Abstract
Men and women differ in cerebral organization and prevalence rates of eating disorders. However, no studies have yet examined sex differences in cerebral responses to the caloric content of food images. Sixteen healthy adults (8 men; 8 women) underwent functional magnetic resonance imaging (fMRI) while viewing images of high- and low-calorie foods. Compared to men, women showed significantly greater activation to calorie-rich foods within dorsolateral, ventrolateral, and ventromedial prefrontal cortex, middle/posterior cingulate, and insula. Men failed to show greater activation in any cortical region compared to women, although amygdala responses were greater in men at a more liberal threshold. When viewing high-calorie food images, women appear more responsive than men within cortical regions involved in behavioral control and self-referential cognition.
Keywords: FMRI, Neuroimaging, Food, Calorie, Sex Differences, Prefrontal Cortex, Limbic System, Amygdala, Insula
The human brain responds differentially to images of high- and low-calorie foods [1], and these responses can be affected by a number of factors, including hunger [2], body mass [3], mood [4], and age [5]. A potential factor that has not been adequately explored regarding its effects on cerebral responses to food is the sex of the individual. Men and women are often socialized differently with regard to many aspects of food. Women tend to be more invested in food-related issues, have greater knowledge of food and nutrition, are more prone to diet, and are more likely to perceive themselves as needing to lose weight than men [6]. Furthermore, there are well-documented differences between the sexes in the organization and structure of the brain, and many of these differences are significantly related to neurocognitive functioning and reward processing [7]. Thus, it is possible that either through socialization or biologically hardwired systems, males and females may respond differently to food-related cues. Understanding sex differences in cerebral responses to food stimuli is of particular importance given the well-established disparity between men and women in the prevalence of many eating disorders [8,9]. Despite the numerous functional magnetic resonance imaging (fMRI) studies recently focusing on cerebral responses to food images, to our knowledge, no studies have directly examined sex differences in the functional brain responses of healthy adults when viewing images of high-versus low-calorie foods.
In the present study, we examined sex differences in cerebral responses to images of foods differing in caloric content using a previously validated paradigm [1]. Healthy male and female adults viewed color photographs depicting foods with high and low calorie density while attending to the images for later recognition. Based on prior evidence that women tend to express greater food-related concerns [10,11] and higher prevalence rates for eating disorders [8,9], it was hypothesized that females would show greater overall activation in response to high-calorie food images, particularly within prefrontal inhibitory and self-monitoring regions compared to males. Furthermore, given the importance of the insula in hunger and visceral sensations [12] and the amygdala in food-related processing [1,2,5,12], it was hypothesized that women would show greater activation than men within these specific regions.
Methods
Participants
Sixteen healthy adults (8 men; 8 women; 12 right-handed by self-report) were recruited from the local community of Belmont, MA. As shown in Table 1, men and women did not differ with regard to age, education level, or body mass index (BMI). Furthermore, when queried about their exercise habits men and women indicated similar levels of physical activity and similar experiences of exhaustion and fatigue as a result of working out. There were also no differences between males and females in self-reported appetite or perceived loss of control over eating behaviors (see Table 1). Participants were in good health, had no history of neurologic or psychiatric problems, and had normal or corrected-normal vision. The protocol was consistent with the guidelines of the McLean Hospital Institutional Review Board and written informed consent was obtained from all participants.
Table 1.
Characteristics of the Male and Female Groups.
