Abstract
BACKGROUND
Endometriosis is a prevalent but enigmatic gynecologic disorder for which few modifiable risk factors have been identified. Fish oil consumption has been associated with symptom improvement in studies of women with primary dysmenorrhea and with decreased endometriosis risk in autotransplantation animal studies.
METHODS
To investigate the relation between dietary fat intake and the risk of endometriosis, we analyzed 12 years of prospective data from the Nurses' Health Study II that began in 1989. Dietary fat was assessed via food frequency questionnaire in 1991, 1995 and 1999. We used Cox proportional hazards models adjusted for total energy intake, parity, race and body mass index at age 18, and assessed cumulatively averaged fat intake across the three diet questionnaires.
RESULTS
During the 586 153 person-years of follow-up, 1199 cases of laparoscopically confirmed endometriosis were reported. Although total fat consumption was not associated with endometriosis risk, those women in the highest fifth of long-chain omega-3 fatty acid consumption were 22% less likely to be diagnosed with endometriosis compared with those with the lowest fifth of intake [95% confidence interval (CI) = 0.62–0.99; P-value, test for linear trend (Pt) = 0.03]. In addition, those in the highest quintile of trans-unsaturated fat intake were 48% more likely to be diagnosed with endometriosis (95% CI = 1.17–1.88; Pt = 0.001).
CONCLUSION
These data suggest that specific types of dietary fat are associated with the incidence of laparoscopically confirmed endometriosis, and that these relations may indicate modifiable risk. This evidence additionally provides another disease association that supports efforts to remove trans fat from hydrogenated oils from the food supply.
Keywords: endometriosis, cohort study, diet, epidemiology, fats
Introduction
Endometriosis is the third leading cause of gynecologic hospitalization in the USA (Eskenazi and Warner, 1997). Despite the high morbidity and health care cost associated with endometriosis, the etiology has not been fully delineated and few modifiable risk factors have been identified. The pathophysiology likely includes hormonal, anatomic, genetic, immune and inflammatory factors. Risk may be associated with factors that increase the volume, frequency and duration of retrograde menstruation and promote implantation and growth of endometrial plaques (Oral and Arici, 1997).
Dietary factors have been the focus of a growing number of endometriosis patient-directed books and web-sites. Unfortunately, there is little direct scientific evidence to support these suggestions. Only one animal study has directly investigated the relation between diet and the incidence of endometriosis (Covens et al., 1988), which suggested that fish oil could induce regression of surgically induced endometriosis. In the one human study focused upon dietary intake, laparoscopically confirmed endometriosis was positively related to red meat consumption [odds ratio (OR) = 2.0, 95% CI = 1.4–2.8] and inversely related to current green vegetable (OR = 0.3, 95% CI = 0.2–0.5) and fruit consumption (OR = 0.6, 95% CI = 0.4–0.8). However, this study did not observe a significant association with butter, margarine or oil intake (Parazzini et al., 2004) Additionally, a study of organochlorines and endometriosis risk observed no relation between specific foods about which intake data were collected based upon their hypothesized potential organochorine content (Heilier et al., 2007).
There is also a growing body of literature suggesting relations between dietary factors and the physiologic processes or symptoms believed to be associated with endometriosis. Smooth muscle contractility, estrogen levels, inflammation, prostaglandin metabolism and menstrual cyclicity are some of the factors that may contribute to endometriosis and can be influenced by diet. For example, specific dietary fatty acids are known to influence the circulating levels of IL-6 and other inflammatory markers found in higher levels among women with endometriosis (Baer et al., 2004). Also, an inverse relation between fish oil supplementation and circulating levels of series 2 prostaglandins and inflammatory symptoms have been observed (Bartram et al., 1993). In addition, a double-blind crossover study involving fish oil supplementation showed a significant reduction in dysmenorrhea (Harel et al., 1996), and another study observed that menstrual pain increased as intake of alpha-linolenic acid decreased (Deutch, 1995). To follow-up on these findings, we used data from the Nurses’ Health Study II, an ongoing, prospective cohort study of premenopausal USA nurses that began in 1989, to evaluate the relation between dietary fat consumption and the incidence of laparoscopically confirmed endometriosis.
Materials and Methods
Study population and data collection
Data for these analyses were collected in the Nurses' Health Study II cohort from September 1989 to 1st June 2001. Questionnaires requesting information on incident diseases and demographic, biologic, environmental and lifestyle risk factors are updated and mailed biennially. A total of 116 607 female registered nurses—ranging in age from 25 to 42 and residing in one of 14 states in the USA—completed the baseline questionnaire. Follow-up of this cohort in each 2-year interval has been consistently ≥90%. This research was approved by the Institutional Review Boards of Brigham and Women's Hospital and the Harvard School of Public Health.
Case ascertainment and analytic definition
In 1993, the women were first asked if they had ‘ever had physician-diagnosed endometriosis’. If ‘yes’, they were asked to report when the diagnosis had occurred and if it had been confirmed by laparoscopy. These questions were asked again in each subsequent questionnaire.
In March 1994, we conducted a study to validate self-reported endometriosis diagnosis within the Nurses’ Health Study II prospective cohort. Supplementary questionnaires were mailed to 200 women who were randomly selected from the then 1766 cases who had reported an incident diagnosis. Among those who reported laparoscopic-confirmation and for whom records were received and reviewed (n = 105), a diagnosis of endometriosis was indicated in the laparoscopic report in 96.2%. However, among those women without laparoscopic confirmation (n = 26), evidence of clinical diagnosis was found in only 53.8% of the records. As part of this validation study, requests for permission to review medical records were also sent to any woman who indicated that she had had a hysterectomy during the time-period of reported endometriosis diagnosis. A diagnosis of endometriosis at time of surgical procedure was confirmed in 79.6% (n = 144/181) of the records received. However, endometriosis was the primary indication for hysterectomy in only 5.5% (n = 9/163) of women for whom an indication was available. Therefore, to reduce the magnitude of misclassification and prevent confounding by indication for hysterectomy, analyses of incident diagnosis of endometriosis were restricted to those women who reported laparoscopic confirmation of their diagnosis.
