Abstract
Objective
Chronic inflammatory bowel disease (IBD) demonstrates some similarities of dysregulated chronic immunoinflammatory lesion of periodontitis. Trinitrobenzene sulfonic acid (TNBS) and dextran sodium sulphate (DSS) administered to rodents have been shown to elicit inflammatory responses that undermine the integrity of the gut epithelium similar to IBD in humans. The objective of this study was to evaluate the ability of these chemicals to elicit periodontal inflammation as a novel model for alveolar bone loss.
Methods
Mice were treated by oral application of TNBS 2 times/week, or with DSS in the diet over a period of 18 weeks. Alveolar bone loss was assessed on defleshed skull using morphometric measures for area of bone resorption.
Results
TNBS-treated animals tolerated oral administration with no clinical symptoms and gained weight similar to normal controls. In contrast, DSS exerted a systemic response including shortening of colonic tissue and liver enzyme changes. Both TNBS and DSS caused a localized action on periodontal tissues with alveolar bone loss observed in both maxilla and mandibles with progression in a time dependent manner. Bone loss was detected as early as week 7, with more severe periodontitis increasing over the 18 weeks (p<0.001). Young (7 month) and old (12 month) SCID mice were treated with TNBS for a period of 7 weeks and did not develop significant bone loss.
Conclusions
These data show that oral administration of TNBS and DSS provoke alveolar bone loss in concert with the autochthonous oral microbiota.
Introduction
There is accumulating evidence for an increasing incidence of chronic diseases in the human population (1-3). Additionally, many of these diseases clearly results from chronic inflammation that contributes to eventual loss of function of cells, tissues, or organs resulting in disease outcomes (4-6). In this regard, the chronic inflammation occurring in inflammatory bowel disease (IBD) has many similarities to the chronic immunoinflammatory response in the oral cavity that destroys the soft and hard tissues of the periodontium (ie. periodontitis), potentially resulting in exfoliation of the teeth (7).
Trinitrobenzene sulfonic acid (TNBS) given rectally and dextran sodium sulphate (DSS) providing orally elicit gastrointestinal inflammatory responses, linking with the natural microbiota of the murine gut (8-10). DSS acts to undermine the epithelial barrier and as an immune cell activator resulting in innate immune damage to the tissues. TNBS appears to function as a hapten to modify autologous proteins and induce a T cell-mediated response, resulting an autoimmune-like inflammatory responses (11). These compounds also up-regulate reactive oxygen species (ROS) creating a reproducible model of inflammatory bowel disease (12-13,8). Chronic inflammatory responses at all sites in the body result in the production of both inflammatory mediators and the production of high levels of these ROS in the local microenvironment of the inflammation. Ample evidence in IBD(12,14) and more limited data in periodontitis(15-17) provide support for a role of ROS in the clinical presentation of these mucosal diseases.
Rodent models of periodontal disease generally have been developed using exogenous oral infections with human pathogens, ie. bacteria not part of the animals commensal oral microbiota, in attempting to mimic some of the chronic inflammatory responses and resulting alveolar bone loss observed in humans. Alternatively, some iterations of these models have evaluated changes in the periodontium following gingival injection of antigens (18-19) or microbial stimulants (20-25). While these models have provided a range of data and new knowledge concerning host responses and tissue destruction, a concern remains regarding the nature of the triggering of periodontitis in humans via commensal bacteria that appear to transition to opportunistic pathogens in the subgingival sulcus versus the microbial approach used in the rodent models. Since the gingival tissues also represent a mucosal surface that is subjected to a complex commensal microbial challenge, we attempted to translate the mucosal immunopathologic findings of IBD models to the oral cavity.
The implementation of the DSS and TNBS models would enable studies of osteoimmunological interactions in the oral cavity, by targeting the innate immune system with DSS and the T-cell mediated immune responses with TNBS. We tested the hypothesis that challenge of the periodontium with TNBS or DSS, agents that are known to elicit chronic inflammation and IBD in murine models, will stimulate periodontitis in these animals. This is the first to document the oral mucosal disease triggered by these compound and should provide seminal data to evaluate the contribution of this model to the studies of gingival inflammation and subsequent alveolar bone loss in periodontitis.
Materials and Methods
Animals
BALB/c mice (11-12 weeks old; Sprague Dawley Harlan Laboratories, Indianapolis IN) were housed in micro-filter top cages in an American Association of Accreditation of Laboratory Animal Care (AAALAC) certified Laboratory Animal Research Resource Facility at the University of Kentucky Medical Center. At day 0 of experiment, mice were weighed and ear-punched for appropriate identification. They were placed in a room maintained at 22°C with a 12:12-hr light: dark cycle and fed rodent chow and water ad libitum.
