Abstract
Structural analysis of an adduct of a thiophene-based cryptand with tosylic acid shows the formation of a hybrid amine-water cyclic pentamer composed of four water molecules and one protonated amine in the charged hydrophobic cavity. The bulky tosylate groups remain outside the cavity, making the ligand favorable for hosting water molecules. Ab initio calculations based on density functional theory (DFT) confirm that the hybrid amine-water pentamer is stabilized inside the hydrophobic cavity of the cryptand.
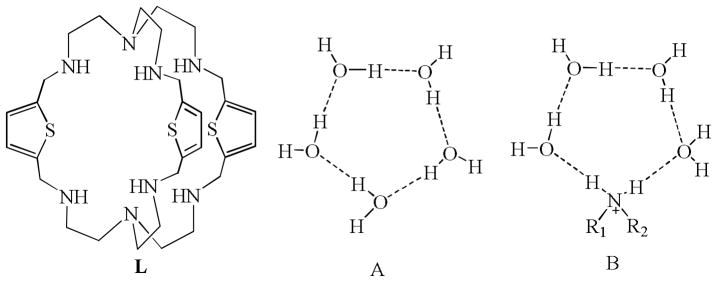
Despite significant progress in experimental and theoretical studies of discrete water clusters, a detailed understanding of molecular water within a confined hydrophobic cavity remains a challenge.[1] Within the peptide walls of a protein, molecular or clustered water can form in the hydrophobic cavity through hydrogen bonding and hydrophobic interactions,[2] playing a critical role in controlling the function, activity, and folding of the protein.[3] Protein-bound water clusters have also been identified structurally, showing stable polygons composed of three to eight water molecules.[4] At the molecular level, water clusters are thought to facilitate the transfer of a proton along the hydrogen-bonded chain,[5] which is not only a fundamental biological process[6] but also a key mechanism for the generation of electrical power in hydrogen fuel cells.7 Interests in this area continue to intrigue the scientific community both in terms of basic research and technological applications.[8] In particular, the structural characterization of water clusters is critical in correlating and predicting the properties of bulk water at a molecular level. In the solid state, water clusters (H2O)n are known to exist as discrete rings, chains, tapes, and layers[9] with different numbers of water molecules. Among these arrangements, cyclic water clusters[10] have been shown to be stabilized as trimers,[10a] tetramers,[10b] pentamers,[10c,d] hexamers,[10e] octamers,[10f] decamers,[10g] undecamers,10h and dodecamers10i in various organic environments. However, in contrast to their presence within crystal lattices or between hosts, cyclic water clusters inside the cavity are rare,[11] with the exception of tricyclic amide with hexamer,[11a] p-xylyl cryptand for quasi-prismatic hexamer,[11b] and self-assembled palladium-based cages for a decamer.[11c] It is known that, in the solid states, the most of the organic hosts (ca. 96%) forming water clusters are directly linked to water molecules via hydrogen bonding interactions.[12] However, to the best of our knowledge, the participation of a heteroatom in forming a ring has not been previously reported within a synthetic molecule. Herein, we present structural evidence and ab initio calculations based on a new type of hybrid pentamer [(H2O)4(NH2)]+ (Chart 1) This unique structure is stabilized within the charged cavity of a hexaprotonated cryptand L, bearing a similar structural topology to the cyclic pentamer, (H2O)5[10c] or cylcopentane.
Chart 1.
Thiophene-based octaazacryptand (L), cyclic water pentamer (A), and hybrid amine-water cyclic pentamer (B).
We recently reported that the bicyclic receptor L effectively binds chloride[13a] and nitrate[13b] ions in aqueous solution through the protonated bridgehead amines. While one chloride was found to be encapsulated linearly within the cavity, three nitrates were bridged in L forming a [NH(NO3)3HN]+ motif in the solid state. The formation of trinitrate complex, however, was inconsistent with a 1:1 binding observed in aqueous solution, suggesting that water molecules occupied the cavity and prevented the competitive binding of more than one nitrate in solution. This observation inspired us to further explore the cryptand with bulky anions that are too large to enter the cavity, thus making an ideal environment in L for hosting water molecules.
