Abstract
We report the full account of our efforts on the lanthanum tricyanide-catalyzed acyl silane-ketone benzoin reaction. The reaction exhibits a wide scope in both acyl silane (aryl, alkyl) and ketone (aryl-alkyl, alkyl-alkyl, aryl-aryl, alkenyl-alkyl, alkynyl-alkyl) coupling partners. The diastereoselectivity of the reaction has been examined in both cyclic and acyclic systems. Cyclohexanones give products arising from equatorial attack by the acyl silane. The diastereoselectivity of acyl silane addition to acyclic α-hydroxy ketones can be controlled by varying the protecting group to obtain either Felkin-Ahn or chelation control. The resultant α-silyloxyketone products can be resolved with selectivity factors from 10 to 15 by subjecting racemic ketone benzoin products to CBS reduction.
Introduction
Polarity umpolung has evolved into a powerful synthetic tool for making otherwise challenging carbon-carbon bonds.1 The archetypical example of this strategy is the benzoin reaction, the mechanism of which was elucidated by Lapworth in 1903 in his report on the homodimerization of aldehydes under cyanide anion catalysis.2 Since then, both N-heterocyclic carbene (NHC) and metallophosphite catalysts have been developed to promote the asymmetric benzoin3 and cross silyl benzoin (acyl silane-aldehyde benzoin)4 reaction, respectively. Despite the recent advances in the scope and utility of the benzoin reaction, the direct catalytic coupling of acyl anion equivalents to ketones has proved more challenging. Stoichiometric methods for ketone acylation do exist; however, general strategies are not in place for performing those reactions asymmetrically.5 Suzuki,6,3c Enders,7,3e and You8 have achieved NHC-catalyzed intramolecular aldehyde-ketone benzoin cyclization for the formation of five- and six-membered rings. Asymmetric variants for the intramolecular reaction have also been reported that proceed in up to 98% yield and 99% ee.6b
An important example of intermolecular catalytic ketone acylation was reported by Demir and coworkers, who described the cyanide-catalyzed coupling of acyl phosphonates9 with ketones in chemical yields of 41–95%.10 Most of the examples employed electron-deficient ketones and enolizable protons were often replaced with flourine. While a limited number of aliphatic ketones could be successfully employed, they required modification of the reaction conditions and/or addition of various co-catalysts. Notably, the reaction was incompatible with methyl-aryl ketones and ortho-substituted aryl ketones. We recently published an initial report of the La(CN)3-catalyzed coupling betweeen acyl silanes and a variety of aryl-alkyl and alkyl-alkyl ketones.11 The purpose of this paper is to fully describe the development and expansion of this methodology, which encompasses a broad range of ketone substrates, achieves substrate-controlled diastereoselectivity, and a enables a subsequent kinetic resolution that allows access to enantioenriched products.
Results
Reaction Development and Optimization
Having previously found La(CN)3 to be an effective catalyst for promoting the cross silyl benzoin between acyl silanes and aldehydes,12 we were hopeful that we might be able to engage ketone electrophiles with acyl silanes using the same catalyst. Gratifyingly, acyl silane 1a reacted with one equivalent of acetophenone in the presence of 20 mol % of La(CN)3 in THF to deliver the desired α-silyloxyketone product in approximately 40% yield within 20 minutes. Competing with desired product formation was the deprotonation of acetophenone, leading to the quenched silyl cyanohydrin (3a). A retro-benzoin pathway was also found to be operative, as subjection of the product 2a to the reaction conditions led to the formation of 3a and acetophenone. Employing two equivalents of ketone proved to be optimal, as the product 2a was obtained in greater that 90% yield when these conditions were applied. In a screen of metal cyanide catalysts, we found that numerous M(CN)n species effectively promoted the reaction and gave complete conversion (Table 1); however, La(CN)3 provided the highest ratio of desired product (2a) to the quenched cyanohydrin (3a). Optimization of the catalyst loading showed that the benzoin product could be obtained in up to 95% yield with 10 mol % catalyst loading. Lowering the catalyst loading to 5 mol % provided the product with no change to the conversion or yield. Upon further reduction of the catalyst loading to 2 mol % and 1 mol %, the reaction stalled with incomplete conversion after 24 h.
Table 1.
Optimization of M(CN)3 Catalysta
 | |||||
|---|---|---|---|---|---|
| entry | catalyst | x mol % | convn (%)b | 2a:3b | Yield (%)c |
| 1 | Ce(CN)3 | 20 | 100 | 3.2:1 | nd |
| 2 | Y(CN)3 | 20 | 100 | 4.5:1 | nd |
| 3 | Yb(CN)3 | 20 | 100 | 6.5:1 | nd |
| 4 | Sc(CN)3 | 20 | 100 | 6.8:1 | nd |
| 5 | Er(CN)3 | 20 | 100 | 8.0:1 | nd |
| 6 | Hf(CN)4 | 20 | 100 | 8.6:1 | nd |
| 7 | La(CN)3 | 20 | 100 | 10.5:1 | nd |
| 8 | La(CN)3 | 10 | 100 | nd | 95 |
| 9 | La(CN)3 | 5 | 100 | nd | 94 |
| 10 | La(CN)3 | 2 | 62 | nd | nd |
| 11 | La(CN)3 | 1 | 7 | nd | nd |
Conditions: 1.0 equiv of 1a, 2.0 equiv of ketone, 0.10 equiv of La(CN)3, THF, [1a]0 = 0.04 M, rt, 20 min.
Determined by 1H NMR spectroscopy.
Yields of analytically pure material after SiO2 column chromatography.
Scope: Ketone Partner
To probe the scope of the reaction, we used acyl silane 1a and varied the ketone employed (Table 2). The reactions proceeded with complete consumption of acyl silane, with isolated yields ranging from 40–95%. All examples employed enolizable electrophiles. The major byproduct in all cases was the quenched cyanohydrin, which accounts for most of the remaining mass balance. For some substrates, it was convenient to deprotect the silyl ether to the alcohol using TBAF at 0 °C in order to separate the benzoin product from the ketone starting material. In all cases, this desilylation proceeded smoothly within 10 minutes, and elimination to the alkene was never observed.