Within this restricted case definition, the relation between endometriosis and infertility status is complex. At baseline, the prevalence of infertility (defined as attempting to become pregnant for >1 year without success) was greater among women with laparoscopic confirmation (20%) than among those who were clinically diagnosed without laparoscopic confirmation (4%), potentially resulting in over-sampling those with otherwise ‘asymptomatic’ disease. Because endometriosis with infertility may be indicative of asymptomatic disease secondary to other primary causes of infertility, the risk factors for endometriosis with infertility could differ from those for endometriosis without concurrent infertility. Hence, we looked at risk factors separately by these two ‘subtypes’ of endometriosis—(i) cases with neither past nor concurrent infertility and (ii) cases with concurrent infertility. Within this cohort, self-reported infertility was validated in a study of 100 randomly selected women who reported ovulatory infertility—95% of the self-reports were confirmed through medical record review (Rich-Edwards et al., 1994).
Assessment of exposures
A semi-quantitative food frequency questionnaire (FFQ) with more than 130 food items was sent to women in 1991, 1995 and 1999 to assess usual dietary intake during the past year. Participants were asked how often, on average, they had consumed each type of food or beverage during the past year. The FFQ had nine possible responses, ranging from never or less than once per month to six or more times per day. Intakes of total and specific types of fat per individual were calculated as the sum of the contributions from all foods on the basis of U.S. Department of Agriculture food composition data (1993), taking into account types of margarine and fats used in cooking and baking. To calculate the percentage of energy contributed by each type of fat, we divided energy intake from each fat by total energy intake.
Because the temporal relation between fat intake and risk of endometriosis is unclear, and the influence of pre-diagnostic symptoms on diet is unknown, we examined timing of dietary exposure in three ways. First, we conducted a baseline only analysis where diet reported in 1991 was related to cases reported during the entire follow-up period (1991–2001). Next, we performed an updated analysis where cases were related to the most recently reported diet; specifically, the 1991 diet was related to the cases reported in the 1991–1995 follow-up period, the 1995 diet was related to the cases reported in the 1995–1999 follow-up period and the 1999 diet was related to the cases reported in the 1999–2001 follow-up period. Lastly, we performed a separate analysis using cumulative averaged intakes using each of the three diet reports to best represent the long-term intake for our primary analyses (Hu et al., 1999). Specifically, the 1991 intake was related to cases reported in the 1991–1995 follow-up period, the average of the 1991 and 1995 intake was related to cases reported in the 1995–1999 follow-up period, and the average of the 1991, 1995 and 1999 intake was related to cases reported in the 1999–2001 follow-up period to maintain a strictly prospective analysis. These three approaches allowed us to observe any differences in latent, short-term and cumulative diet effects. However, the results observed for these three approaches were very similar, and therefore we present only the results of the cumulative averaged intakes as this methodology minimizes the measurement error due to random within-person variation over time (Hu et al., 1999).
The reproducibility and validity of fat intake determined with a similar FFQ have been assessed in cohorts of older women (London et al., 1991; Willett, 1998, Willett et al., 2001). For specific types of fat, Pearson correlation coefficients between energy-adjusted intakes from the average of two 1-week diet records and from the FFQ ranged from 0.48 to 0.73 (0.57 for total fat and 0.68 for saturated fat), with a correction for attenuation resulting from random error in diet records (Willett, 1998). Total fat intake has been validated using changes in blood lipid levels (Willett et al., 2001). Spearman correlation coefficients between the percentage of fat intake calculated from the FFQ and the fatty acid composition of subcutaneous fat aspirates has confirmed that the FFQ measured specific fatty acids from exogenous sources reasonably well (r = 0.51 for trans-unsaturated fat; r = 0.48 for long-chain omega-3 fatty acids; London et al., 1991).
Statistical analysis
Follow-up started in 1991 when diet was first measured. From the 97 807 women who returned the 1991 dietary questionnaire, we excluded women who had an implausible total energy intake (<800 or >4200 kcal/day) or who left more than 70 food items blank in the 1991 FFQ. Those who reported the diagnosis of endometriosis or a history of infertility prior to June 1991 were excluded from all analyses. Analyses were also restricted to those who were premenopausal and had intact uteri, because the occurrence of endometriosis after hysterectomy or in post-menopausal women is rare. Women with prior cancer diagnoses, other than non-melanoma skin cancer, also were excluded.
Women were followed from return of the baseline FFQ until death, cancer diagnosis (other than non-melanoma skin cancer), self-report of laparoscopically confirmed endometriosis diagnosis, hysterectomy, the onset of menopause or end of the study period. In addition, because infertility is so strongly correlated with diagnosis of endometriosis via laparoscopy, we censored at time of self-report of infertility. Therefore, in all analyses our comparison group consists of women with neither diagnosed endometriosis nor infertility.
In all analyses, women were categorized into quintiles of fat intake and risks were compared in specific quintiles relative to the lowest quintile. Incidence rates for each exposure category were computed as the number of incident cases divided by the person-time accumulated. Time-varying Cox proportional hazards models treating age in months and stratifying by 2-year questionnaire cycle as the time scale were used to estimate multivariable (MV) incidence rate ratios (RR) and to calculate 95% confidence intervals (CIs), after adjusting simultaneously for potential confounding variables.