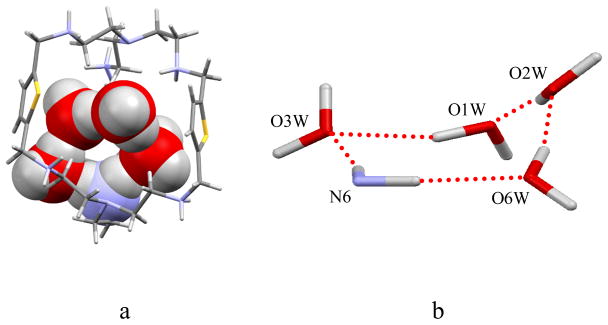
Crystallographic analysis of the tosylate salt of L revealed that the ligand in its hexaprotonated form crystallizes in the monoclinic space group P21/n with six tosylate anions and twelve water molecules.[14] As shown in Figure 1, a cyclic pentamer is formed with four water molecules (O3W, O1W, O2W, and O6W) and one protonated amine (N6) group inside the cavity. The hydrogen-bonding interactions facilitate the formation of a pentamer which adopts a puckered conformation, with one water (OW1) lying at 1.410 A above the mean plane formed by the three oxygens and the linking nitrogen (Figure 1b). The protonated amine involved in the ring acts as two hydrogen bond donors for two waters with 2.894 (2) and 2.754 (2) Å for N6···O3W and N6···O6W, respectively.
Figure 1.
(a) Crystal structure of [H6L(4H2O)]6+ showing the hybrid amine-water pentamer [(H2O)4(NH2)]+ in a space-filling model. (b) Representation of the hybrid amine-water pentamer [(H2O)4(NH2)]+ in puckered conformation in [H6L]6+.
The large anions are located outside the cavity without interacting with the macrocycle. The secondary amines are protonated in the macrocycle exhibiting a C3 symmetry along the bridgehead nitrogens, N1 and N4. The absence of an anion inside the cavity makes L favorable for hosting the water molecules. The distance between the bridgehead nitrogen sites is longer (7.891 Å) than the corresponding distance in the chloride (6.096(4) Å)13a or nitrate (5.528(6) Å)13b complex, where the internally bound anion brings the two bridgehead amines closer through electrostatic interactions. The geometry of the hybrid pentamer is similar to the cyclic pentamer observed in a lattice of hydrated trans-bis(4-pyridyl)ethylene dioxide.21a The O···O (O1W···O3W = 2.885(2) Å, O2W···O1W = 2.868(3) Å, and O6W···O2W = 2.750(16) Å) distances in the hybrid pentamer are comparable to the reported pentamer (2.706(3) to 2.918(3) Å),[10c] liquid water (2.85 Å ),[15] and ab initio calculations (2.813–2.831 Å by Hartree-Fock and 2.689–2.700 Å by DFT).[16] Additional water molecules remain outside the cavity and are involved in various hydrogen-bonding interactions, forming an acyclic trimer with OW9, OW10, and OW11, and a dimer with OW8 and OW12. The trimer is associated to N2 through OW11 with a N···O bond distance of 2.715(3) Å. The water with OW4 and OW5 forms two acceptor hydrogen bonds with N7, giving a bond distance of 2.764(2) and 2.682(2) Å, respectively. Clearly, the absence of interactions between anionic groups with the macrocycle makes the cavity versatile not only for water in this case, but also for other anions.[13]
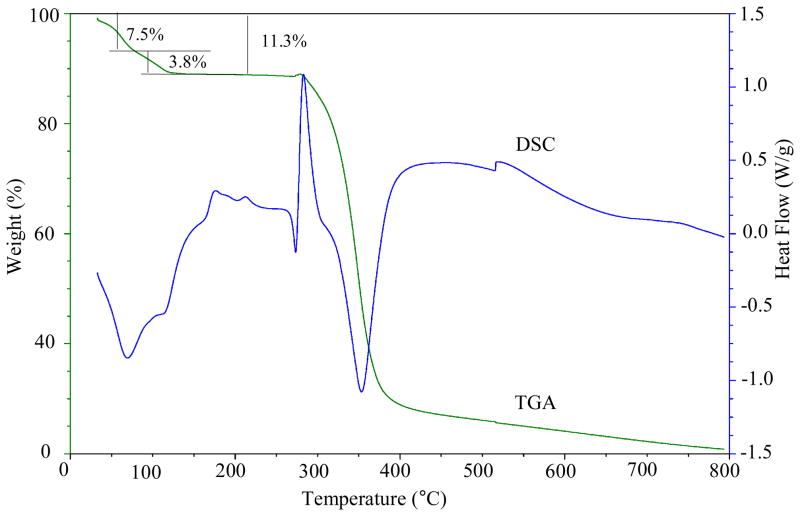
