Abstract
Mesenchymal stem cells (MSCs) represent a promising new approach to the treatment of several diseases that are associated with dismal outcomes. These include myocardial damage, graft versus host disease, and possibly cancer. Although the potential therapeutic aspects of MSCs continue to be well-researched, the possible hazards of MSCs, and in particular their oncogenic capacity are poorly understood. This review addresses the oncogenic and tumor-supporting potential of MSCs within the context of cancer treatment. The risk for malignant transformation is discussed for each stage of the clinical lifecycle of MSCs. This includes malignant transformation in vitro during production phases, during insertion of potentially therapeutic transgenes, and finally in vivo via interactions with tumor stroma. The immunosuppressive qualities of MSCs, which may facilitate evasion of the immune system by a tumor, are also addressed. Limitations of the methods employed in clinical trials to date are reviewed, including the absence of long term follow-up and lack of adequate screening methods to detect formation of new tumors. Through discussions of the possible oncogenic and tumor-supporting mechanisms of MSCs, directions for future research are identified which may eventually facilitate the future clinical translation of MSCs for the treatment of cancer and other diseases.
Keywords: mesenchymal stem cells, stem cell transplantation, oncogenesis
INTRODUCTION
Mesenchymal stem cells (MSCs) have received considerable attention in recent years for several potential therapeutic applications, including myocardial tissue repair, prevention of graft versus host disease, and the treatment of cancer. While the therapeutic promise of MSCs has been reiterated time and again in multiple reviews, the potential hazards of their use are infrequently addressed. This review considers the oncogenic potential of MSCs, with a focus on the use of MSCs to treat cancer. Although almost all animal studies that have employed genetically-modified MSCs for the treatment of cancer have shown therapeutic effects [1], an understanding of oncogenic mechanisms that may give rise to cancers in humans over an extended time frame will be necessary to facilitate the clinical translation of MSCs. The approach in this review is to describe how MSCs may undergo malignant transformation at each phase of their clinical lifecycle, from initial isolation, to expansion in culture, transfection with therapeutic transgenes, and finally, administration to patients. Additionally, the immunosuppressive properties of MSCs, which may promote evasion of the immune system by tumors, are also addressed. By identifying these potential oncogenic and tumor-enhancing mechanisms, we define areas for further research which we hope will facilitate the translation of MSCs to clinical use for the treatment of cancer.
DEFINITION OF A MESENCHYMAL STEM CELL
MSCs are classically defined by the initial experimental protocols that were used to isolate them from bone marrow [2,3]. Bone marrow aspirates can be dissociated into a suspension containing hematopoietic stem cells and marrow stromal cells. The hematopoietic stem cells give rise to erythroid, lymphoid, and myeloid progenitors; whereas the marrow stromal cells support hematopoiesis, comprise the structural matrix of bone marrow, and are capable of differentiating into the osteogenic, chondrogenic, and adipogenic lineages. When cultured, only the marrow stromal cells adhere to the flask, and it is possible to separate these cells by repeatedly changing the media. These adherent cells were termed colony-forming unit fibroblasts due to their fibroblast-like morphology and the propensity to form colonies in culture [3]. They are now referred to as mesenchymal stem cells or marrow stromal cells. Their characteristic morphology is shown in Fig. (1).
Figure 1.
Human mesenchymal stem cells at first passage display characteristic morphology: large nuclei (black arrows), prominent round nucleoli (white arrow), and long thin processes (arrowheads).
The immunophenotypic definition of what constitutes an MSC has only recently been standardized. The minimal criteria that define an MSC, as set forth in 2006 by the International Society of Cellular Therapy (ISCT) are:
Plastic-adherence in standard culture conditions;
Expression of the mesenchyme markers CD105, CD73 and CD90, and no expression of markers of contaminating endothelial, hematopoietic, or immunological cells (CD45, CD34, CD14 or CD11b, CD79α, or CD19 and HLA-DR surface markers); and
The ability to differentiate into osteoblasts, adipocytes and chondroblasts [4,5].
There is no one marker that is specific for MSCs, and combinations of markers must be specified to distinguish cell types with behavior characteristic of MSCs. Although the ISCT definition will help to standardize future research, most of the existing studies published since the initial isolation of MSCs in 1970 have used inconsistent defining characteristics of MSCs, which may in part explain the high prevalence of conflicting experimental results. This inconsistency could call into question the validity of many of the experiments that have used these cells, and at worst, may influence the results of clinical trials [6].
IN VITRO MALIGNANT TRANSFORMATION
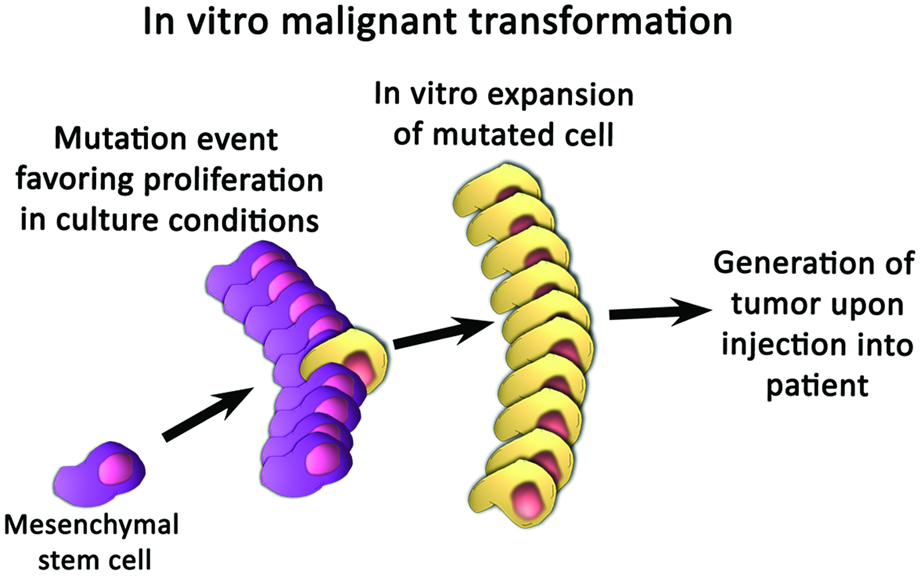
Considerable in vitro expansion is often necessary to achieve adequate numbers of MSCs for therapeutic purposes [7–9]. This in vitro expansion phase is the first point at which MSCs become susceptible to malignant transformation, as shown in Fig. (2a). Rubio et al. showed that human adipose-derived MSCs undergo spontaneous transformation after 4–5 months of culture, through the sequential upregulation of c-myc and downregulation of p16, although this phenomenon was not observed after only 6 to 8 weeks in culture [10]. Wang et al. noted that the in vitro culture of human bone marrow-derived MSCs produces a sub-population of cells with high levels of telomerase activity, chromosomal aneuploidy, and translocations, that are capable of forming tumors in multiple organs in NOD/SCID mice [11]. These findings were not reproduced in a subsequent study, in which chromosomal abnormalities were absent, and normal telomere shortening was observed, in human bone marrow-derived MSCs that were propagated to senescence or 25 passages [12]. Because the results of such experiments conflict with one another, and because available research on in vitro characteristics of MSCs is limited, the possibility of malignant transformation in vitro remains highly controversial. Future studies which employ standardized isolation protocols for MSCs will therefore be needed to elucidate the poorly understood potential for malignant transformation during the in vitro expansion phase [13].