For each type of fat analyzed, we examined two distinct multivariable models. The first multivariable model (total energy substitution) included terms for age, calendar year, age at menarche, length of menstrual cycle, parity, body mass index (BMI) and total energy intake. No other factors, including physical activity, caffeine, cigarette smoking, alcohol intake or oral contraceptive use were observed to be confounders and therefore are not included in the final multivariable models. All covariates except for age at menarche were updated in each questionnaire cycle. The coefficients for specific fats in this first model can be interpreted as the effect of increasing percentage of energy from each type of fat whereas reducing the intake by the same percentage from all other sources of energy while keeping calories constant. The second model (carbohydrate substitution) included additional terms for all other sources of energy, except for carbohydrates. The coefficients in this second model can be interpreted as the effect of increasing intake of the specific fat at the expense of carbohydrates whereas keeping calories constant. This second model allows a direct comparison of the magnitude of the associations with endometriosis risk between different fat types. We also estimated the effect of consuming one type of fat instead of another by modeling the intake of specific fats as continuous variables. We used the difference in coefficients from the same model and their covariance matrix, to calculate the relative risk and 95% CIs associated with these dietary substitutions (Willett, 1998).
Tests for linear trend (Rosner, 1993), which evaluates the presence of a linear or dose–response relation between the exposure and endometriosis, in ordinal categorical exposures were calculated by creating a variable in which the median value of each category was assigned to all participants in that group. Tests for heterogeneity comparing the effect estimates among cases with no past or current infertility to cases with concurrent infertility were calculated with a Wald statistic referred to a χ2 distribution with 1 degree of freedom (Prentice et al., 1978). To evaluate effect modification by BMI (<25 and ≥25 kg/m2), oral contraceptive use (never, ever), parity (nulliparous, parous), physician exam in the past 2 years (yes, no) and cigarette smoking (never, past, current), stratified analyses were conducted and likelihood ratio tests comparing the carbohydrates substitution model with both the main effects and the interaction terms to that with the main effects only were calculated.
Results
After baseline exclusions, a total of 70 709 women contributed 586 153 person-years to these analyses; 1199 incident cases of laparoscopically confirmed endometriosis with no past infertility were reported. These included 970 cases with no past or current infertility and 228 cases who reported an infertility evaluation during the same follow-up period as laparoscopic confirmation of endometriosis. Women with a higher intake of total fat were more likely to be overweight or obese, a current smoker or parous, and were also less likely to have had a recent gynecologic exam (Table I).
Table I.
Distribution of potential risk factors for endometriosis according to total fat intake among women in the Nurses’ Health Study II (n = 70 709).*
| Total fat intake quintile (%) |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| No. of women | 14 095 | 14 320 | 14 170 | 14 099 | 14 025 |
| Total fat median % of energy | 24.4 | 28.7 | 31.6 | 34.5 | 38.7 |
| Age (years) | 35.6 | 35.5 | 35.5 | 35.7 | 35.9 |
| Caucasian | 89.6 | 92.3 | 92.7 | 92.9 | 93.6 |
| BMI (kg/m2) | |||||
| 25–29.9 (overweight) | 14.8 | 17.2 | 18.9 | 19.6 | 20.5 |
| ≥30 (obese) | 7.2 | 9.4 | 11.0 | 14.4 | 18.0 |
| Cigarette smoking | |||||
| Never | 67.2 | 67.2 | 66.9 | 65.8 | 64.2 |
| Past | 23.1 | 22.4 | 22.1 | 21.9 | 20.1 |
| Current | 9.6 | 10.3 | 10.9 | 12.2 | 15.5 |
| Age at menarche | |||||
| <12 years | 23.6 | 23.2 | 22.9 | 23.4 | 24.5 |
| 12 years | 30.2 | 29.9 | 30.3 | 30.8 | 30.5 |
| 13 years | 27.4 | 28.4 | 28.7 | 28.0 | 27.1 |
| ≥14 years | 18.5 | 18.3 | 17.9 | 17.6 | 17.6 |
| Menstrual cycle length | |||||
| < 26 days | 11.5 | 11.2 | 11.2 | 10.8 | 11.8 |
| 26–31 days | 66.4 | 66.8 | 67.3 | 67.6 | 66.7 |
| 32–50 days | 17.4 | 17.6 | 17.2 | 17.2 | 17.0 |
| Oral contraceptive use | |||||
| Ever | 81.7 | 83.3 | 83.3 | 83.9 | 84.7 |
| Nulliparous | 34.9 | 26.4 | 23.4 | 22.7 | 24.4 |
| Lactation (among parous women) | |||||
| None | 8.0 | 9.1 | 10.1 | 11.6 | 14.0 |
| ≥ 24 months | 13.6 | 14.4 | 14.1 | 12.3 | 9.6 |
| Recent gynecologic exam | |||||
| No exam | 12.6 | 12.9 | 14.0 | 15.0 | 17.2 |
*All data shown are standardized to the age distributions of the cohort in 1991.
Total fat intake was not associated with endometriosis (Table II). There was the suggestion of an increased risk of endometriosis with animal fat intake. Women in the upper fifth of animal fat intake had a 20% greater risk of endometriosis compared with those in the lowest fifth [95% CI = 0.99–1.45; P-value, test for linear trend (Pt) = 0.06]. However, intakes of saturated fat and monounsaturated fat, the major components of animal fat, were not associated with endometriosis risk. Interestingly, palmitic acid intake, a saturated fat primarily contributed by animal products, was significantly related to increased endometriosis risk when all other dietary components were held constant [RR = 1.52 comparing the fifth to first quintile of intake (95% CI = 0.94–2.46; Pt = 0.008; data not shown)]. No other saturated (myristic, stearic) and monounsaturated (oleic, palmitoleic) fatty acids was significantly related to endometriosis risk (data not shown).