| Characteristic | Men | Women | p-value |
|---|---|---|---|
| Age | 46.5 (5.4) | 48.0 (5.7) | .60 |
| Education | 16.1 (2.6) | 15.7 (2.1) | .76 |
| Body Mass Index (BMI) | 27.1 (5.5) | 23.5 (4.5) | .19 |
| Engage in regular exercise (%) | 12.5 | 37.5 | .57 |
| “Do you feel exhausted during exercise?” (1 = never; 10 = always) | 2.7 (1.4) | 2.7 (1.5) | .95 |
| “Are you fatigued after exercising?” (1 = never; 10 = always) | 3.3 (1.9) | 4.0 (2.2) | .53 |
| “What is your appetite like?” (1 = always hungry; 10 = never hungry) | 6.4 (1.2) | 7.1 (1.8) | .34 |
| “Do you feel you eat more than you intend to?” (1 = never; 10 = always) | 4.5 (2.5) | 3.5 (1.7) | .37 |
| High Calorie Post-Test Recognition Performance | |||
| % Total Correct | 92.5 (4.1)* | 87.5 (9.9)* | .24 |
| % Hits | 90.0 (5.8)* | 90.0 (10.7)* | 1.00 |
| % False Alarms | 5.0 (5.0)* | 15.0 (13.6)* | .09 |
| Low Calorie Post-Test Recognition Performance | |||
| % Total Correct | 91.4 (3.5)* | 88.8 (6.3)* | .34 |
| % Hits | 90.0 (9.6)* | 87.5 (13.4)* | .69 |
| % False Alarms | 7.1 (4.9)* | 10.0 (10.0)* | .51 |
For post-scan recognition performance, mean index differs from chance value of 50% at p <.0001, indicating that participants attended effectively to the stimuli.
Study Design
Each participant completed a medical and psychiatric interview, questionnaires about food and lifestyle preferences, and underwent an fMRI scan on the same day. Participants were asked to adhere to their normal dietary routine prior to the scan, although no food was permitted in the hour prior to entry into the scanner. During the fMRI session, participants completed two stimulation paradigms that involved full-color visual presentations of high calorie images (e.g., cheeseburgers, hot dogs, ice cream, cake) and low calorie images (e.g., mixed salads, vegetables, whole-grain cereals). These paradigms have been described in detail in several previous reports [1,3–5]. Briefly, each task required participants to attend to a series of colored photographs and try to remember them well enough to discriminate from similar distractor images following the scan. During each task, participants viewed alternating blocks (30 seconds each) depicting either the stimulus images (e.g., high calorie foods) or control images of non-food objects with similar visual complexity, color, and texture (e.g., shrubs, rocks, flowers, bricks, leaves). Each task lasted 150 seconds and included 5 blocks of ten photographs (2500 msec stimulus presentation and a 500 msec inter-stimulus interval). Stimuli were back-projected onto a screen placed at the rear of the scanner, controlled by a Macintosh computer, and viewed via a mirror mounted on the head coil. To ensure attention to the task, all participants completed post-scan recognition tests for the images. Each test randomly presented all 20 previously seen stimulus images (old) along with 20 images from the same category (e.g., high-calorie foods) that had not been seen before (new). Recognition performance was quantified in terms of the percent of correct hits (percent of “old” images correctly identified), percent of false alarms (percent of “new” items incorrectly judged as “old”), and total percent correct ([total hits + total correct rejections]/40).
Imaging Methods
Functional imaging was conducted on a Siemens Trio whole body 3T MRI scanner using a quadrature RF head coil (TR = 3 sec, TE = 30 msec, flip angle = 90 degrees). Using a single-shot, gradient pulse-echo sequence, 35 to 41 coronal slices (5 mm thick, 0 skip) were obtained for each subject employing a 20 cm field of view and a 64 × 64 acquisition matrix (in-plane resolution = 3.125 × 5 × 3.125 mm). Fifty images were collected per slice. At the outset of scanning, three dummy images were acquired to allow the scanner to reach a steady state.
