Abstract
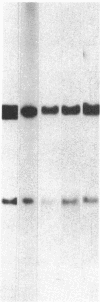
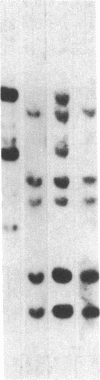
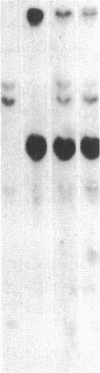
Several genes were found closely associated with major histocompatibility class I and class II beta-chain genes in chicken genomic DNA clusters by hybridizing tissue-specific cDNA probes to cosmid clones. A cDNA probe for one of these genes, probe C12.3 isolated from a chicken liver cDNA library, was used to clone the homologous sequence H12.3 from a human B-lymphoblastoid cell line cDNA library. C12.3 and H12.3 encode exactly the same 317-residue-long protein. The sequence of 12.3 shows significant homology with the two known guanine nucleotide-binding protein beta subunits (GP beta 1 and GP beta 2) and other proteins that all share the same segmented structure with seven internal homologous repeats about 45 residues in length. Unlike the chicken gene, the human H12.3 gene and its mouse counterpart are not located on the same chromosome as the major histocompatibility complex. A possible involvement of the C12.3 gene product in major histocompatibility complex-linked control of lymphocyte proliferation in chickens is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom S. E., Bacon L. D. Linkage of the major histocompatibility (B) complex and the nucleolar organizer in the chicken. Assignment to a microchromosome. J Hered. 1985 May-Jun;76(3):146–154. [PubMed] [Google Scholar]
- Bourlet Y., Béhar G., Guillemot F., Fréchin N., Billault A., Chaussé A. M., Zoorob R., Auffray C. Isolation of chicken major histocompatibility complex class II (B-L) beta chain sequences: comparison with mammalian beta chains and expression in lymphoid organs. EMBO J. 1988 Apr;7(4):1031–1039. doi: 10.1002/j.1460-2075.1988.tb02910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B., Goetsch L. Duplication of spindle plaques and integration of the yeast cell cycle. Cold Spring Harb Symp Quant Biol. 1974;38:123–131. doi: 10.1101/sqb.1974.038.01.016. [DOI] [PubMed] [Google Scholar]
- Calnek B. W. Marek's disease--a model for herpesvirus oncology. Crit Rev Microbiol. 1986;12(4):293–320. doi: 10.3109/10408418509104432. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Fong H. K., Amatruda T. T., 3rd, Birren B. W., Simon M. I. Distinct forms of the beta subunit of GTP-binding regulatory proteins identified by molecular cloning. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3792–3796. doi: 10.1073/pnas.84.11.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong H. K., Hurley J. B., Hopkins R. S., Miake-Lye R., Johnson M. S., Doolittle R. F., Simon M. I. Repetitive segmental structure of the transducin beta subunit: homology with the CDC4 gene and identification of related mRNAs. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2162–2166. doi: 10.1073/pnas.83.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F., Billault A., Pourquié O., Béhar G., Chaussé A. M., Zoorob R., Kreibich G., Auffray C. A molecular map of the chicken major histocompatibility complex: the class II beta genes are closely linked to the class I genes and the nucleolar organizer. EMBO J. 1988 Sep;7(9):2775–2785. doi: 10.1002/j.1460-2075.1988.tb03132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett M. M., Klaus G. G. G protein regulation of receptor signalling. Immunol Today. 1988 Oct;9(10):315–320. doi: 10.1016/0167-5699(88)91325-4. [DOI] [PubMed] [Google Scholar]
- Hartley D. A., Preiss A., Artavanis-Tsakonas S. A deduced gene product from the Drosophila neurogenic locus, enhancer of split, shows homology to mammalian G-protein beta subunit. Cell. 1988 Dec 2;55(5):785–795. doi: 10.1016/0092-8674(88)90134-1. [DOI] [PubMed] [Google Scholar]
- Hála K., Chaussé A. M., Bourlet Y., Lassila O., Hasler V., Auffray C. Attempt to detect recombination between B-F and B-L genes within the chicken B complex by serological typing, in vitro MLR, and RFLP analyses. Immunogenetics. 1988;28(6):433–438. doi: 10.1007/BF00355375. [DOI] [PubMed] [Google Scholar]
- Kanehisa M. Use of statistical criteria for screening potential homologies in nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):203–213. doi: 10.1093/nar/12.1part1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lassila O., Nurmi T., Eskola J. Genetic differences in the mitogenic response of peripheral blood lymphocytes in the chicken. J Immunogenet. 1979 Feb;6(1):37–43. doi: 10.1111/j.1744-313x.1979.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Lee L. F., Bacon L. D. Ontogeny and line differences in the mitogenic response of chicken lymphocytes. Poult Sci. 1983 Apr;62(4):579–584. doi: 10.3382/ps.0620579. [DOI] [PubMed] [Google Scholar]
- Lévi-Strauss M., Carroll M. C., Steinmetz M., Meo T. A previously undetected MHC gene with an unusual periodic structure. Science. 1988 Apr 8;240(4849):201–204. doi: 10.1126/science.3353717. [DOI] [PubMed] [Google Scholar]
- Miggiano V., North M., Buder A., Pink J. R. Genetic control of the response of chicken leukocytes to a T-cell mitogen. Nature. 1976 Sep 2;263(5572):61–63. doi: 10.1038/263061a0. [DOI] [PubMed] [Google Scholar]
- Morrow P. R., Abplanalp H. Genetic control of T-lymphocyte mitogenesis in chickens. Immunogenetics. 1981;13(3):189–200. doi: 10.1007/BF00350785. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymura J. M., Wabl M. R., Klein J. Mouse mitochondrial superoxide dismutase locus is on chromosome 17. Immunogenetics. 1981;14(3-4):231–240. doi: 10.1007/BF00342192. [DOI] [PubMed] [Google Scholar]
- Whiteway M., Hougan L., Dignard D., Thomas D. Y., Bell L., Saari G. C., Grant F. J., O'Hara P., MacKay V. L. The STE4 and STE18 genes of yeast encode potential beta and gamma subunits of the mating factor receptor-coupled G protein. Cell. 1989 Feb 10;56(3):467–477. doi: 10.1016/0092-8674(89)90249-3. [DOI] [PubMed] [Google Scholar]