Table II.
RR and 95% CIs for laparoscopically confirmed endometriosis according to quintile of cumulative averaged fat intake among women in the Nurses’ Health Study II.
| Type of fat | Quintile of intake |
P-value* | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Total fat | ||||||
| Median intake (% of energy) | 24.2 | 28.3 | 31.1 | 33.9 | 38.0 | |
| Number of cases | 257 | 236 | 231 | 240 | 235 | |
| Age-adjusted RR (95% CI) | 1.0 (referent) | 0.91 (0.76–1.08) | 0.91 (0.76–1.08) | 0.95 (0.79–1.13) | 0.95 (0.80–1.14) | 0.89 |
| Multivariable RR (95% CI)† | 1.0 (referent) | 0.98 (0.82–1.17) | 1.00 (0.84–1.20) | 1.07 (0.89–1.27) | 1.05 (0.88–1.26) | 0.38 |
| Multivariable RR (95% CI)‡ | 1.0 (referent) | 0.98 (0.82–1.17) | 1.01 (0.84–1.20) | 1.07 (0.89–1.28) | 1.06 (0.88–1.27) | 0.36 |
| Vegetable fat | ||||||
| Median intake (% of energy) | 9.4 | 11.7 | 13.5 | 15.4 | 18.5 | |
| Number of cases | 252 | 253 | 217 | 242 | 235 | |
| Age-adjusted RR (95% CI) | 1.0 (referent) | 1.01 (0.84–1.20) | 0.86 (0.72–1.04) | 0.99 (0.83–1.18) | 0.99 (0.83–1.19) | 0.95 |
| Multivariable RR (95% CI)† | 1.0 (referent) | 1.02 (0.86–1.21) | 0.87 (0.73–1.04) | 0.99 (0.83–1.18) | 0.96 (0.80–1.15) | 0.69 |
| Multivariable RR (95% CI)‡ | 1.0 (referent) | 1.02 (0.85–1.21) | 0.87 (0.72–1.05) | 0.99 (0.82–1.18) | 0.96 (0.79–1.15) | 0.66 |
| Animal fat | ||||||
| Median intake (% of energy) | 11.8 | 15.0 | 17.2 | 19.4 | 23.0 | |
| Number of cases | 242 | 241 | 233 | 241 | 242 | |
| Age-adjusted RR (95% CI) | 1.0 (referent) | 0.98 (0.82–1.18) | 0.95 (0.79–1.13) | 0.98 (0.82–1.17) | 0.99 (0.83–1.19) | 0.98 |
| Multivariable RR (95% CI)† | 1.0 (referent) | 1.09 (0.91–1.30) | 1.08 (0.90–1.30) | 1.15 (0.96–1.38) | 1.17 (0.98–1.41) | 0.08 |
| Multivariable RR (95% CI)‡ | 1.0 (referent) | 1.10 (0.92–1.32) | 1.10 (0.92–1.33) | 1.18 (0.97–1.42) | 1.20 (0.99–1.45) | 0.06 |
| Trans-unsaturated fat | ||||||
| Median intake (% of energy) | 0.9 | 1.3 | 1.5 | 1.8 | 2.3 | |
| Number of cases | 229 | 219 | 241 | 242 | 268 | |
| Age-adjusted RR (95% CI) | 1.0 (referent) | 0.94 (0.78–1.13) | 1.03 (0.86–1.24) | 1.04 (0.86–1.24) | 1.17 (0.98–1.39) | 0.03 |
| Multivariable RR (95% CI)† | 1.0 (referent) | 1.03 (0.86–1.24) | 1.16 (0.97–1.39) | 1.16 (0.97–1.40) | 1.28 (1.07–1.54) | 0.003 |
| Multivariable RR (95% CI)‡ | 1.0 (referent) | 1.09 (0.89–1.33) | 1.26 (1.03–1.55) | 1.30 (1.04–1.62) | 1.48 (1.17–1.88) | 0.001 |
| Monounsaturated fat | ||||||
| Median intake (% of energy) | 8.9 | 10.6 | 11.9 | 13.1 | 14.8 | |
| Number of cases | 250 | 251 | 223 | 238 | 237 | |
| Age-adjusted RR (95% CI) | 1.0 (referent) | 0.99 (0.83–1.18) | 0.90 (0.75–1.08) | 0.97 (0.81–1.16) | 1.01 (0.84–1.20) | 0.89 |
| Multivariable RR (95% CI)† | 1.0 (referent) | 1.06 (0.89–1.27) | 1.00 (0.83–1.19) | 1.09 (0.91–1.30) | 1.10 (0.92–1.32) | 0.29 |
| Multivariable RR (95% CI)‡ | 1.0 (referent) | 1.08 (0.87–1.33) | 0.97 (0.75–1.24) | 0.98 (0.74–1.30) | 0.91 (0.66–1.26) | 0.38 |
| Polyunsaturated fat | ||||||
| Median intake (% of energy) | 4.1 | 4.8 | 5.3 | 6.0 | 7.0 | |
| Number of cases | 271 | 238 | 235 | 226 | 229 | |
| Age-adjusted RR (95% CI) | 1.0 (referent) | 0.88 (0.74–1.05) | 0.90 (0.75–1.07) | 0.88 (0.74–1.05) | 0.93 (0.78–1.11) | 0.59 |
| Multivariable RR (95% CI)† | 1.0 (referent) | 0.90 (0.76–1.07) | 0.93 (0.78–1.10) | 0.91 (0.76–1.08) | 0.94 (0.79–1.12) | 0.63 |
| Multivariable RR (95% CI)‡ | 1.0 (referent) | 0.86 (0.72–1.03) | 0.87 (0.72–1.05) | 0.84 (0.69–1.02) | 0.87 (0.70–1.06) | 0.31 |
| Long-chain omega-3 fatty acids | ||||||
| Median intake (% of energy) | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | |
| Number of cases | 269 | 247 | 256 | 227 | 200 | |
| Age-adjusted RR (95% CI) | 1.0 (referent) | 0.93 (0.79–1.11) | 0.99 (0.84–1.18) | 0.88 (0.74–1.06) | 0.82 (0.68–0.98) | 0.03 |