Table 2.
Scope of Ketone Coupling Partnera
 | |||
|---|---|---|---|
| entry | product | yield (%)b | |
| 1 | 2a |  |
96 |
| 2 | 2b |  |
92 |
| 3 | 2c |  |
91 |
| 4 | 2d |  |
85 |
| 5 | 2e |  |
77 |
| 6c | 2f |  |
61 |
| 7c | 2g |  |
60 |
| 8 | 2h |  |
73 |
| 9c | 2i |  |
63 |
| 10 | 2j |  |
86 |
| 11 | 2k |  |
80 |
| 12c | 2l |  |
85 |
| 13c | 2m |  |
40 |
Conditions: 1.0 equiv of 1a, 2.0 equiv of ketone, 0.10 equiv of La(CN)3, THF, [1a]0 = 0.04 M, rt, 20 min.
Yields of analytically pure material after SiO2 column chromatography.
Product was treated with 1.0 equiv TBAF at 0 °C for 10 min to enable purification. Yield reported over two steps.
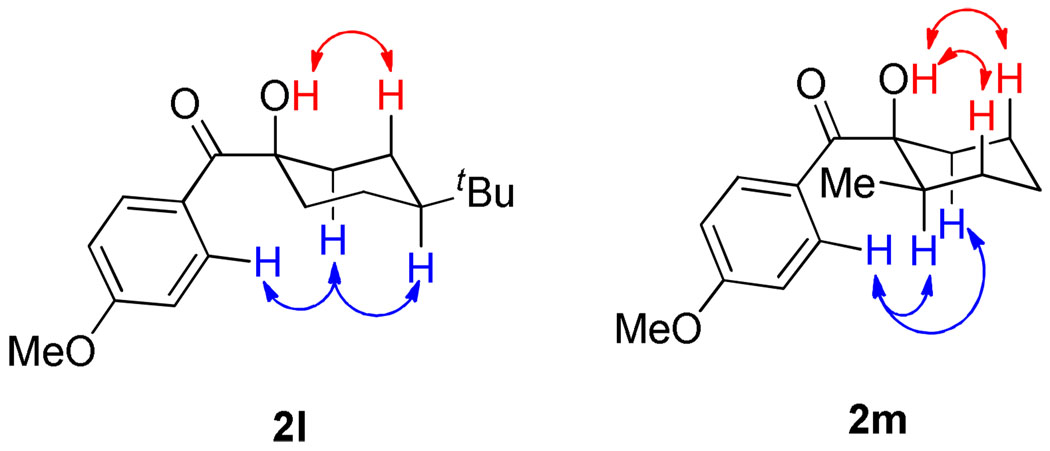
Products 2l and 2m demonstrate the high degree of stereoselectivity possible in the reaction with cyclic electrophiles, each being isolated as a single diastereomer. In order to determine the relative stereochemistry between the existing alkyl group and the newly introduced acyl group, 2-D NOESY was employed (Figure 2). In 2l, an nOe was observed between the hydroxyl proton and the two axial γ-protons, as well as between the ortho aryl protons and the axial β-protons on the cyclohexane ring. Similar nOe’s were observed for compound 2m. This leads us to propose the illustrated stereochemistry with the hydroxyl group cis to the existing alkyl group, arising from an equatorial attack of the acyl silane to generate an axial alcohol.
Figure 2.
NOESY Analysis to Determine Equatorial Attack
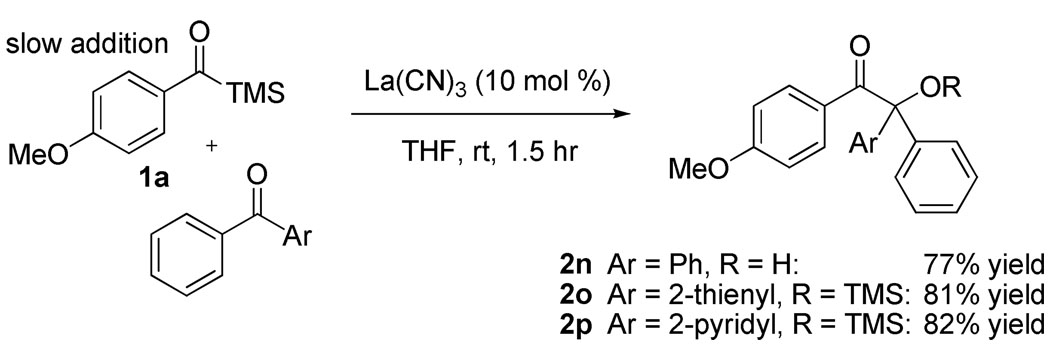
We also hoped to couple acyl silanes with diaryl ketones. While lacking enolizable protons, the additional steric constraints make this a challenging transformation. Initial attempts at employing benzophenone under the normal reaction conditions led to less than 20% yield of the desired coupling product. Cyanohydrin formation (presumably from adventitious water), acyl silane dimerization, and desilylation of the acyl silane were all observed. We found that by freshly distilling the benzophenone prior to use and employing a slow addition of the acyl silane via syringe pump over fifty min allowed us to isolate the desired product in 80% yield after silyl deprotection and column chromatography. This procedure works for both carbocyclic and heterocyclic diaryl ketones, as evidenced by Scheme 1.
Scheme 1.