Severe-combined-immunodeficiency mutation (C.B-17 SCID) congenitc BALB/c mice except carried Igh-1b allele from the C57BL/Ka strain and lack both T and B cells due to a defect in V(D)J recombination (do not mount an antibody response to immunogenic material) originated from Taconic Inc (Hudson, NY) were reared in the knockout rodent facility using autoclaved micro-filter top cages shoebox cages and bedding, and reared using sterilized drinking water and irradiated mouse diet (Purina, Harlan) ad libitum. The animals were either 7 months (similar to age of the BALB/c mice) or 12 months (aged mice) at the time of the TNBS challenge.
This experimental study was approved and performed in accordance with the guidelines for Institutional Animal Care and Use Committee (IACUC). All cages were only opened in biosaftey hood to minimize contamination. The mice were monitored daily for comfort, food and water intake, and clinical symptoms and survival. Animals were weighed weekly. At each time point, mice were weighed and euthanized to provide samples.
TNBS/DSS Models
After one week of acclamation the animals were randomly divided into 3 groups. BALB/c mice were treated biweekly with dextran sodium sulfate (2% DSS, Biochemical International, OH) in the diet followed by one week of abstinence for a period of 7-18 weeks. Trinitrobenzene sulfonic acid (TNBS, 2.5 mg solution; Sigma-Aldrich, St. Louis. MO) was delivered via a micropipette tip (100 μl) orally 2 times/week. Animals received fresh food and water 3 times per week. At each time point 5 animals were euthanized. Normal control animals received a normal diet and sham challenge (13,26). The SCID mice were treated with TNBS or sham as described above. These animals tolerated TNBS for a period of 7 weeks before they started to lose weight and develop clinical symptoms.
Tissue Collection
At each time point, the animals were anesthetized by Halothane inhalation and tissue dissected. Liver tissue was excised flash frozen in liquid nitrogen and kept at -80C for further analysis. Liver cystine concentration was measured, in Dr. Theresa Chen's laboratories, using HPLC as described previously (26). The colonic tissue was removed and perfused with phosphate-buffered saline (PBS) (Sigma, St. Louis, MO). The severity of tissue changes was determined by measurement of tissue-wet length and weight (27).
Analysis of alveolar bone
Five mice from each group were euthanized at each time point. The mouse head was removed and gingival tissue excised. The skulls were autoclaved for 5 min, the alveolar bones separated from the skull, and hemisected. Mandible and maxilla were defleshed, treated overnight with 0.3% H2O2 cleaned and stained with 0.5% methylene blue for 45 sec.
Recent reports indicate that alveolar bone loss in mice can be accurately quantified using microscopic morphometry, histomorphometry, or microcomputed tomography with no significant variations in outcomes (28). Therefore, we used a morphometric approach for the analyses. Digital photographs were prepared under stereomicroscopy on a custom made stage holder with the jaws angled to enhance visualization of the CEJ and bone level, as described previously (29). The images were analyzed using NIH Image J Program with enhancements for estimation of alveolar bone loss area. Using the CEJ of the teeth and the horizontal bone level, the area of bone loss was measured on buccal and lingual surfaces for each segment. Means for the mandibles, maxilla, and total were determined and used for comparisons between treatment and control groups. The area pixel readings for the bone loss were converted to mm2 units for actual CEJ-alveolar bone measurements by inclusion of a standard calibrator into each digital image.
Statistical Analysis
All results are expressed as mean±SEM unless otherwise stated. Data were accordingly evaluated using a Mann Whitney U test, or a one way analysis of variance on ranks (ANOVA), followed by appropriate post hoc test (the Tukey-Kramer Multiple Comparisons Test) using GraphPad Instat version 3 for Windows (GraphPad Software, San Diego, CA). Statistical significance was set at p<0.05.