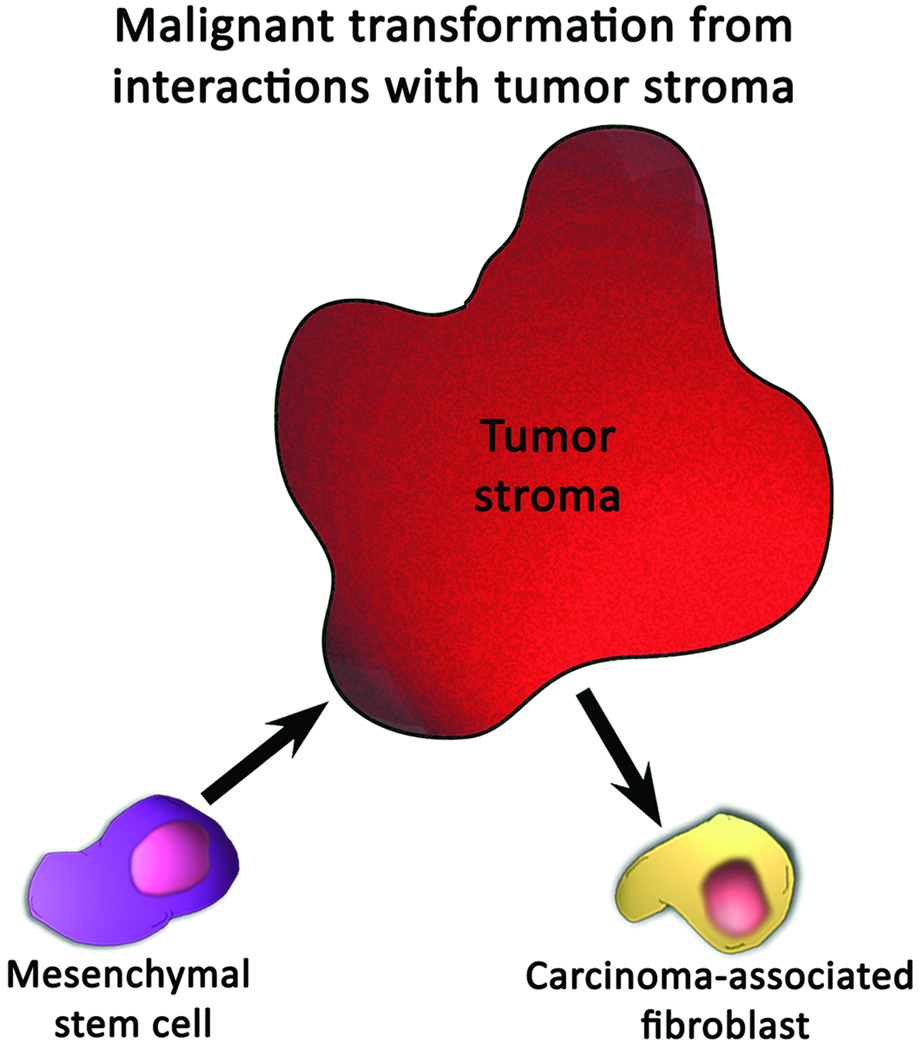
Figure 2.
Mechanisms by which mesenchymal stem cells may undergo carcinogenic transformation. (A) Transformation may occur by the outgrowth of a sub-population of cells that proliferates more favorably in culture conditions. (B) Interactions between tumor stroma and mesenchymal stem cells may cause mesenchymal stem cells to differentiate into carcinoma-associated fibroblasts.
MESENCHYMAL STEM CELLS MIGRATE TO TUMORS
In vivo experimental studies on the migration of MSCs are summarized in Table 1 and Table 2. These tables include several supporting clinical studies that have rigorously tracked the distribution of MSCs administered to patients. Taken together, these studies demonstrate two important in vivo characteristics of MSCs: MSCs migrate toward tumors, but this migration is non-specific [1,13]. The migratory tropism toward tumors has been observed when MSCs are administered by intravenous [14], intraarterial [15], or peritumoral routes [16]. The mechanism of migration is poorly understood, but has been shown to be dependent upon the cytokine/receptor pairs SDF-1/CXCR4 [15,17,18], SCF-c-Kit [19,20], HGF/c-Met [21], VEGF/VEGFR [22], PDGF/PDGFr [15], MCP-1/CCR2 [23], and HMGB1/RAGE [24,25], as well as cellular adhesion molecules [18,26,27]. Migration to tumors, however, is non-specific as exogenously administered MSCs have also been shown to localize to the lung [14,28–32], bone marrow [29,30,33,34], and lymphoid organs [35,36]; and prior whole body irradiation tends to expand the distribution of MSCs in the body to multiple organs [28,30]. Additionally, MSCs appear to migrate to sites of localized chronic inflammation [35,37], which may in part explain the observation that MSCs are recruited in the process of wound repair [38–42]. Although MSCs have been shown to enhance metastatic potential in an animal model of breast carcinoma [43], there have been no reports of tumor seeding by MSCs in normal, non-cancerous tissue. These interactions between MSCs and normal, noncancerous tissue, however, are probably very different from the interactions of MSCs with abnormal, neoplastic tissue. These interactions between MSCs and cancerous tissue have been researched more thoroughly, and are addressed in subsequent sections of this review.
Table 1. In vivo studies of unmodified mesenchymal stem cell migration.
Additional studies on the migration of other cell types, such as neural stem cells, are reviewed elsewhere [1].