Addition of Acyl Silane to Diaryl Ketones
Felkin-Ahn and Chelation Control
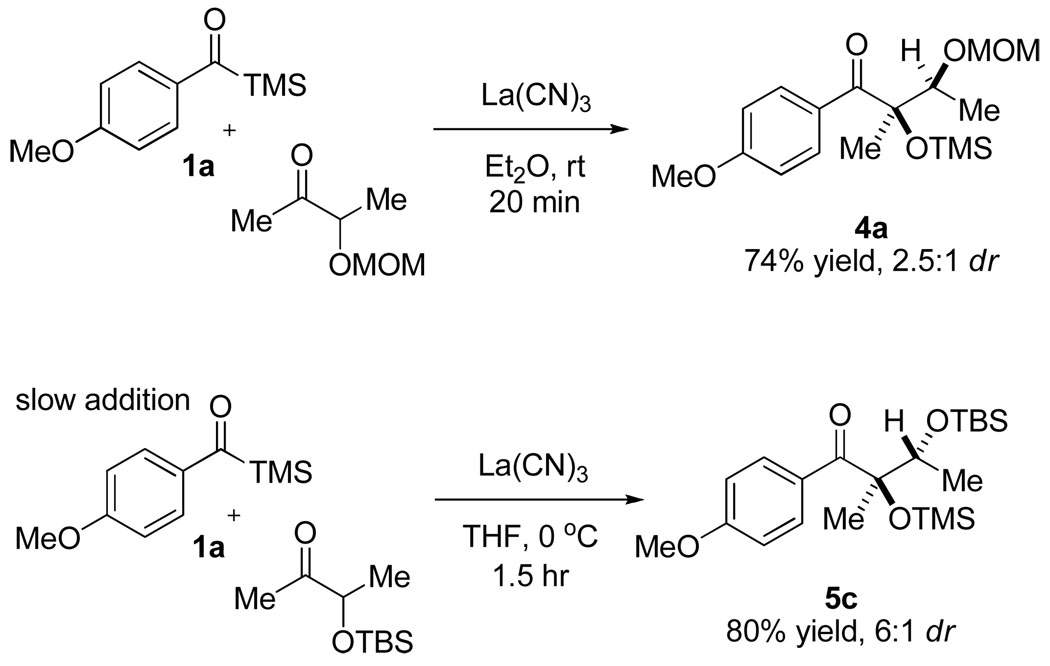
After achieving high levels of diastereocontrol with cyclic electrophiles, we sought to determine if we could extend this control to acyclic systems. We hoped that we could use an α-stereocenter to exert either Felkin-Ahn13 or chelation14 diastereocontrol on the ketone-benzoin coupling. We found that acyl silane 1a did couple with various protected acetoins to yield α,β-dihydroxy ketones. As with the diaryl ketone substrates, a slow addition of the acyl silane to a suspension of the ketone and catalyst provided increased yields. We screened a number of hydroxyl protecting groups and reaction conditions, summarized in Table 3. While we achieved minimal ketone facial selectivity with the benzyl protecting group, we found that the MOM group afforded moderate levels of diastereocontrol (2.7:1) favoring the chelation product (MOM = CH2OCH3). A solvent screen identified Et2O and MTBE as giving similar diastereoselectivity; however, Et2O afforded higher yields in the reaction. Metal salt additives and various Ln(CN)3 catalysts were examined; however, none of the results improved upon the La(CN)3 catalyst system.
Table 3.
Optimization of Chelation Control in Addition to Protected Acetoinsa
 | ||||||
|---|---|---|---|---|---|---|
| entry | R | M(CN)3 | solvent | additive | yield (%)b | 4a:4bb |
| 1c | Bn | La(CN)3 | THF | none | 49 | 1:1.4 |
| 2c | Bn | La(CN)3 | Et2O | none | 45 | 1.1:1 |
| 3 | MOM | La(CN)3 | THF | none | 42 | 1.4:1 |
| 4 | MOM | La(CN)3 | Et2O | none | 80 | 2.7:1 |
| 5 | MOM | La(CN)3 | MeOcC5H9 | none | 10 | 1.2:1 |
| 6 | MOM | La(CN)3 | MeOtBu | none | 71 | 2.1:1 |
| 7 | MOM | La(CN)3 | Et2O | LiCl | 70 | 2.1:1 |
| 8 | MOM | La(CN)3 | Et2O | MgBr2 | NDPd | --- |
| 9 | MOM | La(CN)3 | Et2O | ZnBr2 | NDPd | --- |
| 10 | MOM | Ce(CN)3 | Et2O | none | 76 | 2.0:1 |
| 11 | MOM | Er(CN)3 | Et2O | none | 72 | 1.8:1 |
| 12 | MOM | Yb(CN)3 | Et2O | none | 49 | 1.2:1 |
Conditions: 1.0 equiv of 1a added via slow addition, 2.0 equiv of ketone, 0.10 equiv of La(CN)3, THF, [1a]0 = 0.04 M.
Yields and dr determined by 1H NMR.
1a added via normal addition.
NDP = no desired product
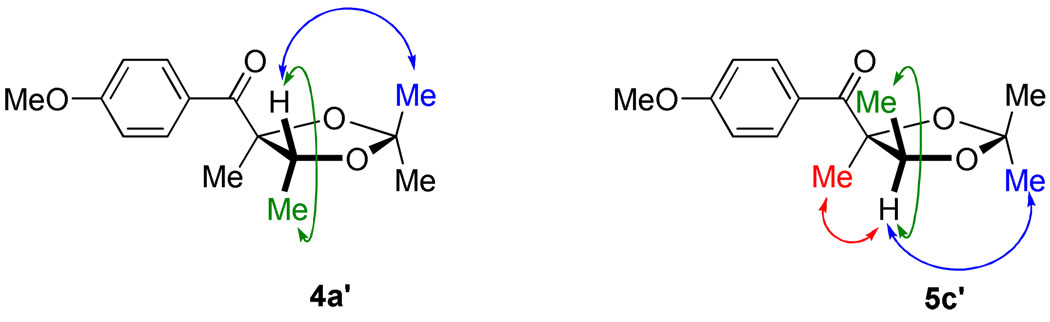
To investigate the feasibility of obtaining the opposite (Felkin-Ahn) diastereomer, we investigated non-chelating silyl protecting groups (Table 4). As expected, we found that as the size of the silyl group was increased from TMS to TES to TBS, the dr likewise increased (this trend did not extend to the TIPS group). Upon varying the reaction temperature, we found that we could increase the dr of the reaction up to 10:1 by running the reaction at −20 °C; however, the diastereoselectivity came at the expense of yield as competing side reactions could not be suppressed even with slow addition of 1a at the lower temperature. To verify chelation and Felkin-Ahn controlled products were formed under the respective conditions, the products were converted to the free diols and thence to their acetonides. 2D-NOESY analysis of the resultant acetonides confirmed that 4a was indeed the syn-diol arising from chelation control and 5c was consistent with the anti-diol and Felkin-Ahn control. Scheme 2 shows the final optimized conditions, yields, and drs for both the chelation and Felkin-Ahn controlled ketone benzoin reactions.