RESULTS
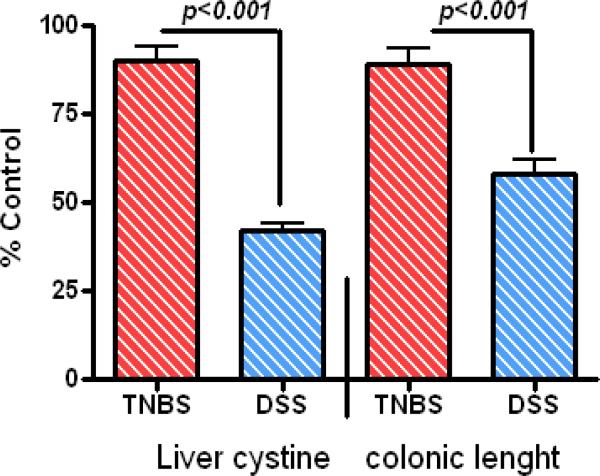
In this investigation low dose DSS was added into the diet for a one week interval. When the clinical features of the gut disease began, the DSS was removed and the diarrhea and bloody stool resolved after 1-2 days. TNBS was applied orally 2 times per week. To evaluate any systemic responses in treated animals we measured body weight, wet-colonic length, and the concentration of liver cystine. The animals tolerated administration of compounds demonstrating a generally normal weight gain during the 18 weeks (Table 1). TNBS-treated animals did not display clinical systemic effects and maintained normal colonic length with no statistical difference with controls (89% of controls), while the colonic length in DSS animals was significantly shortened compared to normal controls (58%) and TNBS-treated animals (p<0.001), consistent with the occurrence of gastrointestinal symptoms during the experimental protocol (Fig. 1). We also measured liver cystine as a marker of the systemic changes. TNBS animals did not show any significant differences in the levels of this tissue marker as compared to the normal controls (90% of control), while the liver cystine levels were decreased significantly in the DSS animals compared to controls (60%) or TNBS-treated mice (p<0.001) (Fig. 1). However, there was no substantial morbidity or mortality in either of the treatment groups during 18 week period.
Table 1.
Body weights of mice.
| Week (weight grams) | Weight gain (%) | ||
|---|---|---|---|
| 0 | 18 | ||
| Control | 20.3±0.5* | 26±0.8 | 30±3 |
| TNBS | 19.9±0.3 | 25.5±0.8 | 28±5 |
| DSS | 20.3±0.4 | 24.4±0.6 | 20±2 |
mean ± SEM.
Figure 1.
amount of Liver cystine and colonic length normalized according to sham control values (i.e cystine= 327±0.6nmol/mg and colonic length 117±0.6mm) after 18 weeks of treatments. Note TNBS exerted its effect locally with no significant difference between TNBS and control values.
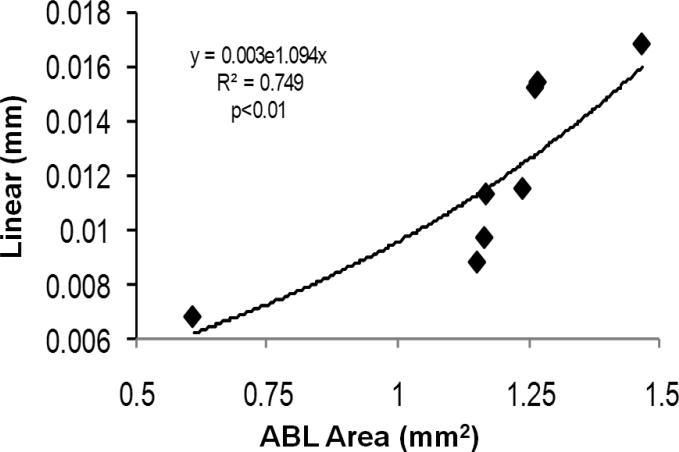
We initially compared evaluation of alveolar bone loss by measuring the total area of maxillary/mandibular bone loss expressed as mm2 with a technique that measured linear dimensions of bone loss at 6 sites (mesial, distal) edge of each molar tooth in the individual quadrants. Our findings indicated a significant correlation between these bone loss measures (Fig. 2).
Figure 2.
Linear correlation between total bone loss with 2 measures/molars (mm) compared to our technique (mm2) in randomly selected mandibles.