In order to characterize the bound and unbound water molecules in the complex, we performed thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) of [H6L(4H2O)](TsO)6·8H2O (Figure 2). As shown on the TGA curve, the complex exhibits a first weight loss of 7.5% before reaching a temperature of 100 °C, displaying a large endothermic peak on the DSC at around 70 °C. The first weight loss, which corresponds to eight water molecules, could be due to the removal of unbound water molecules outside the cavity. The weight loss in the second phase on TGA is about 3.8%, corresponding to four water molecules that are assigned to the internally bound water cluster in the hybrid pentamer observed in the crystal structure. The removal of water clusters results in the appearance of another endothermic peak at 112 °C. Thus, the 11.3% decrease in the original weight below 120 °C corresponds to the twelve water molecules as per the chemical composition of [H6L(4H2O)](TsO)6.8H2O observed in the solid state. After loosing of the crystalline water, the complex appears to be stable up to 275 °C. The exothermic and endothermic peaks at ~275 ° and ~350 °C on the DSC curve could be due to the melting and decomposition of the complex, respectively. Furthermore, the presence of water in the complex was identified by FT-IR spectra (supporting materials). The complex showed a broad band at around 3420 cm−1 that can be assigned to the O··· H stretching vibration of water present in the sample, similar to the calculated frequency, 3443 cm−1 obtained for the pentamer[17] or to the experimental value, 3400 cm−1 observed for other water cluster.10i This band almost disappeared after heating the sample at 120 °C for 12 hours, confirming the exclusion of water molecules from the complex, an observation which is consistent with the TGA observations and theoretical calculations discussed below.
Figure 2.
Thermograms of [H6L(4H2O)](TsO)6·8H2O showing TGA (green) and DSC (blue) at the heating rate of 10 °C/min.
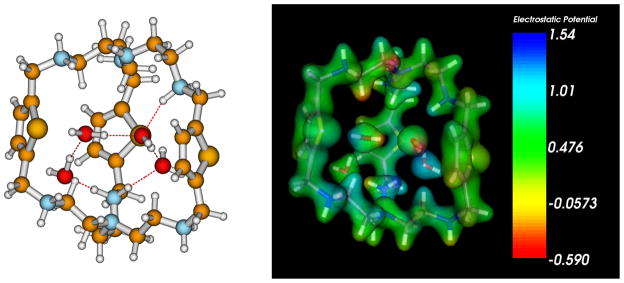
In order to quantitatively understand the unique bonding involved in the hybrid amine-water pentamer, ab initio calculations based on density functional theory (DFT) were carried out. All quantum chemical calculations were carried out with Truhlar’s M06-2X meta-GGA hybrid functional[18] which has been shown to accurately predict the relative energies of water hexamers and other noncovalent bonding interactions in large molecular systems.[19,20] Ground-state geometries were optimized at the M06-2X/6-31G(d,p) level of theory, and harmonic frequencies were computed to verify all structures were true minima. The initial geometry was modeled from the crystallographic coordinates of the protonated cluster-cryptand. At the optimized geometries, single-point energies with a larger 6-311G(d,p) basis set were carried out (a diffuse 6-311+G(d,p) basis set was used for the H, O, and N atoms involved in the amine-water pentamer within the complex). Figures 3a and b show the optimized geometry and electrostatic potential of the cluster-cryptand, respectively. From the DFT-optimized geometry, we find that the bond distances compare excellently with the experimental crystal structure, confirming the puckered conformation of the amine-water pentamer (supporting materials). The electrostatic potential also shows a localization of positive charge on the protonated