| Multivariable RR (95% CI)† | 1.0 (referent) | 0.94 (0.79–1.12) | 0.98 (0.83–1.17) | 0.86 (0.72–1.03) | 0.77 (0.64–0.93) | 0.003 |
| Multivariable RR (95% CI)‡ | 1.0 (referent) | 0.94 (0.79–1.13) | 0.98 (0.81–1.18) | 0.86 (0.70–1.05) | 0.78 (0.62–0.99) | 0.03 |
| Long-chain omega-6 fatty acids | ||||||
| Median intake (% of energy) | 3.5 | 4.2 | 4.7 | 5.3 | 6.4 | |
| Number of cases | 263 | 240 | 236 | 256 | 204 | |
| Age-adjusted RR (95% CI) | 1.0 (referent) | 0.90 (0.75–1.07) | 0.90 (0.75–1.07) | 0.99 (0.84–1.18) | 0.82 (0.68–0.98) | 0.11 |
| Multivariable RR (95% CI)† | 1.0 (referent) | 0.94 (0.79–1.12) | 0.94 (0.79–1.12) | 1.06 (0.89–1.25) | 0.86 (0.71–1.03) | 0.26 |
| Multivariable RR (95% CI)‡ | 1.0 (referent) | 0.93 (0.77–1.12) | 0.95 (0.77–1.16) | 1.08 (0.88–1.34) | 0.93 (0.73–1.20) | 0.91 |
| Saturated fat | ||||||
| Median intake (% of energy) | 8.1 | 9.8 | 10.9 | 12.1 | 13.9 | |
| Number of cases | 257 | 226 | 215 | 243 | 258 | |
| Age-adjusted RR (95% CI) | 1.0 (referent) | 0.86 (0.72–1.03) | 0.82 (0.68–0.98) | 0.90 (0.76–1.08) | 0.98 (0.82–1.16) | 0.70 |
| Multivariable RR (95% CI)† | 1.0 (referent) | 0.93 (0.77–1.11) | 0.92 (0.77–1.10) | 1.03 (0.86–1.22) | 1.10 (0.92–1.31) | 0.10 |
| Multivariable RR (95% CI)‡ | 1.0 (referent) | 0.87 (0.71–1.07) | 0.85 (0.68–1.07) | 0.94 (0.74–1.20) | 1.01 (0.77–1.31) | 0.42 |
*P-value, test for linear trend calculated with median intake of fat in each quintile as a continuous variable.
†Multivariable model was stratified by age in months at start of follow-up and calendar year of the current questionnaire cycle and was simultaneously adjusted for age at menarche (<10, 10, 11, 12, 13, 14, 15, 16, >16 years), length of menstrual cycle (<26, 26–31, 32–50, 51+ days), parity (nulliparous, 1, 2, 3, 4+ pregnancies lasting >6 months), BMI (<19, 19–20.4, 20.5–21.9, 22–24.9, 25–29.9, 30+ kg/m2) and energy (continuous).
‡Total fat multivariable model additionally adjusted for protein. Animal fat multivariable model additionally adjusted protein and vegetable fat. Vegetable fat multivariable model additionally adjusted for protein and animal fat. Trans-unsaturated fat multivariable model additionally adjusted for protein, monounsaturated fat, saturated fat, polyunsaturated fat. Monounsaturated fat multivariable model additionally adjusted for protein, saturated fat, polyunsaturated fat, trans-unsaturated fat. Polyunsaturated fat multivariable model additionally adjusted for protein, monounsaturated fat, saturated fat, trans-unsaturated fat. Omega-3 fat multivariable model additionally adjusted for protein, monounsaturated fat, saturated fat, trans-unsaturated fat, long-chain omega-6 fatty acids. Omega-6 fat multivariable model additionally adjusted for protein, monounsaturated fat, saturated fat, trans-unsaturated fat, long-chain omega-3 fatty acids. Saturated fat multivariable model additionally adjusted for protein, monounsaturated fat, polyunsaturated fat, trans-unsaturated fat.
Intake of trans-unsaturated fats was associated with a higher risk of endometriosis (Table II). This association became stronger when protein and other types of fat were added to the model such that the estimates represent the effect of increasing trans-unsaturated fat at the expense of carbohydrates. The multivariable RR for the highest quintile compared with the lowest quintile was 1.48 (95% CI = 1.17–1.88; Pt = 0.001). However, intake of long-chain omega-3 fatty acids was associated with a lower risk of endometriosis. In the total energy substitution model, high consumption of long-chain omega-3 fatty acids was associated with a 23% lower risk of endometriosis (95% CI = 0.64–0.93; Pt = 0.003; Table II). This relation did not change with the carbohydrate substitution model, i.e. when intakes of protein and other types of fat were added to the model (RR = 0.78; 95% CI = 0.62–0.99; Pt = 0.03).