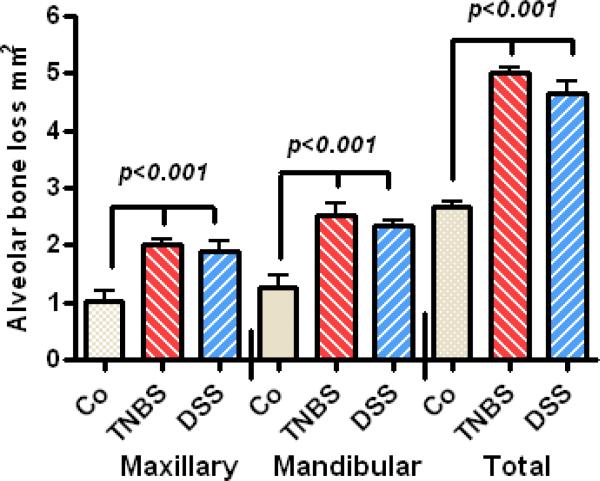
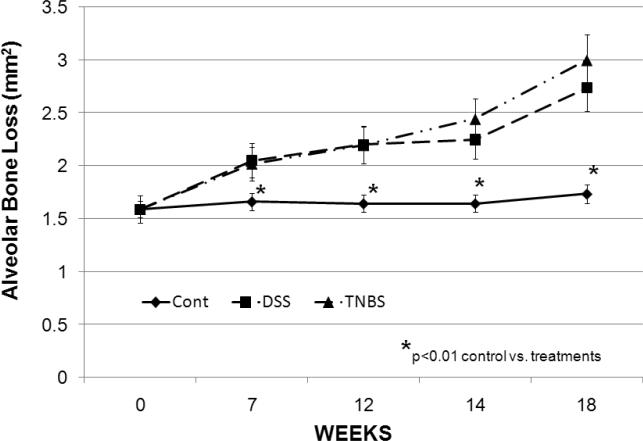
Significant alveolar bone loss was observed in all 4 quadrants in the oral cavity of the treated animals after 18 weeks (Fig. 3) with no statistical difference between the extent of bone loss in the mandibles compared to maxillary bone loss. No major differences were noted between bone loss on the right or left side in treatment groups or controls (data not shown). TNBS and DSS treatments caused significant alveolar bone loss in a time dependent manner. Significant bone loss was detected as early as week 7 and progressed to a more severe periodontitis through week 18 (Fig. 4). No significant difference was noted between the level of alveolar bone loss in mice treated with either TNBS or DSS.
Figure 3.
Significant bone resorption at the levels of Maxillary, mandibular, and total quadrant after 18 weeks treatment with TNBS and DSS (p<0.001).
Figure 4.
Extent of the alveolar bone resorption. Significant mandibular bone loss was detected as early as week 7 and progressed to a more severe periodontitis through week 18. The y-axis denotes mm2 area of bone loss. (n=5/group).
To explore the requirement for an intact host immune system in this model of alveolar bone loss, we examined SCID mice that lack both T and B cell responses. The SCID mice tolerated oral TNBS application for a period of 7 weeks before beginning to demonstrate weight loss and becoming moribund. However, they showed no significant bone loss when treated with TNBS at 7 months of age verses sham controls (Wild-type 0.62mm2 (30%) vs SCID 0.14mm2 (7%) bone loss), comparable to the age of wild-type BALB/c mice, or at 12 months of age consistent with an aged animals (Table 2).
Table 2.
Alveolar bone loss in SCID mice.
| Age (months) | ||
|---|---|---|
| 7 | 12 | |
| Baseline | 2.13±0.13† | 2.14±0.05 |
| Control* | 2.14±0.05 | 2.18±0.11 |
| TNBS | 2.27±0.13 | 2.34±0.12 |
ABL assessed 7 weeks after initiating of control sham or TNBS treatment.
mean ± SEM in mm2.
Discussion
This study demonstrates the induction of alveolar bone loss in BALB/c mice administrated TNBS and DSS orally during an extended time interval of 18 weeks. Periodontitis is a chronic immunoinflammatory disease with progressive loss of attachment of gingival tissues, reflecting destruction of the periodontal ligament and adjacent supporting alveolar bone. The chronic inflammation of periodontitis is initiated by a complex subgingival biofilm comprised of commensal bacteria and microorganisms that represent opportunistic pathogens (21). In response to periodontal pathogens, PMNs and other inflammatory and gingival resident cells can release destructive reactive oxygen radical (15-16,20), proteinases (30), and other factors that can damage host tissues (17,31-32). These molecules induce damage to gingival tissue, periodontal ligament, and elicit osteoclastic bone resorption(17,33-35).
Our data present that mice tolerated the treatments for up to 18 weeks with no mortality or significant clinical side effects. In modifying the protocol for the use of these inflammatory agents, we were able to examine the effects of challenge of TNBS on alveolar bone loss, presumably resulting from a localized inflammatory response in the periodontium and DSS that appears to exert its effect in a more generalized and systemic manner. These agents historically have been used to cause inflammation of the intestine, as models of chronic idiopathic Inflammatory Bowel Disease (IBD), principally ulcerative colitis and Crohn's disease (8-10,14,26,36). Human IBD is a chronic immunoinflammatory condition mediated by aberrant immune responses to the luminal bacterial antigens by activated CD4+ T cells, not dissimilar from the host-microbial interactions that can occur in the oral cavity, resulting in inflammation and tissue destruction. Murine models of IBD (12 review) have also utilized chemical induction of acute and chronic inflammation in the gut to evaluate molecular mechanisms of disease. DSS, a sulfated polysaccharide compound, is commonly given in water for one week (acute) to 6 weeks (chronic) to elicit destructive inflammation of the gut, similar to ulcerative colitis (12,26,37,38). Exposure of animals to DSS induces inflammation and macrophage activation commensurate with activation of innate immune mechanisms, with subsequent loss of epithelial integrity, and increases in the Gram-negative microbiota of the colon (9-10). Our published data, as well as others, show increased activation of NF-κB responsive genes (eg. TNFα) and decreased antioxidant activities in these inflammatory models suggesting the importance of ROS activation in digestive system inflammation (26,36-39). Previous studies demonstrate that TNBS delivered rectally to BALB/c mice induced similar clinical features to human Crohn's disease, predominantly a Th1 activity in the mucosal CD4+ T cell population and transmural infiltration of inflammatory mononuclear cells (40). These colonic inflammatory responses may result from covalent binding of the haptenizing agent to autologous host proteins with subsequent immune reactivity toward TNBS-modified self-antigens (11).