Image Processing and Analysis
Images were motion corrected, realigned, convolved into the standard MNI space, smoothed using an isotropic Gaussian kernel (full width half maximum [FWHM] = 6 mm), and resliced to 2×2×2 mm voxels using SPM99 (Wellcome Trust Centre for Neuroimaging, UK). The high- and low-calorie runs were concatenated to form a single dataset. For each paradigm, contrast images were created using the general linear model to compare activation changes associated with high-calorie images versus low-calorie images. In the second, or “random-effects” level of analysis, these contrast images were entered into an analysis of covariance (ANVOVA) to compare the responses of men versus women after controlling for BMI. Brain activation was evaluated at the whole brain level at a threshold of p < .001 (uncorrected), with an extent threshold of k≥10. In addition, based on prior evidence from several independent laboratories pointing to the importance of the amygdala [1,5,13] and insula [13,14] in responses to food stimuli, we chose to focus additional analyses on these regions. Specifically, two bilateral regions of interest (ROI) masks were created a priori using the WFU Pickatlas utility [15] to restrict analyses to search territories encompassing the amygdala and insula using the published neuroanatomical atlas of Tzourio-Mazoyer and colleagues [16]. Because activation within these search territories was predicted a priori to differ between men and women, a statistical threshold of p < .05, k = 10 contiguous voxels was applied within the search territories.
Results
Behavioral Data
It is clear from the post-scan recognition test data presented in Table 1 that the participants were actively engaged in the task, with the percent of correct hits and total correct discriminations significantly exceeding chance levels, while the percent of false alarms was significantly below chance for high-calorie and low-calorie images (all p-values < .0001). As shown in Table 1, men and women did not differ in their recognition memory for the images.
Males > Females
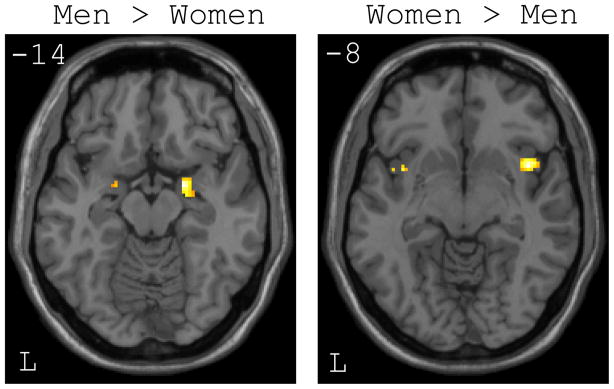
At the whole brain level, there were no regions that were more responsive to high- versus low-calorie foods in males when compared to females (see Table 2). However, at a more liberal threshold within the a priori ROIs, males showed significantly greater bilateral activation within the amygdala (see Figure 1). The activation was greatest within the right amygdala (56 voxels; MNI coordinates: 22, −2, −14; t[13] = 3.87), but was also evident to a smaller extent within the left amygdala (10 voxels; MNI coordinates: −20, 2, −16; t[13] = 2.48). In contrast, even at the liberal threshold, there were no voxels within either insular ROI showing greater activation in males than females.
Table 2.
Local Maxima for Whole Brain Analysis of Sex Differences in Responses to High-versus Low-Calorie Foods.
| Region | Cluster Size (Voxels) | x | y | z | SPM {t} |
|---|---|---|---|---|---|
| Males > Females | |||||
| None | -- | -- | -- | -- | -- |
| Females > Males | |||||
| L Middle Frontal Gyrus | 55 | −22 | 26 | 54 | 7.42 |
| L Inferior Orbitofrontal Gyrus | 23 | −46 | 42 | −16 | 6.71 |
| L Supplementary Motor Area | 47 | 0 | 18 | 60 | 6.47 |
| L Middle Cingulate Gyrus | 56 | −4 | −36 | 38 | 5.66 |
| L Middle Temporal Gyrus | 22 | −52 | 4 | −24 | 5.61 |
| L Superior Medial Frontal Gyrus | 16 | −10 | 28 | 56 | 5.54 |
| L Middle Cingulate Gyrus | 13 | −10 | −2 | 34 | 5.51 |
| L Inferior Orbitofrontal Gyrus | 16 | −38 | 42 | −6 | 5.19 |
| L Gyrus Rectus | 16 | −8 | 50 | −16 | 5.14 |
| L Middle Frontal Gyrus | 17 | −28 | 32 | 48 | 4.47 |
| R Superior Medial Frontal Gyrus | 10 | 12 | 24 | 60 | 4.29 |
P < .001 (uncorrected), k ≥ 10.