Table 4.
Optimization of Felkin-Ahn Control in Addition to Protected Acetoinsa
 | ||||
|---|---|---|---|---|
| entry | R | T (°C) | yield (%)b | 5c:5db |
| 1 | TMS | 0 | 65 | 2:1 |
| 2 | TES | 0 | 70 | 4:1 |
| 3 | TBS | 0 | 80 | 6:1 |
| 4 | TIPS | 0 | 74 | 3.5:1 |
| 5 | TBS | −20 | 48 | 10:1 |
Conditions: 1.0 equiv of 1a, 2.0 equiv of ketone, 0.10 equiv of La(CN)3, THF, [1a]0 = 0.04 M.
Yields and dr were determined by 1H NMR.
Scheme 2.
Optimized Conditions for Achieving Felkin-Ahn or Chelation Control
Scope: Enones
After expanding the scope of the reaction to include alkyl-alkyl, aryl-alkyl, and aryl-aryl ketones, we turned our attention to enones and ynones. Choosing cyclohexenone as our initial model system, we were disappointed to isolate only a small amount (9%) of the desired product. The major product in the reaction was the 4-silyloxycyanohydrin ketone resulting from Stetter-type 1,4-addition to the enone (Table 5, entry 1).15 The La(CN)3 catalyst loading was increased to 33 mol% (stoichiometric in −CN) for substrates that gave a large amount of 1,4 addition by-product (Table 5, entries 1–4 and 6). The silyl cyanohydrins exist as inseparable mixtures of diastereomers. Cleavage of the silyl group with TBAF converted both diastereomers to the 1,4-diketone.
Table 5.
Scope of α,β-Unsaturated Coupling Partnersa
 | |||
|---|---|---|---|
| entry | product | % yield (dr)b | |
| 1c | 6a |  |
9 |
| 1c,d | 6a´ |  |
71 |
| 2c | 6b |  |
35 |
| 2c,d | 6b´ |  |
29 |
| 3c | 6c |  |
45 |
| 3c,d | 6c´ |  |
33 |
| 4c | 6d |  |
41 |
| 5 | 6e |  |
82 |
| 6c | 6f |  |
34 |
| 7 | 6g |  |
69 |
| 8 | 6h |  |
74 (2.5:1) |
| 9 | 6i |  |
83 (2.7:1) |
| 10 | 6j |  |
86 |
Conditions: 1.0 equiv of 1a, 2.0 equiv of ketone, 0.10 equiv of La(CN)3, THF, [1a]0 = 0.04 M, rt, 20 min.
Yields of analytically pure material after SiO2 column chromatography, dr determined by 1H NMR spectroscopy.
Stoichiometric cyanide was employed.
Product was treated with 1.0 equiv TBAF at 0 °C for 10 min to provide diketone which was used for characterization. Yield refers to yield of the silylcyanohydrin.
Fortunately, we found that the yield of the 1,2-addition product increased when enones with more steric hindrance at the β-position were employed. Additionally, acyclic enones gave more of the 1,2-addition product than comparably substituted cyclic alkenes. An ynone were also found to be a competent coupling partner as evidenced by the formation of 6j. No conjugate addition product was observed with this ynone.
Scope: Acyl Silane
In addition to varying the ketone component, we examined the reaction’s tolerance with respect to the variation of the acyl silane coupling partner. The results of this survey are shown in Table 3. Electron-rich acyl silanes deliver a more nucleophilic (silyloxy)nitrile anion intermediate and performed the best, as expected.10,16 Yields of the coupling product decreased as a function of electron density on the aryl ring, as evidenced by entries 1, 4, and 5. The effect of steric hindrance in the acyl silane component was examined with entry 7, which delivered the desired product with a slight decrease in yield. In addition to aromatic acyl silanes, both heteroaromatic and aliphatic silanes were tolerated. The more sterically demanding TES and TBS groups were also tolerated with almost no decrease in yield (7b, 7c).
Kinetic Resolution
Having established the scope of the reaction with respect to both coupling partners, we turned our attention to accessing the products in an asymmetric fashion. Our attempts to develop a catalytic asymmetric variant have been thus far unsuccessful. Demir demonstrated that both Lewis and Brønsted acid co-catalysts were capable of accelerating the intermolecular coupling of acyl phosphonates and ketones.10 We observed that addition of a thiourea co-catalyst did increase the relative rate of the reaction at −30 °C as compared to just the La(CN)3; however, we saw no asymmetric induction in the products when chiral variants were applied. Similarly, box- and pybox-complexes with Ln(CN)3 also led to isolation of racemic product. Upon examining metallophosphite catalysts, which our group has used with success in other acyl silane coupling reactions, we found that no desired product was obtained.4,16,17 We conjecture that this may be due to steric repulsion between the phosphite-silane adduct and the ketone electrophile.