| CITATION | HOST SPECIES |
PRECONDITIONING OR PRE-EXISTING CONDITION |
IMPLANTED CANCER CELL DESCRIPTION |
IMPLANTED MSC DESCRIPTION |
MSC INJECTION ROUTE |
MAIN RESULTS / MSC DISTRIBUTION PATTERNS |
|---|---|---|---|---|---|---|
| Pereira et al. 1998 [30] |
Mouse | Osteogenesis imperfecta animal model; lethal or sublethal irradiation |
None | Murine BM-MSC | IV | MSCs migrated primarily bone marrow, cartilage, lung, but also in spleen, brain, skin. |
| Maestroni et al. 1999 [169] |
Mouse | None | Lewis lung carcinoma, B16 melanoma |
Bone marrow cells, unspecified source | IM | Inhibition of tumor growth and number of metastases. |
| Koç et al. 2000 [170] |
Human | Patients underwent myeloablative therapy and then received hematopoietic stem cell graft |
Pre-existing breast cancer |
Human BM-MSC | IV | MSCs detected in blood up to 1 hour after infusion. Revived neutrophil and platelet count in 8 days. |
| Gao et al. 2001 [29] | Rat | Either sodium Nitroprusside administration or none. |
None | Rat BM-MSC | IA, IV, IP | Migration to lungs, liver, bone marrow. With nitroprusside, high levels in liver and bone marrow. |
| Le Blanc et al. 2004 [36] |
Human | Patient had grade IV acute graft-versus-host disease |
None | Human BM-MSC | IV | Migration to colon and lymph node. Recovery after 1 year. |
| Houghton et al. 2004 [37] |
Mouse | Chronic gastrointestinal inflammation from Helicobacter infection |
None | Endogenous MSCs were studied | N/A | Repopulation of the stomach with MSCs, which progress through metaplasia, dysplasia, and cancer. |
| Khakoo et al. 2006 [171] |
ID Mouse | None | Kaposi’s sarcoma animal model |
Human BM-MSCs | IV | Inhibition of tumor growth. |
| Zhu et al. 2006 [46] |
ID Mouse | None | F6 and SW480 colon cancer |
Fetal and adult human BM-MSC |
SQ | Higher tumor incidence in MSC-treated groups, with elevated proliferation, angiogenesis, and metastatic ability of cancer cells. |
| Ramasamy et al. 2007[34] |
NOD/ SCID Mouse |
None | BV173 chronic myeloid leukemia |
Human BM-MSC co- injected with tumor cells |
SQ | Migration to bone marrow. Higher incidence of tumor formation in MSC- treated groups. Lower rates of cancer cell apoptosis. |
| Karnoub et al. 2007 [43] |
NOD/ SCID Mouse |
None | MCF7/Ras, MDA-MB-231, MDA-MB-435, and HMLER breast cancer |
Human BM-MSC co- injected with tumor cells |
SQ | Enhanced motility, invasion, and metastasis of cancer cells. |
Abbreviations:AMSC = Adipose tissue-derived mesenchymal stem cell; BM-MSC = Bone marrow-derived mesenchymal stem cell; CT = Contralateral to tumor; IA = Intra-arterial; ID = Immunodeficient; IFN = Interferon; IL = Interleukin; IM = Intramuscular; IP = Intraperitoneal; IT = Intratumoral; IV = Intravenous; MFP = Mammary fat pad; MSC = Mesenchymal stem cell; neoR = Neomycin phosphotransferase gene; PT = Peritumoral; SCID = Severe combined immunodeficient; SQ = Subcutaneous; TRAIL = Tumor necrosis factor-related, apoptosis-inducing ligand; tsFlk-1 = Truncated soluble vascular endothelial growth factor receptor gene.
Table 2. In vivo studies of mesenchymal stem cells genetically modified by incorporation of a transgene.
Abbreviations are the same as for Table 1.
| CITATION | HOST SPECIES |
PRECONDITIONING OR PRE-EXISTING CONDITION |
IMPLANTED CANCER CELL DESCRIPTION |
IMPLANTED MSC DESCRIPTION |
MSC TRANSGENE |
MSC INJECTION ROUTE |
MAIN RESULTS / MSC DISTRIBUTION PATTERNS |
|---|---|---|---|---|---|---|---|
| Studeny et al. 2002 [172] |
ID Mouse | None | A375SM melanoma lung metastasis |
Human BM-MSC | IFN β | SQ, IV | Extended survival in tumor implanted animals treated with IV IFN β-secreting MSCs. |
| Horwitz et al. 2002 [33] |
Human | Prior bone marrow transplantation |
None | Human BM-MSC | neoR | IV | Migration to bone marrow, bone, skin. Accelerated growth velocity 6 months following MSC transplant. |
| Devine et al. 2003 [28] |
Baboon | Lethal irradiation + hematopoietic stem cells; or no conditioning |
None | Baboon BM-MSC | GFP | IV | Migration to gastrointestinal tract, kidney, lung, liver, thymus, and skin. Nonconditioned animal had less abundant engraftment. |
| Studeny et al. 2004 [173] |
SCID mouse | None | MDA231 breast cancer, A375SM pulmonary metastasis |
Human BM-MSC | IFN β | IV | Extended survival. |
| Nakamura et al. 2004 [174] |
Rat | None | 9L glioma | Rat BM-MSC | Il-2 | CT, IT | Migration toward tumor. Extended survival, tumor volume reduction. |
| Nakamizo et al. 2005 [15] |
ID Mouse | None | U87, U251, LN229 glioma implantation |
Human BM-MSC | IFN β | IA, IT | Migration toward tumor. Extended survival in U87 implanted animals treated with IFN β-secreting MSCs. |
| Zappia et al. 2005 [35] |
Mouse | Autoimmune encephalomyelitis animal model |
None | Murine BM-MSC | Enhanced GFP | IV | Migration to lymphoid organs, subarachnoid space. Decreased inflammatory infiltrates and demyelination in mice treated with MSCs. |
| Elzaouk et al. 2006 [175] |
Mouse | None | B16F10 local melanoma, and melanoma lung metastasis |
Human MSCs, unspecified source |
Rat IL-12 | IP, IM, IT | Decreased tumor volume and metastasis seeding. |
| Komarova et al. 2006 [176] |
SCID Mouse | None | SKOV 3 ovarian carcinoma |
Human BM-MSC | Ad5/3 | IP | Enhanced survival. |
| Djouad et al. 2006 [44] |
Mouse | None | Renca adenocarcinoma pulmonary metastasis, B16 melanoma, animal model |
Murine MSC | Luciferase | IV | No effect on tumor volume, but earlier onset of tumors in MSC treated groups. |
| Kyriakou et al. 2006 [177] |
ID Mouse | None | Raji Burkitt lymphoma |
Human BM-MSC | tsFlk-1 | SQ | Inhibition of tumor growth |
| Kucerova et al. 2007 [32] |
ID Mouse | None | HT-29 colon adenocarcinoma |
Human AMSC | Cytosine deaminase |
SQ, IV | Migration to lungs and liver, but cleared at 20 days. Inhibition of tumor growth. |
| Miletic et al. 2007 [178] |
Rat | None | 9L glioma | Rat BM-MSC | Thymidine kinase, eGFP |
IT | Extended survival. |
| Hong et al. 2009 [16] |
Mouse | None | Ast11.9-2 glioma | Murine BM-MSC | Murine IL-12 | PT | Migration toward tumor. Extended survival. |
| Duan et al. 2009 [31] |
Mouse | None | TC71 Ewing sarcoma tumors |
Murine BM-MSC | IL-12 | IV | Migration toward tumor, lung, liver, and spleen. Inhibition of tumor growth. |
| Yang et al. 2009 [14] |
ID Mouse | None | U87 glioma | Human BM-MSC | TRAIL | IV | Migration to tumor and kidney, with lower levels in liver, lung, spleen. Extended survival in MSC-TRAIL groups. |
| Sasportas et al. 2009 [168] |
ID Mouse | None | GBM8 CD133+ human glioma |
Human BM-MSC | TRAIL, luciferase |
PT | Migration toward tumor. Extended survival in MSC-TRAIL groups. |
IMMUNOSUPPRESSION BY MESENCHYMAL STEM CELLS MAY FAVOR TUMOR GROWTH
In several animal tumor models, including melanoma [44,45], colon adenocarcinoma [46], multiple myeloma [47], lung cancer [48], and glioblastoma [48], the presence of exogenous MSCs was shown to enhance tumor formation. Such studies provide indirect evidence that there may be a cancer-promoting interaction between MSCs and tumors. One potential mechanism underlying these observations is immunosuppression. MSCs exert an immunosuppressive effect by interacting with almost all cells of the innate and adaptive immune systems, and these interactions may enhance the ability of some tumors to evade immune surveillance [13].