Figure 1.
Axial slices showing significant differences between men and women within the amygdala and insula regions of interest (ROIs) for the high-calorie > low-calorie food contrast. Left: Men showed greater activation than women bilaterally within the amygdala. Right: Women showed greater activation than men bilaterally within the insular cortex.
Females > Males
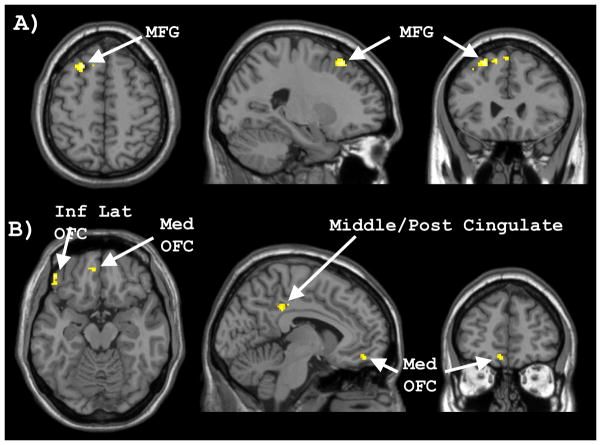
Whole brain analysis revealed a number of clusters that showed significantly greater activation within females compared to males for the high- versus low-calorie food contrast (see Table 1). As evident in Figure 2, females showed greater responses than males in the left middle frontal gyrus, left inferior lateral orbitofrontal cortex, left ventromedial/orbitofrontal cortex, and left middle/posterior cingulate gyrus. At a more liberal threshold within the a priori established ROIs, females showed greater activation than males within the right (76 voxels; MNI coordinates: 42, 10, −8; t[13] = 3.53) and left (17 voxels; MNI coordinates: −36, 10, −10; t[13] = 3.30) insular cortices. However, despite the lower statistical thresholds applied to the ROIs, neither amygdala showed evidence of greater activation in females than males.
Figure 2.
Whole brain activation showing significantly greater activation for women compared to men during the high-calorie > low-calorie food image contrast. A) Women showed significantly greater activation than men within the middle frontal gyrus (MFG), as evident in the axial (left), sagittal (middle), and coronal planes (right). B) Women showed significantly greater activation than men within the inferior lateral orbitofrontal gyrus (Inf Lat OFC), medial orbitofrontal cortex (Med OFC), and middle/posterior cingulate gyrus.
Discussion
We observed significant sex differences in cerebral responses to images of high- versus low-calorie foods. Based on previous evidence suggesting that women tend to be more concerned with food, weight, and body image than men [6], and evidence of sex differences in neurocognitive functioning and reward processing [7], we hypothesized that women would show relatively greater responses within prefrontal cortical regions involved in behavioral control and self-referential cognition. This hypothesis was confirmed. Women showed significantly greater whole brain responses to images of calorie-dense relative to calorie-lean foods than men, particularly within dorsolateral and inferior lateral orbitofrontal cortex, medial orbitofrontal cortex, and posterior/middle cingulate gyrus, as well as greater activation within the insular cortex ROI. In contrast, there were no regions where men showed greater activation than women when evaluated at the whole brain level, although at a more liberal statistical threshold, men did show greater bilateral activation within the amygdala. Thus, clear sex-related differences in cortico-limbic functional responses to the calorie content of foods were found.