In lieu of a direct asymmetric addition, we explored other possibilities for obtaining optically enriched products. When the silyloxyketone 2a was subjected to a CBS reduction,18 the remaining starting material was enantioenriched when the reaction was quenched at partial conversion. We therefore decided to examine a kinetic resolution of the silyloxyketones obtained from the acyl silane-ketone benzoin reaction using the CBS reduction catalyst.19
We chose to use silyloxyketone 2a in optimizing the kinetic resolution. Upon screening a number of reaction conditions, we found BH3·THF to be the optimal borane source. Two equivalents of the hydride source worked best, as lower loadings often resulted in the reaction stalling at low conversions and er. The reaction proceeded in both ethereal and aromatic solvents, but Et2O gave the shortest reaction time while maintaining high selectivity factors. Higher selectivity factors were obtained at lower temperatures at the expense of reaction rate, with −10 °C being the best compromise.
With the optimized conditions in hand, we sought to explore the scope of the kinetic resolution. Selectivity factors obtained with methyl-aryl ketones were between 10 and 15 (Scheme 3). The reaction works well for aryl-methyl ketones; however, other substrates (including dialkyl, vinyl-alkyl, and alkynyl-alkyl) resulted in very little discrimination between enantiomers. The bulkier TBS group can be substituted for the TMS protecting group which results in a slightly higher selectivity factor.
Scheme 3.
Kinetic Resolution of α-Silyloxy Ketones via CBS Reduction
The absolute stereochemistry of product (+)−7d was determined to be R by comparison to a sample of ent-7d, prepared by Sharpless asymmetric dihydroxylation, oxidation, and silylation (Scheme 3). The remaining absolute configurations were assigned by analogy. Silyloxyketone 2a was subjected to the CBS reduction and the reaction monitored by SFC of aliquots. Following chromatography, enantioenriched (+)−2a was recovered in 44% yield and 88% ee.
Discussion
Challenges to the Ketone Benzoin
As can be inferred from the relative dearth of literature precedent, the intermolecular ketone-benzoin reaction poses significant challenges as compared to the intermolecular aldehyde-benzoin and the intramolecular ketone benzoin. Some of these potential pitfalls are illustrated in Figure 4 below.
Figure 4.
Proposed mechanistic pathway for acyl silane-ketone benzoin reaction (black) and possible side reactions (gray).
The principle source of the challenges lies in the lower reactivity of ketones relative to aldehydes due to the extra steric demand posed by the second carbonyl substituent. This lower desired reactivity may lead to side reactions, including dimerization of the acyl silane starting material or deprotonation of the ketone by the silyloxycyanohydrin anion. A third potential side reaction is a retro-benzoin reaction. Bode has recently reported an NHC-catalyzed retro-benzoin reaction of α-hydroxyketones as a strategy to mask unstable enal functionality (Figure 5).20 The viability of retro-benzoin reaction was established in the following control experiments: when products 2a or 7f were treated with La(CN)3 in THF, acetophenone and the corresponding silyloxycyanohydrin were obtained.
Figure 5.
NHC-catalyzed retro-benzoin reaction of tertiary α-hydroxyketone
In the course of our studies, we encountered by-products arising from each of the three possible side reactions noted. Ketone enolization was largely a function of the steric hindrance and acidity of the ketone substrate, and manipulation of the reaction conditions was typically unsuccessful in suppressing this pathway. For example, sterically hindered pinacolone and enolizable methyl pyruvate failed to provide useful yields of the coupling products under a variety of conditions. Entries 8 and 9 (in Table 2) and isobutyrophenone (not tabulated) demonstrate the sensitivity to even small changes in sterics. While 2h can be obtained in good yield (73%) from propiophenone, employing isobutyrophenone as the ketone leads to a yield of <10%, despite propiophenone having a lower pKa (24.4 as compared to 26.3).21 However, cyclobutyl phenyl ketone coupled to give 2i in 63% yield, despite possessing a very similar steric environment to isobutyrophenone. It should be noted that, with the exception of the diaryl ketones, all of the substrates employed are enolizable. In some substrates, drying and distillation of the ketone to remove adventitious water slightly reduced the amount of quenched silyloxycyanohydrin obtained. Dimerization of the acyl silane was observed with some of the more encumbered ketones, notably when diaryl ketones and protected acetoins were employed. We were pleased to find that a slow addition of the acyl silane effectively generated a large excess of ketone, which inhibited silane dimerization with challenging substrates.
Scope of the Reaction
The system described herein for acyl silane-ketone benzoin reactions offers a substantial improvement in the reaction scope of ketone-benzoin methodology. As Demir had illustrated the coupling of electron-deficient and non-enolizable ketones, we chose to address substrates with no general procedure for their synthesis in place. The coupling of aryl-methyl ketones was accomplished in a very general manner: electron-rich, neutral, and heteroaromatic substrates were tolerated, as well as those with ortho substituents (albeit in moderately lower yield). Dialkyl substrates were well tolerated, although α-branching exerts a strong negative impact on the yields via competitive quenching of the silylcyanohydrin. Sterically encumbered diaryl ketones underwent coupling in high yields once side reactions were minimized via a slow addition.
In exploring the diastereoselectivity of the reaction, we found that with cyclohexanone-derived ketones, the acyl silane adds equatorially to avoid 1,3-diaxial interactions, even in the case of 2-methylcyclohexanone, where the vicinal methyl group also occupies the equatorial position. In each case, a single diastereomer was obtained. In the fused decalin systems, the dr was decreased to 2.5:1. Diastereocontrol was also achieved in acyclic systems by employing a protected α-hydroxy group to direct either Felkin-Ahn or chelation control. Chelation control was achieved by employing the MOM protecting group in conjunction with a non-coordinating ethereal solvent, albeit in a modest 2.7:1 dr. Attempts to achieve higher dr were made by adding a soluble metal co-catalyst. The La(CN)3 is a heterogeneous catalyst, which we felt might preclude efficient chelation to the protected acetoin. The soluble metal additives we examined led to either no change or a decrease in the reaction yield and dr. Switching to a coordinating solvent (THF) and a TBS protecting group allowed us to access the Felkin-Ahn product in 6:1 dr and 80% yield.