In the adaptive immune system, MSCs interact with both T-cells and B-cells. The effects of MSCs upon T cells are two-fold. First, MSCs tend to support the survival of T cells that are in a quiescent state. T cells that are exposed to MSCs are arrested at the G1 phase of the cell cycle, in a state that resembles division arrest anergy [49]. This process may be dependent on inhibition of cyclin D2 and the upregulation of CDKN1B [49]. Furthermore, MSCs protect unstimulated T cells from activation induced cell death through downregulation of the Fas ligand and receptor [50].
Second, MSCs suppress proliferation of T-cells that are in an activated state. This T cell suppression occurs by one of three mechanisms. The first mechanism involves the concerted action of IFN γ with one of 3 pro-inflammatory cytokines: TNF α, IL-1α, or IL-1β [51]. IFN γ causes T cells to produce the enzyme indoleamine 2–3 dioxygenase (IDO) [51–53]. IDO is important because it depletes the essential amino acid tryptophan, which is required for lymphocyte proliferation. The pro-inflammatory cytokines TNF α, IL-1α, and IL-1, cause MSCs to secrete iNOS and the chemokines CXCL-9 and CXCL-10 [51]. The elevated expression of iNOS results in high local levels of nitric oxide, which inhibits T lymphocytes in part by suppressing STAT-5 phosphorylation [54]. The chemokines attract T cells into proximity with MSCs [51]. These T lymphocytes which have migrated to a microenvironment that includes high levels of IDO and nitric oxide, then become suppressed. Therefore, MSCs suppress the function of T cells via IFN γ and pro-inflammatory cytokines. The second mechanism of T cell suppression by MSCs involves the non-classical human leukocyte antigen (HLA) class I molecule HLA-G5, which has been shown to suppress T-cell proliferation and increase production of T regulatory cells [55–57]. When MSCs make physical contact with stimulated lymphocytes, they are capable of secreting HLA-G5 in an IL-10-dependent manner [56]. Finally, T cells are indirectly suppressed by the actions of MSCs on dendritic cells (DCs), which are described below. When T cells are suppressed by MSCs, they shift to an anti-inflammatory state. This anti-inflammatory state is characterized by decreased IFN γ production by TH1 cells [57], decreased production of IL-4 production by TH2 cells [57], and diminished production of TNF α [35]. Therefore, MSCs suppress T cells through the actions of IFN γ, HLA-G5, or the suppression of DCs, causing a shift to an anti-inflammatory state.
Direct suppression of B cells by MSCs may occur to a limited extent [58]. As with T cell suppression, a mechanism involving arrest at the G0/G1 phase appears to be involved in B cell suppression, which impairs production of IgM, IgG, and IgA [59]. Cell surface interactions, including the engagement of PD-1 receptor, are also necessary for this effect [60]. Direct suppression of B cells by MSCs remains controversial, as other studies have demonstrated conflicting results [52]. Most likely, the dominant mechanism for the suppression of B cells is indirectly through suppression of T-cells.
MSCs suppress the innate immune system primarily through their effects on dendritic cells (DCs). Dendritic cells process antigenic material, mature, and then function as antigen presenting cells to naive T lymphocytes. MSCs inhibit three critical functions of dendritic cells: maturation, antigen presentation, and secretion of pro-inflammatory compounds. MSCs inhibit the maturation of DCs, or more specifically, the commitment of CD 34+ cell-derived [61] and monocyte-derived precursors [61–64] to differentiate into DCs. This inhibition occurs by blocking DC precursors from entering the G1 cell cycle phase, and through the downregulation of cyclin D2 [62]. Furthermore, activation of the Notch signaling pathway appears to be involved, as the administration of inhibitors of Notch signaling to cocultures of MSCs and DC precursors reverses the effects of MSCs on DC cell maturation [65]. MSCs also inhibit antigen presenting functions by DCs [62]. Co-culture of MSCs with DCs or DC precursors results in diminished levels of cell surface molecules associated with antigen presentation, including MHC class II, CD1a, CD40, and CD 86 [63,64]. Finally, MSCs suppress DC cell function by inhibiting their secretion of the pro-inflammatory compounds TNF α and IL-10 [57]. Therefore MSCs interact with dendritic cells to inhibit their maturation, antigen presentation, and secretion of pro-inflammatory compounds.
MSCs further suppress the innate immune system by acting on NK cells and neutrophils. The interactions between MSCs and NK cells represent an example of reciprocal inhibition. The activation of NK cells is highly dependent on cell surface receptors. These cell surface receptors allow NK cells to recognize target cells and are required for NK cell-mediated lysis to occur. The cell surface receptors NKp30, NKG2D, DNAM-1, and LFA1, which are present on the surface of NK cells, are activated by ligands that are present on the surface of MSCs: ULBP, PVR, Nectin-2, and ICAM-1, respectively [66,67]. Through their interactions with these cell surface receptors, activated NK cells are able to lyse autologous and allogeneic MSCs [66,68]. However, MSCs exert an opposing effect by down-regulating the expression of NKp30 and NKG2D, ultimately inhibiting the cytotoxic activity of NK cells, cytokine production, and proliferation [68,69]. Furthermore, the HLA-G5 and IDO systems discussed previously also appear to inhibit NK cells [56,69]. MSCs act on both resting and activated neutrophils by dampening the respiratory burst [70]. However, MSCs also inhibit apoptosis of neutrophils through the IL-6 induced upregulation of STAT-3, and do not impair phagocytosis, or expression of adhesion molecules on neutrophils.[70] These effects on the immune system may contribute in part to tumor-enhancing properties of MSCs. However, a second factor—the interaction between MSCs and tumor stroma—likely plays a siginificant role. These interactions are discussed next.
MALIGNANT TRANSFORMATION FROM INTERACTIONS WITH TUMOR STROMA
A second mechanism that accounts for enhancement of tumor formation by MSCs is a possible transforming effect exerted by tumor stromal cells upon mesenchymal stem cells [71]. Tumors are comprised of a heterotypic array of malignant cells in communication with stromal cells. This tumor stroma is composed of endothelial cells, immune cells, and fibroblasts, which are thought to support the neoplastic properties of cancer cells [72–77]. Several studies suggest that MSCs could be a source of one type of stromal cell in breast cancer—the carcinoma-associated fibroblast (CAF), as shown in Fig. (2b) [71,74,78]. CAFs are a mixed population of myofibroblasts and activated fibroblasts which have been found to support growth of cells and angiogenesis in breast cancer [79]. Notably MSCs can assume a CAF-like phenotype after prolonged exposure to tumor-conditioned media [78]. These CAF-like MSCs possess a phenotype similar to CAFs, enhance tumor growth both in vivo and in vitro, and demonstrate sustained expression of the stromal-derived factor-1 (SDF-1) and the myofibroblast marker α-smooth muscle actin (α-SMA) [78,79]. Additionally, MSCs have also been shown to adopt a myofibroblast phenotype in response to transforming growth factor-β (TGF-β) [78,80]. Taken together, these observations have led some authors to believe that MSCs exposed to the local environment of a tumor could differentiate into CAFs and may therefore enhance tumor growth or spread [71].