While the present study design cannot definitively isolate the causal factors for these sex differences, there are at least three potential candidates, including sex-specific socialization, genetic/structural differences, and circulating sex hormones, each of which may have contributed to some extent. From a young age, women are socialized differently than men with regard to a variety of issues surrounding food. In many cultures, women have traditionally functioned as the primary preparers of food within the family, and women consistently show more interest in nutrition, healthy eating, dieting, body-image, and weight control [17], are more likely to report needing to lose weight despite having lower mean BMI scores [6], and generally feel more guilt and conflict over eating than men [10,11]. The present findings are consistent with the possibility that, due to prior socialization and experience, images of high-calorie foods may induce greater cognitive analysis, self-reflection, and inhibitory processing for women than men. Specifically, we found that women showed greater calorie-mediated activation to food images within the inferior lateral orbitofrontal cortex, a region believed to be critical to response inhibition [18], and dorsolateral prefrontal cortex, which is involved in a variety of high level decision-making capacities, including selecting, planning, and executing motor responses [19]. Women also showed greater activation than men within the ventromedial/orbitofrontal cortex and posterior/middle cingulate gyrus, regions that are believed to be involved in self-reflection, self-referential thought, and imagining future personal events [20,21]. Though speculative, the greater activation of these regions in healthy women may reflect increased engagement of prefrontal evaluative, decision-making, inhibitory, and self-referential cognitive systems when confronted with images of high- versus low-calorie foods relative to the responses evoked in healthy men. It is conceivable that dysfunction of these same prefrontal cortical systems may be associated with the higher prevalence rates among women for eating disorders such as anorexia nervosa and bulimia nervosa [8,9].
In addition to the role that socialization and prior experience may play in affecting cognitive processing of food-related stimuli, it is also possible that genetic, neuroendocrine, or structural dimorphisms within the brain may also contribute to the observed functional differences. For instance, women tend to have a relatively greater proportion of cortical gray matter, thicker cortical mantle, and higher cerebral blood flow than men [7,22], which may have affected the functional cortical processing of the stimuli. Because the present experimental design cannot directly test the potential contribution of these factors, it will be important for future research to clarify the extent to which sex differences in brain responses to food are due to consistent patterns of socialization and experience versus sexually dimorphic hardwired physiological systems.
We also found sex-related differences within pre-specified limbic/paralimbic regions known for their involvement in emotion, motivation, and monitoring of bodily states. Albeit at a lower statistical threshold, women showed significantly greater activation within the insula to the high-calorie foods, whereas men showed significantly greater activation of the amygdala in response to the same stimuli. Because the insula has been implicated in feelings of hunger, visceral sensations, and evaluation of current need states [12,23], this finding raises the possibility that women may perceive food as a particularly salient stimulus that activates a spectrum of somatic sensations and associated cognitions. Men, in contrast, activated the amygdala, a more primal limbic structure involved in detecting biologically relevant stimuli and determining the appetitive value or attractiveness of food [12]. This finding reinforces the notion that women may process food imagery at a more complex cognitive/somatic level, whereas men may process food stimuli at a less complex hedonic approach-withdrawal level. The conjoint finding of greater amygdala response and reduced cortical activation among men when confronted with images of calorie-rich foods is consistent with reports that men tend to consume more calories than women but experience less cognitive conflict and guilt associated with food and eating behaviors [10,11]. The present results are also consistent with recent functional neuroimaging studies of appetitive brain responses to biologically salient stimuli, such as erotic visual imagery, where men show greater responsiveness of the amygdala than women despite equivalent ratings of physical arousal and attractiveness of the stimuli by both sexes [24]. Interestingly, a recent study showed that men were more effective than women at deliberately inhibiting activation of the amygdala, insula, and other reward-related areas, when confronted with actual desired foods [25], again potentially linking sex differences in cortico-limbic inhibitory responses to food stimuli with known sex differences in the expression of eating disorders [8,9]. Future research examining the relationship between cognitive responses to particular foods and how these cerebral responses differ between men and women may provide further understanding of the neural systems involved in eating disorders.
Conclusion
Men and women showed different patterns of cortico-limbic responses to images of high-calorie relative to low-calorie foods. Men showed greater functional responsiveness to calorie-rich foods within primitive limbic regions involved in assessing affective and appetitive value whereas women showed greater activation within a distributed system of lateral prefrontal and midline cortical regions involved in cognitive analysis, behavioral control, and self-referential cognition. These findings may contribute to a neurobiological understanding of the sex differences associated with food-related attitudes and eating behaviors.