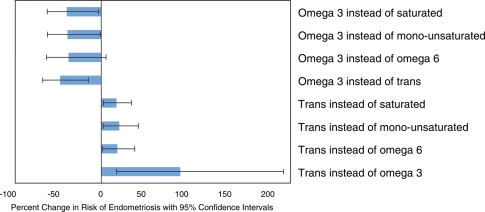
In addition, we estimated the effects of consuming one specific type of fat instead of another (Fig. 1). Consuming each additional 1% of energy from omega-3 fatty acids (e.g. increasing from 1 to 2% of total energy intake) rather than from saturated, monounsaturated or omega-6 polyunsaturated fats was associated with approximately a 50% lower risk of endometriosis, although none of these estimates reached statistical significance. However, each 1% of energy from omega-3 fatty acids rather than from trans fats was associated with nearly a 50% lower risk of endometriosis (RR = 0.52; 95% CI = 0.32–0.85). Also, each 1% of energy from trans fats rather than from any other type of fat was associated with a significantly higher risk of endometriosis.
Figure 1.
Impact of fatty acid substitution on the risk of endometriosis.
Estimated percent changes in the risk of laparoscopically confirmed endometriosis associated with isocaloric substitutions of 1% of energy from one dietary component for another. The I bars represent 95% CIs.
We then evaluated the relation between fatty acid intake among cases who had never reported infertility and separately among cases who concurrently reported laparoscopic endometriosis diagnosis and infertility (Table III). Women in the highest fifth of trans-unsaturated fat consumption had a 48% greater risk if the cases had never been infertile but were at 72% greater risk of endometriosis if the case women were concurrently infertile. Conversely, the relation between long-chain omega-3 fatty acid intake and the risk of endometriosis was similar between the case groups. Whereas all of the tests for linear trend were statistically significant among never infertile women, none were significant among those cases who were concurrently infertile. However, none of the tests for heterogeneity (comparing the effects observed among the women who were never infertile to the effects among those who were concurrently infertile) were statistically significant (lowest P-value, test for heterogeneity = 0.22). Results for total energy substitution models were similar (data not shown). No significant differences in risk between case infertility status were noted for any other types of fat.
Table III.
RR and 95% CIs for laparoscopically confirmed endometriosis according to quintile of trans-unsaturated fat intake and long-chain omega-3 fatty acid intake by infertility status in premenopausal women enrolled in the Nurses' Health Study II (carbohydrate substitution‡).
| Quintile | Case definition |
||||||
|---|---|---|---|---|---|---|---|
| All women (n = 1199) |
Never infertile* (n = 970) |
Concurrent infertility* (n = 228) |
P-value¥ | ||||
| Cases | MV RR (95% CI) | Cases | MV RR (95% CI) | Cases | MV RR (95% CI) | ||
| Trans-unsaturated fat | |||||||
| 1 | 229 | 1.00 (referent) | 177 | 1.00 (referent) | 51 | 1.00 (referent) | 0.22 |
| 2 | 219 | 1.09 (0.89–1.33) | 164 | 1.00 (0.79–1.25) | 54 | 1.56 (1.03–2.36) | |
| 3 | 241 | 1.26 (1.03–1.55) | 195 | 1.21 (0.96–1.53) | 46 | 1.56 (0.99–2.45) | |
| 4 | 242 | 1.30 (1.04–1.62) | 207 | 1.31 (1.02–1.67) | 33 | 1.24 (0.74–2.08) | |
| 5 | 268 | 1.48 (1.17–1.88) | 227 | 1.48 (1.14–1.93) | 44 | 1.72 (1.00–2.96) | |
| Test for linear trend | P-value = 0.0008 | P-value = 0.0007 | P-value = 0.16 | ||||
| Long-chain omega-3 fatty acid | |||||||
| 1 | 269 | 1.00 (referent) | 217 | 1.00 (referent) | 52 | 1.00 (referent) | 0.97 |
| 2 | 247 | 0.94 (0.79–1.13) | 201 | 0.92 (0.75–1.12) | 44 | 0.95 (0.62–1.44) | |
| 3 | 256 | 0.98 (0.81–1.18) | 210 | 0.96 (0.78–1.18) | 48 | 1.09 (0.71–1.69) | |
| 4 | 227 | 0.86 (0.70–1.05) | 181 | 0.82 (0.65–1.03) | 45 | 0.94 (0.59–1.48) | |
| 5 | 200 | 0.78 (0.62–0.99) | 161 | 0.76 (0.59–0.99) | 39 | 0.80 (0.47–1.35) | |
| Test for linear trend | P-value = 0.03 | P-value = 0.03 | P-value = 0.36 | ||||
*Infertility is defined as attempting to become pregnant for >1 year without success. Cases with ‘no past or concurrent infertility’ are women who never reported infertility. Cases with ‘concurrent infertility’ are women who reported an infertility evaluation in the same follow-up cycle as laparoscopic-confirmation of endometriosis.
†Total energy substitution multivariable models were stratified by age in months at start of follow-up and calendar year of the current questionnaire cycle and was simultaneously adjusted for age at menarche (<10, 10, 11, 12, 13, 14, 15, 16, >16 years), length of menstrual cycle (<26, 26–31, 32–50, 51+ days), parity (nulliparous, 1, 2, 3, 4+ pregnancies lasting >6 months), BMI (<19, 19–20.4, 20.5–21.9, 22–24.9, 25–29.9, 30+ kg/m2) and energy (continuous).