Further understanding of such interactions between MSCs and tumor stromal cells will require more refined animal models than are currently available [81]. Standard xenograft models are of limited use in studying the interactions between MSCs and tumor stroma because they fail to fully capture the complexity of the tumor stroma as it occurs in patients [82,83]. Most animal tumor models are created by first culturing human cancer cells or cancer stem cells in vitro and engrafting the cells into an animal. During the in vitro culturing phase, cells lines adapt to and proliferate in culture conditions independent of contact with stromal and epithelial cells, and this process may eventually result in cells with a phenotype that differs markedly from that found in patients [82]. Once these cells are engrafted into an animal, the stroma that forms is derived from the animal’s own cells, resulting in a chimeric tumor that does not precisely represent the human condition. Because human cellular signaling pathways differ from those of mice [84] and other animals, these chimeric tumors most likely do not reproduce the interactions that would occur between human MSCs and human tumor stroma. Animal models that more accurately recapitulate the tumor stromal characteristics observed in patients, and that are capable of engrafting human cell types that comprise the tumor stroma, are therefore required for definitive studies on the interactions of MSCs with tumor cells, and in particular, the stromal component. The new generations of immunocompromised mice, such as the NOG (NOD/Shi-scid/IL-2Rγnull) mouse, which accept heterologous cell populations more readily than wildtype animals, represent one new advancement in this direction [85].
MIGRATION AND TUMOR FORMATION: PARALLELS WITH THE BRAIN TUMOR STEM CELL HYPOTHESIS
The notion that stem cells can migrate toward a tumor, develop into a malignant phenotype, and subsequently contribute to the tumor mass, parallels a broader line of reasoning which has been proposed to explain the origins of brain cancer. This line of reasoning is termed the brain tumor stem cell (BTSC) hypothesis [86–89]. The BTSC hypothesis proposes that oncogenic alterations in neural stem cells (NSCs), or NSC-like cells, may give rise to brain tumors [90]. Therefore, brain cancers represent clonally-derived tissues that arise from a single abnormal stem cell. The BTSC hypothesis arose from observations that normal, non-cancerous neurogenesis continues to take place in the adult brain [90–96]. These mechanisms of neurogenesis in the adult brain rely on NSCs which reside in 3 different niches—the subventricular zone of the lateral ventricles (SVZ), the subgranular layer of the hippocampal dentate gyrus, and the olfactory bulbs [97–99]. From these niches, NSCs most likely migrate into the brain parenchyma [100], and serve as precursors for new neurons, oligodendrocytes, and astrocytes. The BTSC hypothesis proposes that these normal mechanisms of neural development that stem from normal NSCs parallel the abnormal oncogenesis that results from abnormal NSCs.
The BTSC further proposes that the development of a brain tumor parallels normal adult neurogenesis by recruiting NSCs to the site of the tumor. In support of this claim is the observation that NSCs show extensive migratory tropism to brain tumors, to the extent that they can even be modified genetically to serve as gene delivery vehicles for the treatment of cancer [1,101]. Furthermore, the proximity of brain tumors to germinal areas such as the SVZ affects their clinical characteristics, suggesting that cells in these niches may affect the process of tumor formation [102,103]. In a recent series, Lim et al. found that 56% of GBM tumors in contact with the SVZ and cortex were multifocal, but tumors not in contact with the SVZ or cortex were not multifocal [102]. Furthermore, patients with GBM tumors that are not in close proximity to germinal zones survive 3 months longer from the time of diagnosis, than patients with tumors in close proximity to germinal zones [103]. These findings support the hypothesis that brain tumors recruit stem cells from germinal zones, such as the SVZ, which migrate to the tumor, and contribute to tumor formation.
We speculate that there are several parallels that remain to be explored between NSC and MSC migratory characteristics within the context of brain cancer. NSCs are recruited to a neural niche in gliomas, where they contribute to tumor formation. Similarly, it is possible that MSCs may be recruited to the stromal portion of tumors, which could serve as a mesenchymal niche, and thus contribute to tumor formation. As previously discussed, this stromal portion could favor malignant transdifferentiation of MSCs, which would in turn account for the oncogenic properties of MSCs documented in some studies [44–48]. Additional research is necessary to determine if any such parallels exist between the migratory characteristics of NSCs and MSCs.
MALIGNANT TRANSFORMATION FROM INCORPORATION OF TRANSGENES
The observation that MSCs migrate toward tumors has prompted a new approach to treating cancer in which genetically-modified MSCs serve as tumor-selective gene delivery vehicles (Table 2). The basic approach, which has only been employed in animal models thus far, calls for the harvest of MSCs, modification of the MSCs such that they secrete an anti-neoplastic compound, and finally administration of the MSCs into an animal possessing a tumor. Stem cells have already been genetically modified to secrete 11 different compounds with varying success in prolonging survival and reducing tumor mass (reviewed in Aboody et al. [1]). Although this approach to treating cancer holds tremendous promise, the genetic modification of MSCs—or any cell type which is to be administered to patients—may also be hazardous.