Acknowledgments
This study was supported by Kyowa Hakko Kogyo Co., Ltd., JAPAN and by NIDA 1R01 DA020269 (DYT).
References
- 1.Killgore WDS, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 2.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 3.Killgore WDS, Yurgelun-Todd DA. Body mass predicts orbitofrontal activity during visual presentations of high-calorie foods. Neuroreport. 2005;16:859–863. doi: 10.1097/00001756-200505310-00016. [DOI] [PubMed] [Google Scholar]
- 4.Killgore WDS, Yurgelun-Todd DA. Affect modulates appetite-related brain activity to images of food. Int J Eat Disord. 2006;39:357–363. doi: 10.1002/eat.20240. [DOI] [PubMed] [Google Scholar]
- 5.Killgore WDS, Yurgelun-Todd DA. Developmental changes in the functional brain responses of adolescents to images of high and low-calorie foods. Dev Psychobiol. 2005;47:377–397. doi: 10.1002/dev.20099. [DOI] [PubMed] [Google Scholar]
- 6.Davy SR, Benes BA, Driskell JA. Sex differences in dieting trends, eating habits, and nutrition beliefs of a group of midwestern college students. J Am Diet Assoc. 2006;106:1673–1677. doi: 10.1016/j.jada.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 8.Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Current opinion in psychiatry. 2006;19:389–394. doi: 10.1097/01.yco.0000228759.95237.78. [DOI] [PubMed] [Google Scholar]
- 9.Striegel-Moore RH, Bulik CM. Risk factors for eating disorders. Am Psychol. 2007;62:181–198. doi: 10.1037/0003-066X.62.3.181. [DOI] [PubMed] [Google Scholar]
- 10.Narchi I, Walrand S, Boirie Y, Rousset S. Emotions generated by food in elderly French people. J Nutr Health Aging. 2008;12:626–633. doi: 10.1007/BF03008273. [DOI] [PubMed] [Google Scholar]
- 11.Rolls BJ, Fedoroff IC, Guthrie JF. Gender differences in eating behavior and body weight regulation. Health Psychol. 1991;10:133–142. doi: 10.1037//0278-6133.10.2.133. [DOI] [PubMed] [Google Scholar]
- 12.Piech RM, Lewis J, Parkinson CH, Owen AM, Roberts AC, Downing PE, et al. Neural correlates of appetite and hunger-related evaluative judgments. PLoS ONE. 2009;4:e6581. doi: 10.1371/journal.pone.0006581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: An fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 14.Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex. 2005;15:1602–1608. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- 15.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 16.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 17.Wardle J, Haase AM, Steptoe A, Nillapun M, Jonwutiwes K, Bellisle F. Gender differences in food choice: the contribution of health beliefs and dieting. Ann Behav Med. 2004;27:107–116. doi: 10.1207/s15324796abm2702_5. [DOI] [PubMed] [Google Scholar]
- 18.Leung HC, Cai W. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. J Neurosci. 2007;27:9893–9900. doi: 10.1523/JNEUROSCI.2837-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pochon JB, Levy R, Poline JB, Crozier S, Lehericy S, Pillon B, et al. The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: an fMRI study. Cereb Cortex. 2001;11:260–266. doi: 10.1093/cercor/11.3.260. [DOI] [PubMed] [Google Scholar]
- 20.D’Argembeau A, Stawarczyk D, Majerus S, Collette F, Van der Linden M, Feyers D, et al. The Neural Basis of Personal Goal Processing When Envisioning Future Events. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21314. [DOI] [PubMed] [Google Scholar]
- 21.Moran JM, Heatherton TF, Kelley WM. Modulation of cortical midline structures by implicit and explicit self-relevance evaluation. Social neuroscience. 2009;4:197–211. doi: 10.1080/17470910802250519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS ONE. 2009;4:e6310. doi: 10.1371/journal.pone.0006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci. 2004;7:411–416. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- 25.Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci U S A. 2009;106:1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]