‡Trans-unsaturated fat carbohydrate substitution multivariable model additionally adjusted for protein, monounsaturated fat, saturated fat, polyunsaturated fat; omega-3 fat carbohydrate substitution multivariable model additionally adjusted for protein, monounsaturated fat, saturated fat, trans-unsaturated fat, long-chain omega-6 fatty acids.
¥P-value, test for heterogeneity comparing the effect of fat consumption among women with no past or current infertility to those with concurrent infertility.
Finally, we examined whether the associations of dietary fats with endometriosis differed according to BMI, oral contraceptive use, parity, recent physician exam or cigarette smoking. The association with trans-unsaturated fat consumption differed significantly by cigarette smoking status (P-value, test for heterogeneity = 0.03), with ever smokers at greatest risk (multivariable RR for the highest quintile compared with the lowest quintile = 1.61, 95% CI = 1.16–2.23; Pt = 0.002) compared with never smokers (multivariable RR for the highest quintile compared with the lowest quintile = 1.15, 95% CI = 0.93–1.43; Pt = 0.18). No other significant differences were observed (data not shown).
Discussion
In this large, prospective study among premenopausal women, we observed a significantly lower rate of diagnosis of laparoscopically confirmed endometriosis among women with greater long-term intake of long-chain omega-3 fatty acid consumption. Conversely, trans-unsaturated fat consumption and, potentially, a diet with greater animal fat consumption were associated with an increased risk. These associations suggest that diet, a potentially modifiable lifestyle factor, may be important in the pathogenesis of endometriosis. Associations remained consistent regardless of timing of dietary exposure (ranging from 2 to 10 years prior to diagnosis)—suggesting equivalent latent, short-term and cumulative diet effects, although this may be due to relatively consistent diet over time.
Although data regarding the relation between dietary fat intake and endometriosis in humans is scarce, our results are consistent with some animal studies. In vitro survival of endometrial cells from women with and without endometriosis appears to be influenced by fatty acid content of the culture media (Gazvani et al., 2001). Endometrial cell survival is decreased in cultures containing a high proportion of long-chain n-3 fatty acids (i.e. eicosapentaenoic acid). However, cell survival is not affected in cell cultures containing a high proportion of long-chain n-6 fatty acids (i.e. arachidonic acid) or equal amounts of n-3 and n-6 fatty acids. A similar survival pattern of endometrial explants in the peritoneum would be consistent with our findings.
Likewise, in a rabbit model of surgically induced endometriosis, alpha-linolenic acid (an n-3 fatty acid) supplementation decreased concentrations of series 2 prostaglandins and endometrial implant diameter (Covens et al., 1988). In addition, ligands of the peroxisome-proliferator activated receptor-γ (PPAR-γ) have been found to induce regression of surgically induced endometriosis in rodents (Lebovic et al., 2004) and baboons (Lebovic et al., 2007). Our results are also consistent with these models, since trans fatty acids can down-regulate PPAR-γ expression by about 40% (Saravanan et al., 2005), in contrast to the up-regulating effects of cis-poly-unsaturated fatty acids, which are thought to be a natural ligand for PPAR-γ (Desvergne and Wahli, 1999; Berger and Moller, 2002). Furthermore, trans fat intake increases the circulating levels of several inflammatory markers, including IL-6 (Baer et al., 2004; Mozaffarian et al., 2004a, b) and the markers of TNF system activation (Mozaffarian et al., 2004a, b) which are thought to be involved in the pathogenesis of endometriosis (Lebovic et al., 2001).
Within this population of USA registered nurses, the major dietary contributors to long-chain omega-3 fatty acids included salad dressing, tuna and dark fish, although the major contributors to trans-unsaturated fatty acids were commercially (i.e. away from home) fried foods, margarine and crackers. Seventy-eight percent of the trans fat contributors were likely industrially produced due to partial hydrogenation of vegetable oils. The primary contributors to palmitic acid consumption were animal products—meats and dairy foods—which perhaps supports the observation of increased endometriosis likelihood with greater red meat consumption observed within the sole human study published to date (Parazzini et al., 2004). In this study, Parrazini et al. collected current dietary information from 504 case:control pairs and observed a significant increased risk of laparoscopically confirmed endometriosis with greater red meat consumption. However, this study included cross-sectionally collected food group-level dietary data using an unvalidated questionnaire that did not allow for quantification of and thus adjustment for total energy intake. Within our study, we observed the suggestion of an increased risk of endometriosis with animal fat consumption and specifically a significantly increased risk of nearly 80% with palmitic acid intake.
The suggestion of a stronger relation with dietary fat consumption among women who have never reported infertility is interesting. We hypothesize that diet may be most strongly associated with the chronic pelvic pain symptoms of endometriosis as supported by the literature suggesting a beneficial affect of fish oil consumption on primary dysmenorrheal (Deutch, 1995). We may assume that cases with no infertility who have had a laparoscopic diagnosis are ‘symptomatic’, otherwise a surgical evaluation would not have been conducted. Reciprocally, a larger proportion of women diagnosed during an infertility evaluation will have been ‘asymptomatic’. It is also possible that those experiencing pain versus those experiencing sub-fecundity differ between a primary etiology of smooth muscle contractility impairment and aberrant inflammatory response, respectively, and these pathways may be differentially influenced by dietary fat intake. Although the statistical test for heterogeneity was not significant, this may be underpowered given the relatively small infertile case sample size and warrants evaluation by others.