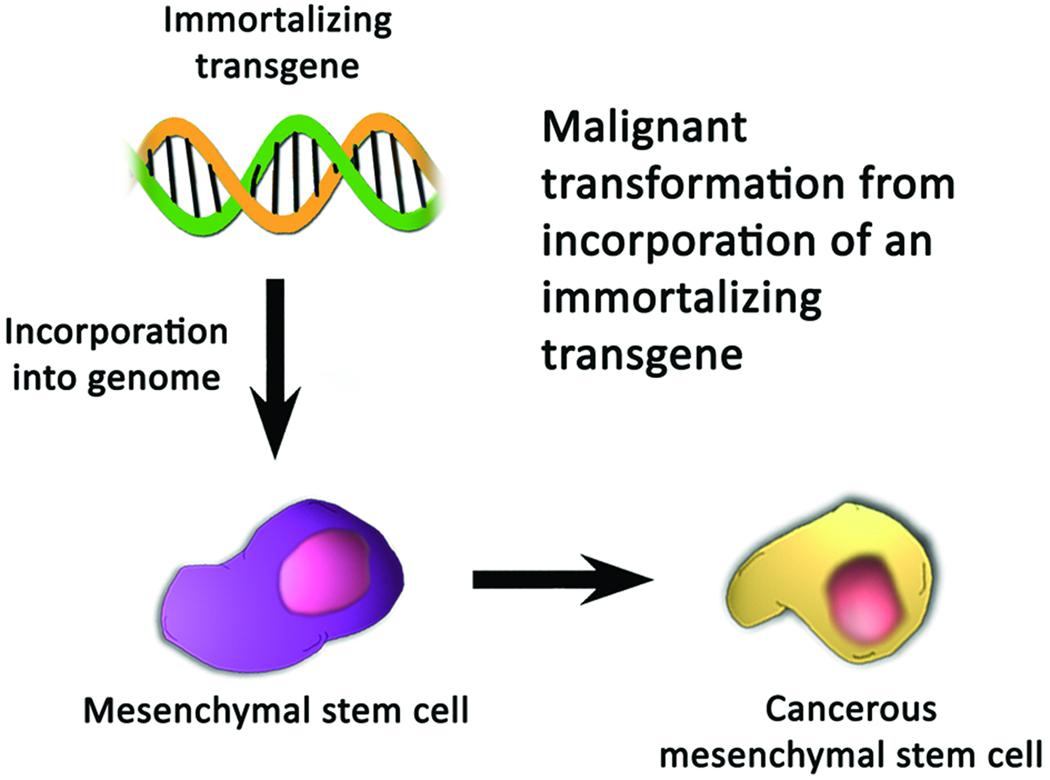
The possible dangers of transgenic cell therapy stem from one of two possibilities: that either the transgene is tumorigenic, or that its insertion disrupts a genomic locus that is critical for tumor suppression, as shown in Fig. (3). The tumorigenicity of a transgene is of concern within the context of using genetic modification to immortalize stem cell lines. Incorporation of an immortalizing transgene may be necessary for therapeutic application of MSCs because MSCs have traditionally been derived from small tissue samples (usually bone marrow) that must be expanded to billions of cells in order to reach therapeutic levels. Significant expansion of an unmodified cell line, however, is difficult since MSCs become senescent after several passages [104]. Therefore the incorporation of immortalizing genes has been explored as an approach to maintain cell lines indefinitely [1]. Immortalizing transgenes include the proto-oncogene v-myc [101,105–110], human telomerase reverse transcriptase (hTERT) [111–118], human papillomavirus type 16 E6/E7 [119–122], Bmi-1 [123–129], and the N-terminal fragment of SV40 large T-antigen [1,130–133]. Notably, the potential tumorigenic capacity of these immortalized cells lines is not well-studied, and it is unknown whether loss of normal cell-cycle checkpoint controls, karyotypic instability, or other undesired changes may occur after transfection [1,134]. Although the proliferative characteristics of hTERT are well documented, aberrant karyotypic structures can be detected at early passages [135,136]. Perhaps the best hope for circumventing the uncertainties of immortalized cell lines is to harvest MSCs from more plentiful sources of tissue, such as umbilical cord blood or adipose tissue, which, by virtue of sheer volume at the time of harvest, can provide sufficient numbers of cells within a few passages. Notably, adipose-derived MSCs are similar to those isolated from bone marrow in terms of morphology, the success rate of isolating MSCs, expansion potential, differentiation capacity, and immunophenotype [137,138], suggesting that they may be an ideal alternative source of MSCs.
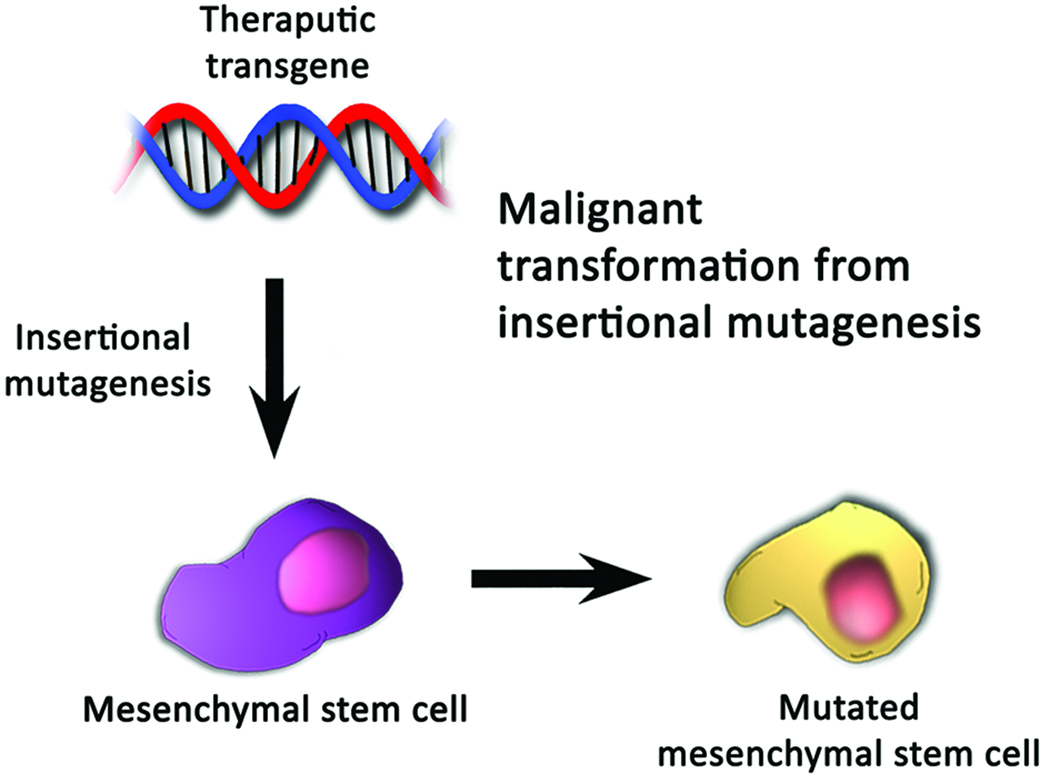
Figure 3.
Mechanisms by which mesenchymal stem cells may become oncogenic after incorporation of a transgene. (A) Incorporation of an immortalizing transgene may result in the loss of normal cell-cycle checkpoints, karyotypic instability, or other changes which cause uninhibited proliferation of cells. (B) Insertional mutagenesis may disrupt a critical regulatory locus, resulting in dysfunction of the normal mechanisms of tumor suppression.
A second potentially harmful aspect of transgenic cell therapy is the possibility of insertional mutagenesis. The term insertional mutagenesis refers to the insertion of an otherwise non-cancerous transgene at a critical genetic locus, resulting in dysregulation of the normal mechanisms of tumor suppression, and ultimately, oncogenic consequences [139,140]. The dangers of insertional mutagenesis were highlighted in a French clinical trial for severe combined immunodeficiency that employed uncloned autologous hematopoietic stem cells transduced with a retrovirus encoding the IL2RG gene [141–143]. The exogenous nucleotide sequences were found adjacent to loci encoding LMO2, LYL1, c-Jun, Bmi1, and CCND2, upregulating the expression of these genes, which in turn transformed T cells [142,144]. Four of the 10 patients in the trial developed a T-cell acute lymphoblastic leukemia-like disease [144–148]. Such devastating outcomes suggest that in transgenic cell-based therapies, either well-characterized cell lines must be used, or robust methods for characterizing the potential insertion sites must be developed, to lower the potential for insertional mutagenesis. This potential for insertional mutagenesis during the genetic modification of MSCs will be both difficult and costly to overcome, and may very well remain one of the greatest limiting factors in the translation of MSCs as gene-delivery vehicles.