Numerous animal models have been used to evaluate the clinical, microbiological, and immunological aspects of this oral disease in attempts to recapitulate features of human disease (20-22). Specifically, studies using rodents (mice, rats, hamsters) have elicited disease via placement of ligatures in the gingival sulcus around the molar teeth of rodents (41) increasing biofilm accumulation, as well as disrupting the gingival epithelium, enhancing osteoclastogenesis and bone loss. In alternative models, these animals are orally infected with select human pathogens, attempting to document the virulence potential of these species in rodents (20,23). This approach has also enabled the use of genetically manipulated mice to focus on individual components of the host response to describe their role in the disease process (42-43). More recently, various laboratories have performed gingival tissue injections of microorganisms(18) or their products (44-45) to elicit periodontitis. However, since current models to examine molecular aspects of alveolar bone loss in rodents generally use an oral bacterial infection or challenge with microbial products derived from human bacteria that are not a part of the oral autochthonous microbiota of rats/mice, we suggest that additional models of alveolar bone loss would benefit our ability to most clearly understand the molecular mediators of tissue destruction in periodontitis. We demonstrated that the bone loss involves both maxillary and mandibular areas. Finally, TNBS treatment, which primarily mimics the induction of an autoimmune type of destructive inflammation, and DSS treatment that undermines the integrity of the epithelium, as well as activating various inflammatory cells, both elicited significant bone loss.
Previous studies have proven that DSS and TNBS exert their inflammatory effects through ROS up-regulation (26,36-38,46). The role that these reactive intermediates play in triggering/regulating molecular aspects of the inflammatory and innate immune responses in oral tissues remains to be determined.
T cell functions are also a critical portion of the periodontal milieu at sites of alveolar bone loss caused by the oral microbial biofilms (45,47-48). Mice deficient in major histocompatibility complex (MHC) class II-responsive CD4+ T cells illustrated decreased bone loss, but no change in bone loss was detected in those mice deficient in MHC class I-responsive CD8+ T cells or NK1+ T cells (49). Mice lacking the cytokines interferon-gamma or IL-6, both Th1 cytokines, also demonstrated decreased bone loss (50). Studies in T cell deficient or adoptively transferred rats have demonstrated the characteristics of T cells in periodontal bone loss in rodents (43, 51-52). Finally, after an oral infection with Porphyromonas gingivalis, severe combined immunodeficient (SCID) mice exhibited considerably less bone loss compared to immunocompetent mice, suggesting the crucial role for host responses in the disease process (49). In our model, SCID mice showed no significant difference in bone loss between sham and TNBS treated animals, in younger or older animals. These results illustrated that alveolar bone loss in this model may be instigated, and/or regulated by T and B cell dependent responses, supporting that exaggerated bone loss cannot progress in the absence of host responses in this model.
In conclusion, these data indicate that intermittent oral administration of TNBS or DSS induced alveolar bone resorption in a time dependent manner. The findings support that these chemicals likely caused similar alterations of host responses at oral mucosal surfaces as occur in the gastrointestinal tract, and provide a useful model system for examining molecular aspects of destructive periodontal inflammation leading to bone loss.
Acknowledgments
Supporting Grants: This research was supported by the National Institutes of Health grant NCRR P20RR020145 and NCCAM-AT1490.