amine groups within the cryptand structure. From the optimized geometry, the stabilization energy of complexation was calculated as Es = E (cluster-cryptand) − E (cryptand) − 4*E (water) and was found to be −68.5 kcal/mol, resulting from strong electrostatic interactions between the hydrogen-bonded water molecules and the highly-charged macrocycle, in addition to strong hydrogen bonds which stabilize the entire complex. In order to compare this binding energy with other complexes, we also calculated stabilization energies for an isolated water pentamer (Scheme 1A) and an isolated NH4+··· water pentamer (Scheme 1B). At the M06-2X/6-311+G(d,p) level of theory, we find that the NH4+··· water complex has a binding energy of −71.2 kcal/mol while the isolated water pentamer complex has a smaller binding energy of −40.4 kcal/mol. The cluster-cryptand stabilization energy is slightly less (more positive) than the NH4+··· water complex due to additional steric interactions (cf. Figure 3b) within the cryptand.
Figure 3.
(a) Optimized geometry of the amine-water cluster, [H6L(4H2O)]6+ at the M06-2X/6-31G(d,p) level of theory. (b) Electrostatic potential of [H6L(4H2O)]6+
In conclusion, we have structurally identified a novel hybrid amine-water cyclic pentamer formed inside the cavity of a cryptand. Ab initio calculations not only support the stabilization of the hybrid pentamer inside the hydrophobic cavity, but also reveal that the hybrid amine water pentamer is energetically more stable than the conventional water pentamer. The tosylate group used as a counter anion is not complementary in size to the cryptand, allowing neutral water molecules to reside inside the cavity. This observation clearly indicates that an appropriate selection of counter anions makes the ligand useful for caging water in the charged hydrophobic environment. The hybrid pentamer, in which a protonated amine is an important component in the ring, adopts a puckered conformation similar to cyclopentane or cyclic water pentamer. In vivo water clusters formed within a protein cavity or cleft are known to be stabilized predominantly through hydrogen-bonding interactions with CO groups at the C termini and NH groups at the N termini[21] as well as with the charged amino acid sidechains.[22] While there is an abundance of discrete water clusters reported in the literature, the formation of the hybrid pentamer described in this work has not been observed before in a synthetic host. Given the novel role of water clusters in chemical and biological systems, the present findings provide insight and serve as a prototype for stabilizing other pure and hybrid water clusters.
Supplementary Material
Acknowledgments
The project described was supported by Grant Number G12RR013459 from the National Center for Research Resources. This material is based upon work supported by the National Science Foundation under CHE-0821357. Purchase of the diffractometer was made possible by grant No. LEQSF (1999-2000)-ENH-TR-13, administered by the Louisiana Board of Regents.