LIMITATIONS OF EVIDENCE FROM CLINICAL TRIALS
Available results from clincial trials do not represent the optimal source of evidence on the potential tumorigenic capacity of MSCs, as these trials rarely focused on parameters which are relevant for assessing the development of new cancer. Twenty four clinical trials employing MSCs have been published to date [149–162]. Most of these trials have evaluated the safety and efficacy of MSCs within the context of non-cancerous conditions, such as myocardial damage [152,157,162,163] and prevention of graft versus host disease [36,153,164–166]. There have been no reports of neither acute nor long-term adverse events thus far. This includes no reports of carcinogenesis or major adverse events from allogeneic transplants. Although these clinical trials represent the best source of evidence for the efficacy of MSCs in the treatment of mostly non-cancerous disease, initial clinical trials of novel therapies, including those using MSCs, are usually insufficient to detect the carcinogenic potential of the agent that is being tested. The longest follow-up interval in published clinical trials employing MSCs is about 3 years [149]. As carcinogenesis is often a prolonged process, more rigorous and long-term follow-up will be required to adequately detect the formation (or prolongation) of cancer in patients treated with MSCs. A second limitation of existing trials of MSCs is that they have only been conducted in very ill patients with poor prognoses, which may obscure any possible harmful effects of MSCs. Third, clinical trials to date have failed to employ imaging modalities that are capable of detecting the presence of cancer in many locations in the body. Finally, genetically-modified MSCs have rarely been used in published clinical trials [33]. Although clinical trials to date have not revealed any evidence of carcinogenesis, their reliability in determining carcinogenic potential is limited by the factors described above.
The gold standard for establishing carcinogenic potential of an agent is the triad of epidemiological studies demonstrating association, animal studies demonstrating causality, and experimental studies demonstrating mechanism [167]. Although considerable advances have been made with regard to the potentially carcinogeneic mechanisms of MSCs, animal studies require much more refined cancer models, and epidemiological studies will require more refined clinical methods. As longer follow up intervals and patient enrollment are achieved in clinical studies, in combination with animal tumor models that recapitulate the stromal environment of human tumors, it may one day be possible to understand more completely the risks of MSC-based therapies.
CONCLUSION: ARE MESENCHYMAL STEM CELLS SAFE TO ADMINISTER TO PATIENTS?
To summarize, the overall impression that emerges from the published literature on MSCs is that they hold tremendous promise for the treatment of cancer as well as many other diseases. These claims of therapeutic efficacy have been substantiated in large part by the few clinical trials that have been conducted to date using MSCs in the treatment of myocardial damage [152,157,162,163] and graft versus host disease [36,153,164–166]. In addition, almost all animal studies that have employed genetically-modified MSCs for the treatment of cancer (Table 2) have demonstrated therapeutic—not harmful—effects. However, the oncogenic potential of MSCs remains a poorly explored, although tremendously important, component to realizing the full clinical potential of MSCs. This review summarized how MSCs may undergo malignant transformation in vitro during production phases, by interactions with tumor stroma in vivo, or through genetic modifications with transgenes. Furthermore, the limitations of existing clinical trials, and in particular, the need for adequate follow-up protocols to screen for the presence of developing cancers, were addressed. Rigorous evaluations of the oncogenic risk of MSCs are currently needed, as well as more thorough elucidation of the molecular mechanisms that underlie their documented biological properties. Both will help to define the scope of limitations of MSC-based therapies, and will underlie the development of future techniques that, it is hoped, can overcome these limitations and facilitate the translation of MSCs to clinical use. The tremendous therapeutic potential of MSCs for the treatment of cancer [1,14,16,31,168] heightens the urgency of such studies that can both define and overcome their potential dangers.
ACKNOWLEDGEMENTS
This work was supported by grants from the Maryland Stem Cell Foundation and NIH KO8 to AQH. The Doris Duke Charitable Foundation supplied a grant to the Johns Hopkins School of Medicine to fund ENM. The Howard Hughes Medical Institute has supported the work of HAZ and AQH. The authors have no other financial interests or conflicts of interest in the subject matter or materials discussed in the manuscript. We thank Pragathi Achanta, Tomas Garzon-Muvdi, and Hugo Guerrero-Cazares, for helpful edits in the preparation of the manuscript.
REFERENCES
- 1.Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15:739–752. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 3.Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 4.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 5.Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 6.Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 7.Pytlik R, Stehlik D, Soukup T, Kalbacova M, Rypacek F, Trc T, Mulinkova K, Michnova P, Kideryova L, Zivny J, Klener P, Jr, Vesela R, Trneny M, Klener P. The cultivation of human multipotent mesenchymal stromal cells in clinical grade medium for bone tissue engineering. Biomaterials. 2009;30:3415–3427. doi: 10.1016/j.biomaterials.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Sotiropoulou PA, Perez SA, Papamichail M. Clinical grade expansion of human bone marrow mesenchymal stem cells. Methods Mol Biol. 2007;407:245–263. doi: 10.1007/978-1-59745-536-7_17. [DOI] [PubMed] [Google Scholar]
- 9.Sensebe L. Clinical grade production of mesenchymal stem cells. Biomed Mater Eng. 2008;18:S3–S10. [PubMed] [Google Scholar]
- 10.Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Huso DL, Harrington J, Kellner J, Jeong DK, Turney J, McNiece IK. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy. 2005;7:509–519. doi: 10.1080/14653240500363216. [DOI] [PubMed] [Google Scholar]
- 12.Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, Zuffardi O, Locatelli F. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 13.Lazennec G, Jorgensen C. Concise review: adult multipotent stromal cells and cancer: risk or benefit? Stem Cells. 2008;26:1387–1394. doi: 10.1634/stemcells.2007-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang B, Wu X, Mao Y, Bao W, Gao L, Zhou P, Xie R, Zhou L, Zhu J. Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells. Neurosurgery. 2009;65:610–624. doi: 10.1227/01.NEU.0000350227.61132.A7. discussion 624. [DOI] [PubMed] [Google Scholar]
- 15.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 16.Hong X, Miller C, Savant-Bhonsale S, Kalkanis SN. Antitumor treatment using interleukin- 12-secreting marrow stromal cells in an invasive glioma model. Neurosurgery. 2009;64:1139–1146. doi: 10.1227/01.NEU.0000345646.85472.EA. discussion 1146–1137. [DOI] [PubMed] [Google Scholar]
- 17.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 19.Erlandsson A, Larsson J, Forsberg-Nilsson K. Stem cell factor is a chemoattractant and a survival factor for CNS stem cells. Exp Cell Res. 2004;301:201–210. doi: 10.1016/j.yexcr.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Sun L, Lee J, Fine HA. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J Clin Invest. 2004;113:1364–1374. doi: 10.1172/JCI20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heese O, Disko A, Zirkel D, Westphal M, Lamszus K. Neural stem cell migration toward gliomas in vitro. Neuro Oncol. 2005;7:476–484. doi: 10.1215/S1152851704000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt NO, Przylecki W, Yang W, Ziu M, Teng Y, Kim SU, Black PM, Aboody KS, Carroll RS. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005;7:623–629. doi: 10.1593/neo.04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Widera D, Holtkamp W, Entschladen F, Niggemann B, Zanker K, Kaltschmidt B, Kaltschmidt C. MCP-1 induces migration of adult neural stem cells. Eur J Cell Biol. 2004;83:381–387. doi: 10.1078/0171-9335-00403. [DOI] [PubMed] [Google Scholar]