In the addition of acyl silanes to enones, we encountered competing 1,2-benzoin-type and 1,4-Stetter-type reactivity. In the Stetter-type reaction, the resultant enolate was not sufficiently nucleophilic to undergo silyl transfer and initiate catalyst turnover. This is consistent with previous work in our lab on the metallophosphite-catalyzed alkene silylacylation reaction, where α,β-unsaturated amides were necessary as enolates derived from enones were not sufficiently nucleophilic to initiate silyl transfer and achieve catalyst turnover.16 Thus, the reactions between acyl silanes and some enones became stoichiometric in cyanide and required use of a full equivalent to achieve complete conversion in the cases where substantial 1,4-addition occurred. In cyclohexene-derived enones, γ-substitution was particularly effective at preventing conjugate addition as the substituents extend above or below the plane of the alkene to block the incoming nucleophile.
Kinetic Resolution
In order to obtain enantioenriched products from this reaction, we developed a kinetic resolution based on the CBS reduction. While there are reports employing the CBS catalyst in kinetic resolutions,19 none of the accounts examined α-hydroxyketones as the substrate. We found that the silyl protecting group was necessary for successful kinetic resolution: when the free α-hydroxyketone was employed, no rate difference was observed for the two enantiomers. Thus, the acyl silane-ketone benzoin reaction directly furnishes the substrate necessary for the resolution. It stands to reason that the tertiary carbinol acts as Rlarge, with the aryl group functioning as Rsmall, as increasing the steric bulk of the silyl group from TMS to TBS led to an increase in the selectivity factor.
Conclusion
In conclusion, we have developed a broadly applicable and operationally simple method for intermolecular ketone acylation through a La(CN)3-catalyzed silyl benzoin reaction. Various ketone classes including aryl-alkyl, alkyl-alkyl, aryl-aryl, enones, and ynones have been employed. The reaction can be done in a diastereoselective fashion on both cyclic and acyclic systems. In cyclic systems, equatorial attack is favored, and in acyclic systems both chelation and Felkin-Ahn products can be selected for by judicious choice of protecting group and solvent with acetoin electrophiles. Products arising from aryl-methyl ketones can be resolved through a novel kinetic resolution that makes use of the CBS reduction.
Experimental Section
General Procedure for La(CN)3-Catalyzed Ketone Benzoin Reaction (A)
In the glovebox, a 25-mL round bottomed flask was charged with LaCl3 (12 mg, 0.049 mmol), a stir bar, and THF (3 mL). A second 10-mL flask was charged with 0.48 mmol of acyl silane, 1.00 mmol of ketone, and 8 mL of THF. The flasks were capped with a septum, removed from the glovebox, and placed under positive pressure of N2. The suspension of LaCl3 was cooled to −78 °C for 5 min, then nBuLi (1.4 M in hexanes, 105 µL, 0.147 mmol) was added. The cloudy suspension was stirred for 10 min at −78 °C and warmed to 0 °C for 15 minutes. TMSCN was added as a solution in THF (~0.5 M, 15 mg, 0.152 mmol). The catalyst suspension was stirred at 0 °C for 10 min and allowed to warm to rt over 30 min. After the catalyst suspension reached room temperature, the solution of acyl silane (0.48 mmol, 1 equiv) and ketone (0.96 mmol, 2 equiv) was added via syringe. The reaction was monitored by TLC (20% EtOAc/petroleum ether) and all substrates showed complete consumption of starting material within 20 min. Upon completion, the reaction mixture was poured into 40 mL of 1:1 Et2O/H2O in a separatory funnel. The layers were separated and the aqueous layer was extracted with two 20 mL portions of Et2O. The organic extracts were combined, dried over MgSO4, filtered, and concentrated on a rotary evaporator. Column chromatography of the crude material on silica gel using 5:95 Et2O/petroleum ether as the eluent furnished the desired product in analytically pure form or as a mixture of product and ketone starting material (see General Procedure for Deprotection of α-Silyloxyketones).
1-(4-methoxyphenyl)-2-phenyl-2-(trimethylsilyloxy)propan-1-one (Table 2, 2a)
The title compound was prepared according to General Procedure A using 100 mg of acyl silane, 120 mg of acetophenone, 12 mg LaCl3, 105 µL of nBuLi, and 15 mg of TMSCN in 11 mL of THF. After chromatography, 151 mg (96% yield) of the title compound was isolated as a colorless oil. Analytical data for title compound: IR (thin film, cm−1) 2959, 1675, 1600, 1508, 1253, 1175, 1154, 1127, 868, 844; 1H NMR (400 MHz, CDCl3) δ 7.93 (dd, J = 6.8, 2.0 Hz, 2H), 7.48-7.46 (m, 2H), 7.33-7.29 (m, 2H), 7.23-7.19 (m, 1H), 6.74 (dd, J = 6.8, 2.0 Hz, 2H), 3.78 (s, 3H), 1.76 (s, 3H), 0.06 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 199.1, 162.7, 145.5, 133.3, 128.4, 127.6, 127.0, 124.1, 112.9, 83.5, 55.2, 30.2, 1.8; TLC (20% EtOAc/petroleum ether) Rf = 0.44, (5% Et2O/petroleum ether) Rf = 0.29; LRMS (ESI) Calcd for C19H25O3Si, 329.2; Found, 329.0.
General Procedure for Deprotection of α-Silyloxyketones (B)
The title compound was dissolved in dry THF (10 mL) in a 100-mL flame dried round bottomed flask and cooled to 0 °C. Tetrabutylammonium flouride (0.50 mL, 1.0 M solution in THF, 1.0 equivalents based on 100% yield from initial coupling) was added. The reaction turned yellow and rapidly faded to colorless. The reaction was monitored by TLC (20% EtOAc/petroleum ether) and was complete within 10 min for all substrates. The reactions were poured into 1:1 Et2O/H2O (40 mL), and the aqueous layer was extracted with two 20 mL portions of Et2O. The organic extracts were combined, dried over MgSO4, and concentrated on a rotary evaporator. The crude material was purified by flash chromatography on silica gel using 3:7 Et2O/petroleum ether as the eluent to furnish the desired product in analytically pure form. Yields for these products are reported over two steps.