Abbreviations
- ABL
Alveolar bone loss
- CEJ
Cemento-enamel junction
- DSS
Dextran sodium sulphate
- IBD
Inflammatory bowel disease
- IL-6
Interleukin-6
- MHC
Major histocompatibility complex
- PBS
phosphate-buffered saline
- ROS
Reactive oxygen species
- SCID
Severe-combined-immunodeficiency
- TNBS
Trinitrobenzene sulfonic acid
References
- 1.Williams RC, Barnett AH, Claffey N, Davis M, Gadsby R, Kellett M, Lip GY, Thackray S. The potential impact of periodontal disease on general health: a consensus view. Curr Med Res Opin. 2008;24:1635–43. doi: 10.1185/03007990802131215. [DOI] [PubMed] [Google Scholar]
- 2.Dhawan SS, Quyyumi AA. Rheumatoid arthritis and cardiovascular disease. Curr Atheroscler Rep. 2008;10:128–33. doi: 10.1007/s11883-008-0019-x. [DOI] [PubMed] [Google Scholar]
- 3.Cashman KD. Altered bone metabolism in inflammatory disease: role for nutrition. Proc Nutr Soc. 2008;67:196–205. doi: 10.1017/S0029665108007039. [DOI] [PubMed] [Google Scholar]
- 4.Dongari-Bagtzoglou AI, Ebersole JL. Increased presence of interleukin-6 (IL-6) and IL-8 secreting fibroblast subpopulations in adult periodontitis. J Periodontol. 1998;69:899–910. doi: 10.1902/jop.1998.69.8.899. [DOI] [PubMed] [Google Scholar]
- 5.Craig RG. Interactions between chronic renal disease and periodontal disease. Oral Dis. 2008;14:8–9. doi: 10.1111/j.1601-0825.2007.01430.x. [DOI] [PubMed] [Google Scholar]
- 6.Novak MJ, Potter RM, Blodgett J, Ebersole JL. Periodontal disease in Hispanic Americans with type 2 diabetes. J Periodontol. 2008;79:629–36. doi: 10.1902/jop.2008.070442. [DOI] [PubMed] [Google Scholar]
- 7.Grössner-Schreiber B, Fetter T, Hedderich J, Kocher T, Schreiber S, Jepsen S. Prevalence of dental caries and periodontal disease in patients with inflammatory bowel disease: a case-control study. J Clin Periodontol. 2006;33:478–84. doi: 10.1111/j.1600-051X.2006.00942.x. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Lafhente A, Antolh M, Guarner F, et al. Incrimination of anaerobic bacteria in the induction of experimental colitis. Am J Physiol. 1997;272:G10–G15. doi: 10.1152/ajpgi.1997.272.1.G10. [DOI] [PubMed] [Google Scholar]
- 9.Ohkawara T, Nishihira J, Takeda H, et al. Amelioration of dextran sulfate sodium-induced colitis by anti-macrophage migration inhibitory factor antibody in mice. Gastroenterology. 2002;123:256–70. doi: 10.1053/gast.2002.34236. [DOI] [PubMed] [Google Scholar]
- 10.Kim HS, Berstad A. Experimental colitis in animal model. Scan J Gastroentrol. 1992;27:529–37. doi: 10.3109/00365529209000116. [DOI] [PubMed] [Google Scholar]
- 11.Fiorucci S, Mencarelli A, Palazzetti B, Sprague AG, Distrutti E, Morelli A, ovobrantseva TI, Cirino G, Koteliansky VE, de Fougerolles AR. Importance of innate immunity and collagen binding integrin alpha1beta1 in TNBS-induced colitis. Immunity. 2002;17:769–80. doi: 10.1016/s1074-7613(02)00476-4. [DOI] [PubMed] [Google Scholar]
- 12.Bilsborough J, Viney JL. From model to mechanism: lessons of mice and men in the discovery of protein biologicals for the treatment of inflammatory bowel disease. Expert Opin Drug Discov. 2006;1:69–83. doi: 10.1517/17460441.1.1.69. (review) [DOI] [PubMed] [Google Scholar]
- 13.Oz HS, Zhong J, de Villiers W. Osteopontin ablation protects against progression of acute and chronic stages of TNBS-induced colitis. Gastroenterology Supl. 2008;134:A-525–T1296. [Google Scholar]
- 14.Oz HS, Ebersole JL. Application of prodrugs to inflammatory diseases of the gut. Molecules. 2008;13:452–474. doi: 10.3390/molecules13020452. review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24:287–296. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 16.Chapple IL, Brock G, Eftimiadi C, Mathews JB. Glutathione in gingival crevicular fluid and its relation to local antioxidant capacity in periodontal health and disease. J Clin Patho: Mol Pathol. 2002;55:367–373. doi: 10.1136/mp.55.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waddington RJ, Moseley R, Embery G. Reactive oxygen species: a potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000;6:138–151. doi: 10.1111/j.1601-0825.