Footnotes
Supporting Information available: One crystallographic file in CIF format, synthetic procedures, hydrogen bonding interactions, ORTEP diagram with numbering scheme, FTIR spectra, and experimental and computed bond distances within the amine-water complex in PDF format. This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Rasaiah JC, Garde S, Hummer G. Annu Rev Phys Chem. 2008;59:713–714. doi: 10.1146/annurev.physchem.59.032607.093815. [DOI] [PubMed] [Google Scholar]
- 2.(a) Peters J, Baumeister W, Lupas A. J Mol Biol. 1996;257:1031–1041. doi: 10.1006/jmbi.1996.0221. [DOI] [PubMed] [Google Scholar]; (b) Yin H, Hummer G, Rasaiah JC. J Am Chem Soc. 2007;129:7369–7377. doi: 10.1021/ja070456h. [DOI] [PubMed] [Google Scholar]
- 3.(a) Otwinowski Z, Schevitz RW, Zhang RG, Sigler PB. Nature. 1988;335:321–329. doi: 10.1038/335321a0. [DOI] [PubMed] [Google Scholar]; (b) Sleigh SH, Tame JRH, Dodson EJ, Wilkinson A. J Biochemistry. 1997;36:9747–9748. doi: 10.1021/bi970457u. [DOI] [PubMed] [Google Scholar]
- 4.Lee L, Kim SH. Protein Sci. 2009;18:1370–1376. doi: 10.1002/pro.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Z, Siu CK, Balaj OP, Gruber M, Bondybey VE, Beyer MK. Angew Chem Int Ed. 2006;45:4027–4030. doi: 10.1002/anie.200504135. [DOI] [PubMed] [Google Scholar]
- 6.Taraphder S, Hummer G. J Am Chem Soc. 2003;125:3931–3941. doi: 10.1021/ja016860c. [DOI] [PubMed] [Google Scholar]
- 7.Kreuer KD. Chem Mater. 1996;8:610–641. [Google Scholar]
- 8.(a) Massera C, Melegari M, Ugozzoli F, Dalcanale E. Chem Commun. 2010;46:88–90. doi: 10.1039/b917931c. [DOI] [PubMed] [Google Scholar]; (b) Gutberlet A, Schwaab G, Birer O, Masia M, Kaczmarek A, Forbert H, Havenith M, Marx D. Science. 2009;324:1545–1548. doi: 10.1126/science.1171753. [DOI] [PubMed] [Google Scholar]; (c) Buch V, Milet A, Vacha R, Jungwirth P, Devlin JP. PNAS. 2007;104:7342–7347. doi: 10.1073/pnas.0611285104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Mohammed OF, Pines D, Dreyer J, Pines E, Nibbering ETJ. Science. 2005;310:83–86. doi: 10.1126/science.1117756. [DOI] [PubMed] [Google Scholar]; (e) Mir MH, Vittal JJ. Angew Chem Int Ed. 2007;46:5925–5928. doi: 10.1002/anie.200701779. [DOI] [PubMed] [Google Scholar]; (f) Headrick JM, Diken EG, Walters RS, Hammer NI, Christie RA, Cui J, Myshakin EM, Duncan MA, Johnson MA, Jordan KD. Science. 2005;308:1765–1769. doi: 10.1126/science.1113094. [DOI] [PubMed] [Google Scholar]; (g) Lee SW, Freivogel P, Schindler T, Beauchamp JL. J Am Chem Soc. 1998;120:11758–11765. [Google Scholar]
- 9.Infantes L, Motherwell S. CrystEngComm. 2002;4:454–461. [Google Scholar]
- 10.(a) MacGillivray LR, Atwood JL. J Am Chem Soc. 1997;119:2592–2593. [Google Scholar]; (b) Zuhayra M, Kampen WU, Henze E, Soti Z, Zsolnai L, Huttner, Oberdorfer FA. J Am Chem Soc. 2006;128:424–425. doi: 10.1021/ja057142j. [DOI] [PubMed] [Google Scholar]; (c) Ma BQ, Sun HL, Gao S. Chem Commun. 