2-hydroxy-2-(2-methoxyphenyl)-1-(4-methoxyphenyl)propan-1-one (Table 2, 2f)
The title compound was prepared according to General Procedure A, followed by General Procedure B, using 100 mg of acyl silane, 145 mg of 2´-methoxyacetophenone, 12 mg LaCl3, 105 µL of nBuLi, and 15 mg of TMSCN in 11 mL of THF. After chromatography, 84 mg (61% yield) of the title compound was isolated as a white solid (mp 89–90 °C). Analytical data for title compound: IR (thin film, cm−1) 3435, 2965, 2932, 1667, 1600, 1509, 1489, 1464, 1251, 1174, 1123, 1026, 965, 844; 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 8.8 Hz, 2H), 7.66 (d, J = 7.6 Hz, 1H), 7.30 (t, J = 8.0 Hz, 1H), 7.06 (t, J = 7.2 Hz, 1H), 6.79-6.73 (m, 3H), 4.98 (s, 1H), 3.79 (s, 3H), 3.49 (s, 3H), 1.85 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 200.4, 163.1, 157.0, 132.5, 131.6, 129.6, 126.2, 121.1, 113.3, 112.1, 76.7, 55.3, 55.2, 26.2; TLC (20% EtOAc/petroleum ether) Rf = 0.12, (30% Et2O/petroleum ether) Rf = 0.11; LRMS (ESI) Calcd for C17H18O4Na, 309.1; Found, 309.1.
General Procedure for Kinetic Resolution of α-Silyloxyketones (C)
The α-silyloxyketone was massed into a dry Teflon coated screw-cap vial with a stir bar, and purged with N2. Diethyl ether (2 mL) was added, and the reaction was capped and brought into the glovebox. A 0.1 M toluene solution of the CBS catalyst (10 mol %) was added, and the reaction was capped and removed from the glovebox. The reaction was then cooled to −10 °C, and a 1.0 M THF solution of BH3•THF was added to the reaction. The reaction was monitored by quenching aliquots in MeOH. To stop the reaction, MeOH was added, and the contents were poured into 1:1 Et2O/saturated NH4Cl. The organic layer was separated, washed with brine, dried over MgSO4, filtered and concentrated. The resolved material was separated from the monosilylated diol by column chromatography on silica gel with 1:9 Et2O/petroleum ether as the eluent.
(R)-1-(4-methoxyphenyl)-2-phenyl-2-((trimethylsilyl)oxy)propan-1-one (Scheme 3, (+)−2a)
The title compound was prepared according to General Procedure C using 30 mg of (±)−2a, 100 µL of the CBS catalyst as a 0.1 M solution in toluene, and 75 µL of BH3•THF as a 1.0 M solution in THF. The reaction was stopped at partial conversion after 3 h. Conversion was determined by 1H NMR spectroscopy and the enantiomeric excess of the recovered starting material was determined by SFC (OD column, 150 psi, 1.5 mL/min, and 0.5% MeOH modifier, tmajor = 12.6 min, tminor = 13.5 min). The reaction was stopped 56% conversion and the starting material was recovered in 85% ee, with a selectivity factor of s = 13.2. The starting material was separated from the diol by flash chromatography. Analytical data is identical to (±)−2a, [α]D25 = 136.0 (c = 3.30, CH2Cl2). Deprotection of (+)−2a with TBAF cleanly yielded the free hydroxyl product, [α]D25 = −30.4 (c = 0.70, CH2Cl2).
Supplementary Material
Figure 1.
Previous examples of intra- and intermolecular-ketone benzoin reactions
Figure 3.
NOESY Analysis to Confirm Felkin-Ahn and Chelation Controlled Addition
Scheme 4.
Determination of Absolute Configuration of Enantioenriched Product
Table 6.
Scope of Acyl Silane Coupling Partner
Conditions: 1.0 equiv of 1a, 2.0 equiv of ketone, 0.10 equiv of La(CN)3, THF, [1a]0 = 0.04 M, rt, 20 min.
Yields of analytically pure material after SiO2 column chromatography.
Product was treated with 1.0 equiv TBAF at 0 °C for 10 min to enable purification. Yield reported over two steps.
Acknowledgements
Funding for this work was provided by the National Institutes of Health (National Institute of General Medical Sciences – GM068443) and Novartis (Early Career Award to J.S.J.).
Footnotes
Supporting Information Available: Characterization data for all compounds, copies of 1H and 13C NMR spectra, and racemic and enantioenriched SFC traces of compounds subjected to kinetic resolution. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Corey EJ, Seebach D. Angew. Chem. Int. Ed. 1965;4:1077–1078. [Google Scholar]
- 2.Lapworth A. J. Chem. Soc. 1903;83:995–1005. [Google Scholar]
- 3.(a) Enders D, Kalfass U. Angew. Chem., Int. Ed. 2002;41:1743–1745. doi: 10.1002/1521-3773(20020517)41:10<1743::aid-anie1743>3.0.co;2-q. and references therein. [DOI] [PubMed] [Google Scholar]; (b) Dunkelmann P, Kolter-Jung D, Nitsche A, Demir AS, Siegert P, Lingen B, Baumann M, Pohl M, Muller M. J. Am. Chem. Soc. 2002;124:12084–12085. doi: 10.1021/ja0271476. [DOI] [PubMed] [Google Scholar]; (c) Hachisu Y, Bode JW, Suzuki K. Adv. Synth. Catal. 2004;346:1097–1100. [Google Scholar]; (d) Enders D, Oliver N. Synlet. 2004:2111–2114. [Google Scholar]
- 4.Linghu X, Potnick JR, Johnson JS. J. Am. Chem. Soc. 2004;126:3070–3071. doi: 10.1021/ja0496468. [DOI] [PubMed] [Google Scholar]
- 5. Deprotonation of silyl cyanohydrins: Bunnelle EM, Smith CR, Lee SK, Singaram SW, Rhodes AJ, Sarpong R. Tetrahedron. 2008;64:7008–7014. doi: 10.1016/j.tet.2008.02.103. Wessig P, Glombitza C, Mueller G, Teubner J. J. Org. Chem. 2004;69:7582–7591. doi: 10.1021/jo040173x. Lithiation of vinyl ethers: Palomo C, Oiarbide M, Arceo E, Garcia JM, Lopez R, Gonzalez A, Linden A. Angew. Chem., Int. Ed. 2005;44:6187–6190. doi: 10.1002/anie.200502308. Deprotonation of dithianes: Nicolaou KC, Magolda RL, Sipio WJ, Barnette WE, Lysenko Z, Joullie MM. J. Am. Chem. Soc. 1980;102:3784–3793.