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima K, Hamada N, Takahashi Y, et al. Restraint stress enhances alveolar bone loss in an experimental rat model. J Periodont Res. 2006;41:527–534. doi: 10.1111/j.1600-0765.2006.00901.x. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki Y, Hara Y, Koji T, Shibata Y, Nakane PK, Kato I. Differential expression of IFN-γ, IL-4, IL10, and IL-1b mRNAs in decalcified tissue sections of mouse lipopolysaccharide-induced periodontitis mandibles assessed by in situ hybridization. Histochem Cell Biol. 1998;109:339–347. doi: 10.1007/s004180050234. [DOI] [PubMed] [Google Scholar]
- 20.Kesavalu L, Bakthavatchalu V, Rahman MM, Su J, Raghu B, Dawson D, Fernandes G, Ebersole JL. ω-3 fatty acid regulates inflammatory cytokine/mediator messenger RNA expression in Porphyromonas gingivalis-induced experimental periodontal disease. Oral Microbiol Immunol. 2007;22:232–9. doi: 10.1111/j.1399-302X.2007.00346.x. [DOI] [PubMed] [Google Scholar]
- 21.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 22.Klausen B. Microbiological and immunobiological aspects of experimental periodontal disease in rats: a review article. J Periodontol. 1999;62:59–73. doi: 10.1902/jop.1991.62.1.59. [DOI] [PubMed] [Google Scholar]
- 23.Kesavalu L, Sathishkumar S, Bakthavatchalu V, Matthews C, Dawson D, Steffen M, Ebersole JL. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect Immun. 2007;75:1704–1712. doi: 10.1128/IAI.00733-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kesavalu L, Chandrasekar B, Ebersole JL. In vivo induction of proinflammatory cytokines in mouse tissue by Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans. Oral Microbiol Immunol. 2002;17:177–80. doi: 10.1034/j.1399-302x.2002.170307.x. [DOI] [PubMed] [Google Scholar]
- 25.Holt SC, Ebersole JL, Felton J, Brunsvold M, Kornman KS. Implantation of Bacteroides gingivalis in on-human primates initiates progression of periodontitis. Science. 1988;239:55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- 26.Oz HS, Chen T, Nagasawa H. Comparative Efficacies of Two Cysteine Prodrugs and a Glutathione Delivery Agent in a Colitis Model. Translational Research. 2007;150:122–129. doi: 10.1016/j.trsl.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Axelsson LG, Landström E. Bylund-Fellenius AC. Experimental colitis induced by dextran sulphate sodium in mice: beneficial effects of sulphasalazine and olsalazine. Aliment Pharmacol Ther. 1998;12:925–34. doi: 10.1046/j.1365-2036.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- 28.Li CH, Amar S. Morphometric, histomorphometric, and microcomputed tomographic analysis of periodontal inflammatory lesions in a murine model. J Periodontol. 2007;78:1120–8. doi: 10.1902/jop.2007.060320. [DOI] [PubMed] [Google Scholar]
- 29.Rivaldo EG, Padilha AMP, Hugo FN, Hilgert JB, Rybu BR. Reproducibility of a hemi mandible positioning device and a method for measuring alveolar bone loss area in mice. J Oral Science. 2007;49:13–17. doi: 10.2334/josnusd.49.13. [DOI] [PubMed] [Google Scholar]
- 30.Kinane DF, Podmore M, Murray MC, Hodge PJ, Ebersole J. Etiopathogenesis of periodontitis in children and adolescents. Periodontol 2000. 2001;26:54–91. doi: 10.1034/j.1600-0757.2001.2260104.x. [DOI] [PubMed] [Google Scholar]
- 31.Enwonwu CO. Cellular and molecular effects of malnutrition and their relevance to periodontal diseases. J Clin Periodontol. 1994;21:643–657. doi: 10.1111/j.1600-051x.1994.tb00782.x. [DOI] [PubMed] [Google Scholar]
- 32.Enwonwu CO. Interface of malnutrition and periodontal diseases. Am J Clin Nutr. 1995;61:430S–436S. doi: 10.1093/ajcn/61.2.430S. [DOI] [PubMed] [Google Scholar]
- 33.Kawashi Y, Jaccard F, Cimasoni G. Sulcular polymorphonuclear leukocytes and gingival exudates during experimental gingivitis in man. J Periodontol Res. 1980;15:151–158. doi: 10.1111/j.1600-0765.1980.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 34.Gustafsson A, Asman B. Increased release of free oxygen radicals from peripheral neutrophils in adult periodontitis after Fc delta-receptor stimulation. J Clin Periodontol. 1996;23:38–44. doi: 10.1111/j.1600-051x.1996.tb00502.x. [DOI] [PubMed] [Google Scholar]