The large, prospective cohort design of this study allowed for investigation of dietary exposure temporality and variation by diagnostic pathway associated with the rate of laparoscopically diagnosed endometriosis. The results of this study suggest that increasing long-chain omega-3 fatty acid intake and decreasing trans-unsaturated fatty acid intake may be the first identified modifiable risk factors for endometriosis. In addition, this evidence provides another disease association that supports efforts to remove trans-unsaturated fats from the food supply.
Funding
This study was supported by research grants HD48544 and HD52473 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and CA50385 from the National Cancer Institute.
Acknowledgements
We gratefully acknowledge the Nurses’ Health Study II participants for their continuing participation.
References
- Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004;79:969–973. doi: 10.1093/ajcn/79.6.969. [DOI] [PubMed] [Google Scholar]
- Bartram HP, Gostner A, Scheppach W, Reddy BS, Rao CV, Dusel G, Richter F, Richter A, Kasper H. Effects of fish oil on rectal cell proliferation, mucosal fatty acids, and prostaglandin E2 release in healthy subjects. Gastroenterology. 1993;105:1317–1322. doi: 10.1016/0016-5085(93)90135-y. [DOI] [PubMed] [Google Scholar]
- Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Agriculture USDo., editor. Washington, DC: Department of Agriculture, Government Printing Office; 1993. Composition of foods – raw, processed, and prepared. 1963–1992. [Google Scholar]
- Covens AL, Christopher P, Casper RF. The effect of dietary supplementation with fish oil fatty acids on surgically induced endometriosis in the rabbit. Fertil Steril. 1988;49:698–703. doi: 10.1016/s0015-0282(16)59842-2. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Deutch B. Menstrual pain in Danish women correlated with low n-3 polyunsaturated fatty acid intake. Eur J Clin Nutr. 1995;49:508–516. [PubMed] [Google Scholar]
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- Gazvani MR, Smith L, Haggarty P, Fowler PA, Templeton A. High omega-3:omega-6 fatty acid ratios in culture medium reduce endometrial-cell survival in combined endometrial gland and stromal cell cultures from women with and without endometriosis. Fertil Steril. 2001;76:717–722. doi: 10.1016/s0015-0282(01)01991-4. [DOI] [PubMed] [Google Scholar]
- Harel Z, Biro FM, Kottenhahn RK, Rosenthal SL. Supplementation with omega-3 polyunsaturated fatty acids in the management of dysmenorrhea in adolescents. Am J Obstet Gynecol. 1996;174:1335–1338. doi: 10.1016/s0002-9378(96)70681-6. [DOI] [PubMed] [Google Scholar]
- Heilier JF, Donnez J, Nackers F, Rousseau R, Verougstraete V, Rosenkranz K, Donnez O, Grandjean F, Lison D, Tonglet R. Environmental and host-associated risk factors in endometriosis and deep endometriotic nodules: a matched case–control study. Environ Res. 2007;103:121–129. doi: 10.1016/j.envres.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1–10. doi: 10.1016/s0015-0282(00)01630-7. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Kir M, Casey CL. Peroxisome proliferator-activated receptor-gamma induces regression of endometrial explants in a rat model of endometriosis. Fertil Steril. 2004;82:1008–1013. doi: 10.1016/j.fertnstert.2004.02.148. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Mwenda JM, Chai DC, Mueller MD, Santi A, Fisseha S, D'Hooghe T. PPAR-gamma receptor ligand induces regression of endometrial explants in baboons: a prospective, randomized, placebo- and drug-controlled study. Fertil Steril. 2007;88:1108–1119. doi: 10.1016/j.fertnstert.2006.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SJ, Sacks FM, Caesar J, Stampfer MJ, Siguel E, Willett WC. Fatty acid composition of subcutaneous adipose tissue and diet in postmenopausal US women. Am J Clin Nutr. 1991;54:340–345. doi: 10.1093/ajcn/54.2.340. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Pischon T, Hankinson SE, Rifai N, Joshipura K, Willett WC, Rimm EB. Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr. 2004a;79:606–612. doi: 10.1093/ajcn/79.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC. Trans fatty acids and systemic inflammation in heart failure. Am J Clin Nutr. 2004b;80:1521–1525. doi: 10.1093/ajcn/80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oral E, Arici A. Pathogenesis of endometriosis. Obstet Gynecol Clin North Am. 1997;24:219–233. doi: 10.1016/s0889-8545(05)70301-6. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Chiaffarino F, Surace M, Chatenoud L, Cipriani S, Chiantera V, Benzi G, Fedele L. Selected food intake and risk of endometriosis. Hum Reprod. 2004;19:1755–1759. doi: 10.1093/humrep/deh395. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- Rich-Edwards JW, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, Manson JE. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171:171–177. doi: 10.1016/0002-9378(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. 4th edn. Belmont, CA: Wadsworth Publishing Co; 1993. [Google Scholar]
- Saravanan N, Haseeb A, Ehtesham NZ, Ghafoorunissa Differential effects of dietary saturated and trans-fatty acids on expression of genes associated with insulin sensitivity in rat adipose tissue. Eur J Endocrinol. 2005;153:159–165. doi: 10.1530/eje.1.01946. [DOI] [PubMed] [Google Scholar]
- Willett W. Nutritional Epidemiology. 2nd edn. NY: Oxford University Press, Inc.; 1998. [Google Scholar]
- Willett W, Stampfer M, Chu NF, Spiegelman D, Holmes M, Rimm E. Assessment of questionnaire validity for measuring total fat intake using plasma lipid levels as criteria. Am J Epidemiol. 2001;154:1107–1112. doi: 10.1093/aje/154.12.1107. [DOI] [PubMed] [Google Scholar]