- 6.(a) Hachisu Y, Bode JW, Suzuki K. J. Am. Chem. Soc. 2003;125:8432–8433. doi: 10.1021/ja035308f. [DOI] [PubMed] [Google Scholar]; (b) Takikawa H, Hachisu Y, Bode JW, Suzuki K. Angew. Chem. Int. Ed. 2006;45:3492–3494. doi: 10.1002/anie.200600268. [DOI] [PubMed] [Google Scholar]; (c) Takikawa H, Suzuki K. Org. Lett. 2007;9:2713–2716. doi: 10.1021/ol070929p. [DOI] [PubMed] [Google Scholar]
- 7.(a) Enders D, Niemeier O, Balensiefer T. Angew. Chem. Int. Ed. 2006;45:1463–1467. doi: 10.1002/anie.200503885. [DOI] [PubMed] [Google Scholar]; (b) Enders D, Niemeier O, Raabe G. Synlett. 2006:2431–2434. [Google Scholar]
- 8.Li Y, Feng Z, You S-L. Chem. Commun. 2008:2263–2265. doi: 10.1039/b801004h. [DOI] [PubMed] [Google Scholar]
- 9.(a) Bausch CC, Johnson JS. Adv. Synth. Catal. 2005;347:1207–1211. [Google Scholar]; (b) Demir AS, Reis B, Reis O, Eymur S, Gollu M, Tural S, Saglam G. J. Org. Chem. 2005;72:7439–7442. doi: 10.1021/jo0710073. [DOI] [PubMed] [Google Scholar]
- 10.Demir AS, Esiringu I, Gollu M, Reis O. J. Org. Chem. 2009;74:2197–2199. doi: 10.1021/jo8026627. [DOI] [PubMed] [Google Scholar]
- 11.Tarr JC, Johnson JS. Org. Lett. 2009;11:3870–3873. doi: 10.1021/ol901314w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Linghu X, Bausch CC, Johnson JS. J. Am. Chem. Soc. 2005;127:1833–1840. doi: 10.1021/ja044086y. [DOI] [PubMed] [Google Scholar]; (b) Bausch CC, Johnson JS. J. Org. Chem. 2004;69:4283–4285. doi: 10.1021/jo0496143. [DOI] [PubMed] [Google Scholar]
- 13.(a) Cherest M, Felkin H, Prudent N. Tetrahedron Lett. 1968;9:2199–2204. [Google Scholar]; (b) Anh NT, Eisenstein O. Tetrahedron Lett. 1976;17:155–158. [Google Scholar]; (c) Anh NT, Eisenstein O. Top. Curr. Chem. 1980;88:145–162. [Google Scholar]
- 14.(a) Cram DJ, Abd Elhafez FA. J. Am. Chem. Soc. 1952;74:5828–5835. [Google Scholar]; (b) Reetz MT, Hullmann M, Seitz T. Angew. Chem. Int. Ed. 1987;26:477–480. [Google Scholar]
- 15.Degl’Innocenti A, Ricci A, Mordini A, Reginato G, Colotta V. Gazz. Chim. Ital. 1987;117:645–648. [Google Scholar]
- 16.Nahm MR, Potnick JR, White PS, Johnson JS. J. Am. Chem. Soc. 2006;128:2751–2756. doi: 10.1021/ja056018x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Nahm MR, Linghu X, Potnick JR, Yates CM, White PS, Johnson JS. Angew. Chem. Int. Ed. 2005;44:2377–2379. doi: 10.1002/anie.200462795. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Garrett MR, Tarr JC, Johnson JS. J. Am. Chem. Soc. 2007;129:12944–12945. doi: 10.1021/ja076095n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Corey EJ, Shibata S, Bakshi RK. J. Org. Chem. 1988;53:2861–2863. [Google Scholar]; (b) Corey EJ, Helal CJ. Angew. Chem. Int. Ed. 1998;37:1986–2012. doi: 10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 19.(a) Schmalz H-G, Jope H. Tetrahedron. 1998;54:3457–3464. [Google Scholar]; (b) Bringmann G, Hinrichs J, Pabst T, Henschel P, Peters K, Peters E-M. Synthesis. 2001:155–167. [Google Scholar]; (c) Dorizon P, Martin C, Darah J-C, Fiaud J-C, Kagan HB. Tetrahedron: Asymmetry. 2001;12:2625–2630. [Google Scholar]; (d) Velcicky J, Lanver A, Lex J, Prokop A, Wieder T, Schmalz H-G. Chem. Eur. J. 2004;10:5087–5110. doi: 10.1002/chem.200400079. [DOI] [PubMed] [Google Scholar]
- 20.Chiang P-C, Kaeobamrung J, Bode JW. J. Am. Chem. Soc. 2007;129:3520–3521. doi: 10.1021/ja0705543. [DOI] [PubMed] [Google Scholar]
- 21.Bordwell FG, Harrelson JA., Jr Can. J. Chem. 1990;68:1714–1718. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.