Villa L, Rinaldi D, Hecht JR. Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J Clin Oncol. 2003;21:807–814. doi: 10.1200/JCO.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 14.Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB., 3rd Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270–3275. doi: 10.1200/JCO.2002.11.149. [DOI] [PubMed] [Google Scholar]
- 15.Poplin E, Feng Y, Berlin J, Rothenberg ML, Hochster H, Mitchell E, Alberts S, O’Dwyer P, Haller D, Catalano P, Cella D, Benson AB., 3rd Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:3778–3785. doi: 10.1200/JCO.2008.20.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocha Lima CM, Green MR, Rotche R, Miller WH, Jr, Jeffrey GM, Cisar LA, Morganti A, Orlando N, Gruia G, Miller LL. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776–3783. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 17.Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, Mineishi S, Tarassoff P, Satterlee W, Raber MN, et al. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991;9:491–498. doi: 10.1200/JCO.1991.9.3.491. [DOI] [PubMed] [Google Scholar]