- 35.Key LL, Wolfe WC, Gundberg CM, et al. Superoxide and bone resorption. 1994;15:431–6. doi: 10.1016/8756-3282(94)90821-4. [DOI] [PubMed] [Google Scholar]
- 36.Neuman MG. Immune dysfunction in inflammatory bowel disease. Transl Res. 2007;149:173–86. doi: 10.1016/j.trsl.2006.11.009. (review) [DOI] [PubMed] [Google Scholar]
- 37.Oz HS, Chen T, deVilliers W, McClain C. Metallothionein overexpression does not protect against inflammatory bowel disease in a DSS murine colitis model. Med Sci Monit. 2005;11:BR69–73. [PubMed] [Google Scholar]
- 38.Oz HS, Chen TS, McClain CJ, de Villiers WJ. Antioxidants a novel therapy in a murine model of colitis. J Nutri Biochem. 2005;16:297–304. doi: 10.1016/j.jnutbio.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Ardite E, Sans M, Panes J, Romero FJ, Pique JM, Fernandez-Checa JC. Replenishment of glutathione levels improves mucosal function in experimental acute colitis. Lab Invest. 2000;80:735–44. doi: 10.1038/labinvest.3780077. [DOI] [PubMed] [Google Scholar]
- 40.Sartor RB. Induction of mucosal immune responses by bacteria and bacterial components. Curr Opin Gastroenterol. 2001;17:555–61. doi: 10.1097/00001574-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Cai X, Li C, Du G, Cao Z. Protective effects of baicalin on ligature-induced periodontitis in rats. J Periodont Res. 2008;43:14–21. doi: 10.1111/j.1600-0765.2007.00989.x. [DOI] [PubMed] [Google Scholar]
- 42.Yu JJ, Ruddy MJ, Wong JC, Sfintescu C, Baker PJ, Smith JB, Evans RT, Gaffen SL. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. 2007;109:3794–3802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alayan J, Ivanovski S, Farah CS. Alveolar bone loss in T helper 1/T helper 2 cytokine-deficient mice. J Periodont Res. 2007;42:97–103. doi: 10.1111/j.1600-0765.2006.00920.x. [DOI] [PubMed] [Google Scholar]
- 44.Kirkwood KL, Cirelli JA, Rogers JE, Giannobile WV. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol 2000. 2007;43:294–315. doi: 10.1111/j.1600-0757.2006.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. J Periodontol. 2005;76:2033–41. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 46.Je JH, Lee TH, Kim DH, Cho YH, Lee JH, Kim SC, Lee SK, Lee J, Lee MG. Mitochondrial ATP synthase is a target for TNBS-induced protein carbonylation in XS-106 dendritic cells. Proteomics. 2008;8:2384–93. doi: 10.1002/pmic.200700962. [DOI] [PubMed] [Google Scholar]
- 47.Yoshie H, Taubman MA, Olson CL, Ebersole JL, Smith DJ. Periodontal bone loss and immune characteristics after adoptive transfer of Actinobacillus-sensitized T cells to rats. J Periodontal Res. 1987;22:499–505. doi: 10.1111/j.1600-0765.1987.tb02061.x. [DOI] [PubMed] [Google Scholar]
- 48.Yoshie H, Taubman MA, Ebersole JL, Smith DJ, Olson CL. Periodontal bone loss and immune characteristics of congenitally athymic and thymus cell-reconstituted athymic rats. Infect Immun. 1985;50:403–8. doi: 10.1128/iai.50.2.403-408.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67:2804–9. doi: 10.1128/iai.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasai M, Saeki Y, Ohshima S, Nishioka K, Mima T, Tanaka T, Katada Y, Yoshizaki K, Suemura M, Kishimoto T. Delayed onset and reduced severity of collagen-induced arthritis in interleukin-6-deficient mice. Arthritis Rheum. 1999;42:1635–43. doi: 10.1002/1529-0131(199908)42:8<1635::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 51.Sasaki H, Suzuki N, Kent R, Jr, Kawashima N, Takeda J, Stashenko P. T cell response mediated by myeloid cell-derived IL-12 is responsible for Porphyromonas gingivalis-induced periodontitis in IL-10-deficient mice. J Immunol. 2008;180:6193–8. doi: 10.4049/jimmunol.180.9.6193. [DOI] [PubMed] [Google Scholar]
- 52.Baker PJ, Howe L, Garneau J, Roopenian DC. T cell knockout mice have diminished alveolar bone loss after oral infection with Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 2002;34:45–50. doi: 10.1111/j.1574-695X.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]