2004:2220–2221. doi: 10.1039/b404364b. [DOI] [PubMed] [Google Scholar]; (d) Day MB, Kirschner KN, Shields GC. J Phys Chem A. 2005;109:6773–6778. doi: 10.1021/jp0513317. [DOI] [PubMed] [Google Scholar]; (e) Wang J, Zheng LL, Li CJ, Zheng YZ, Tong ML. Cryst Growth Des. 2006;6:357–359. [Google Scholar]; (f) Atwood JL, Barbour LJ, Ness TJ, Raston CL, Raston PL. J Am Chem Soc. 2001;123:7192–7193. doi: 10.1021/ja015757k. [DOI] [PubMed] [Google Scholar]; (g) Barbour LJ, Orr GW, Atwood JL. Nature. 1998;393:671–673. [Google Scholar]; (h) Lakshminarayanan PS, Suresh E, Ghosh P. Angew Chem Int Ed. 2006;45:3807–3811. doi: 10.1002/anie.200600254. [DOI] [PubMed] [Google Scholar]; (i) Ma BQ, Sun HL, Gao S. Angew Chem, Int Ed. 2004;43:1374–1376. doi: 10.1002/anie.200353097. [DOI] [PubMed] [Google Scholar]
- 11.(a) Kang SO, Powell D, Day VW, Bowman-James K. Cryst Growth Des. 2007;7:606–608. [Google Scholar]; (b) Li Y, Jiang L, Feng TB, Lu XL. Cryst Growth Des. 2008;8:3689–3694. [Google Scholar]; (c) Yoshizawa M, Kusukawa T, Kawano M, Ohhara T, Tanaka I, Kurihara K, Niimura N, Fujita M. J Am Chem Soc. 2005;127:2798–2799. doi: 10.1021/ja043953w. [DOI] [PubMed] [Google Scholar]
- 12.Steiner T. Acta Crystallogr, Sect D. 1995;51:93–97. doi: 10.1107/S0907444994007614. [DOI] [PubMed] [Google Scholar]
- 13.(a) Saeed MA, Fronczek FR, Hossain MA. Chem Commun. 2009:6409–6411. doi: 10.1039/b916099j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Saeed MA, Fronczek FR, Huang MJ, Hossain MA. Chem Commun. 2010;46:404–406. doi: 10.1039/b923013k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crystal data for [H6L(H2O)4](C7H7O3S)6·8(H2O): C72H120N8O30S9, M = 1866.30, crystal size: 0.27 × 0.25 × 0.12 mm3, Monoclinic, P21/n, a =16.8712(10), b=23.3371(15), c=23.1050(15) Å, β = 98.551(3)o, V = 8995.9(10) Å3, Z = 4, dcalc =1.378 g cm−3, T = 90.0 K, KappaCCD (with Oxford Cryostream) diffractometer, Mo-Kα radiation, λ = 0.71073 Å, μ(Mo-Kα) = 0.30 mm−1, 27409 independent reflections (18460 observed), 1150 parameters, Rint = 0.052, R =0.053, wR(F2) = 0.141. CCDC 755465.
- 15.Narten AH, Thiessen WE, Blum L. Science. 1982;217:1033–1034. doi: 10.1126/science.217.4564.1033. [DOI] [PubMed] [Google Scholar]
- 16.Xantheas SS, Dunning TH., Jr J Chem Phys. 1993;98:8037–8040. [Google Scholar]
- 17.Sediki A, Lebsir F, Martiny L, Dauchez M, Krallafa A. Food Chem. 2008;106:1476–1484. [Google Scholar]
- 18.Zhao Y, Truhlar DG. Theor Chem Acc. 2008;120:215–241. [Google Scholar]
- 19.Dahlke E, Olson RM, Leverentz HR, Truhlar DG. J Phys Chem A. 2008;112:3976–3984. doi: 10.1021/jp077376k. [DOI] [PubMed] [Google Scholar]
- 20.(a) Zhao Y, Truhlar DG. J Phys Chem A. 2005;109:4209–4212. doi: 10.1021/jp050932v. [DOI] [PubMed] [Google Scholar]; (b) Zhao Y, Truhlar DG. Phys Chem Chem Phys. 2005;7:2701–2705. doi: 10.1039/b507036h. [DOI] [PubMed] [Google Scholar]; (c) Wong BM. J Comput Chem. 2009;30:51–56. doi: 10.1002/jcc.21022. [DOI] [PubMed] [Google Scholar]
- 21.Kodandapani MR, Vijayan M. Acta Cryst. 1993;D49:234–245. doi: 10.1107/S090744499200653X. [DOI] [PubMed] [Google Scholar]
- 22.(a) Reichmann D, Phillip Y, Carmi A, Schreiber G. Biochemistry. 2008;47:1051–1060. doi: 10.1021/bi7019639. [DOI] [PubMed] [Google Scholar]; (b) Raschke TM. Curr Opin Struct Biol. 2006;16:152–159. doi: 10.1016/j.sbi.2006.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.