- 24.Palumbo R, Bianchi ME. High mobility group box 1 protein, a cue for stem cell recruitment. Biochem Pharmacol. 2004;68:1165–1170. doi: 10.1016/j.bcp.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 25.Palumbo R, Galvez BG, Pusterla T, De Marchis F, Cossu G, Marcu KB, Bianchi ME. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J Cell Biol. 2007;179:33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 27.Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, Mills M, Wanzeck J, Janowska-Wieczorek A, Ratajczak MZ. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105:40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 28.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 29.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 30.Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan X, Guan H, Cao Y, Kleinerman ES. Murine bone marrow-derived mesenchymal stem cells as vehicles for interleukin-12 gene delivery into Ewing sarcoma tumors. Cancer. 2009;115:13–22. doi: 10.1002/cncr.24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007;67:6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- 33.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21:304–310. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 35.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 36.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 37.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 38.Fu X, Fang L, Li X, Cheng B, Sheng Z. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen. 2006;14:325–335. doi: 10.1111/j.1743-6109.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 40.Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, Lee JH. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26:1047–1055. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- 41.Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka F, Tominaga K, Ochi M, Tanigawa T, Watanabe T, Fujiwara Y, Ohta K, Oshitani N, Higuchi K, Arakawa T. Exogenous administration of mesenchymal stem cells ameliorates dextran sulfate sodium-induced colitis via anti-inflammatory action in damaged tissue in rats. Life Sci. 2008;83:771–779. doi: 10.1016/j.lfs.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 44.Djouad F, Bony C, Apparailly F, Louis-Plence P, Jorgensen C, Noel D. Earlier onset of syngeneic tumors in the presence of mesenchymal stem cells. Transplantation. 2006;82:1060–1066. doi: 10.1097/01.tp.0000236098.13804.0b. [DOI] [PubMed] [Google Scholar]
- 45.Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noel D, Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 46.Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, Cao W, Han C, Chen Y. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80:267–274. doi: 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 48.Yu JM, Jun ES, Bae YC, Jung JS. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 2008;17:463–473. doi: 10.1089/scd.2007.0181. [DOI] [PubMed] [Google Scholar]
- 49.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 50.Benvenuto F, Ferrari S, Gerdoni E, Gualandi F, Frassoni F, Pistoia V, Mancardi G, Uccelli A. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells. 2007;25:1753–1760. doi: 10.1634/stemcells.2007-0068. [DOI] [PubMed] [Google Scholar]
- 51.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 53.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 54.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 55.Maccario R, Podesta M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, Pozzi S, Frassoni F, Locatelli F. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- 56.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 57.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 58.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 59.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 60.Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 61.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 62.Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 63.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 64.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noel D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 65.Li YP, Paczesny S, Lauret E, Poirault S, Bordigoni P, Mekhloufi F, Hequet O, Bertrand Y, Ou-Yang JP, Stoltz JF, Miossec P, Eljaafari A. Human mesenchymal stem cells license adult CD34+ hemopoietic progenitor cells to differentiate into regulatory dendritic cells through activation of the Notch pathway. J Immunol. 2008;180:1598–1608. doi: 10.4049/jimmunol.180.3.1598. [DOI] [PubMed] [Google Scholar]
- 66.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 67.Poggi A, Prevosto C, Massaro AM, Negrini S, Urbani S, Pierri I, Saccardi R, Gobbi M, Zocchi MR. Interaction between human NK cells and bone marrow stromal cells induces NK cell triggering: role of NKp30 and NKG2D receptors. J Immunol. 2005;175:6352–6360. doi: 10.4049/jimmunol.175.10.6352. [DOI] [PubMed] [Google Scholar]
- 68.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 69.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 70.Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26:151–162. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 71.Mishra PJ, Mishra PJ, Glod JW, Banerjee D. Mesenchymal stem cells: flip side of the coin. Cancer Res. 2009;69:1255–1258. doi: 10.1158/0008-5472.CAN-08-3562. [DOI] [PubMed] [Google Scholar]
- 72.Tlsty TD. Stromal cells can contribute oncogenic signals. Semin Cancer Biol. 2001;11:97–104. doi: 10.1006/scbi.2000.0361. [DOI] [PubMed] [Google Scholar]
- 73.Tlsty TD, Hein PW. Know thy neighbor: stromal cells can contribute oncogenic signals. Curr Opin Genet Dev. 2001;11:54–59. doi: 10.1016/s0959-437x(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 74.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 75.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 76.Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res. 2001;264:169–184. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- 77.Moinfar F, Man YG, Arnould L, Bratthauer GL, Ratschek M, Tavassoli FA. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res. 2000;60:2562–2566. [PubMed] [Google Scholar]
- 78.Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 80.Wang D, Park JS, Chu JS, Krakowski A, Luo K, Chen DJ, Li S. Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor beta1 stimulation. J Biol Chem. 2004;279:43725–43734. doi: 10.1074/jbc.M407368200. [DOI] [PubMed] [Google Scholar]
- 81.Rangarajan A, Weinberg RA. Opinion: Comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer. 2003;3:952–959. doi: 10.1038/nrc1235. [DOI] [PubMed] [Google Scholar]
- 82.Kim JB, O'Hare MJ, Stein R. Models of breast cancer: is merging human and animal models the future? Breast Cancer Res. 2004;6:22–30. doi: 10.1186/bcr645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat Rev Cancer. 2002;2:331–341. doi: 10.1038/nrc795. [DOI] [PubMed] [Google Scholar]
- 85.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 86.Zaidi HA, Kosztowski T, DiMeco F, Quinones-Hinojosa A. Origins and clinical implications of the brain tumor stem cell hypothesis. J Neurooncol. 2009;93:49–60. doi: 10.1007/s11060-009-9856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 88.Piccirillo SG, Vescovi AL. Brain tumour stem cells: possibilities of new therapeutic strategies. Expert Opin Biol Ther. 2007;7:1129–1135. doi: 10.1517/14712598.7.8.1129. [DOI] [PubMed] [Google Scholar]
- 89.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 90.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 91.Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser RA, Goldman SA. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb Cortex. 1994;4:576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- 92.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pincus DW, Keyoung HM, Harrison-Restelli C, Goodman RR, Fraser RA, Edgar M, Sakakibara S, Okano H, Nedergaard M, Goldman SA. Fibroblast growth factor-2/brain-derived neurotrophic factor-associated maturation of new neurons generated from adult human subependymal cells. Ann Neurol. 1998;43:576–585. doi: 10.1002/ana.410430505. [DOI] [PubMed] [Google Scholar]
- 94.Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O'Brien TF, Kusakabe M, Steindler DA. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 95.Arsenijevic Y, Villemure JG, Brunet JF, Bloch JJ, Deglon N, Kostic C, Zurn A, Aebischer P. Isolation of multipotent neural precursors residing in the cortex of the adult human brain. Exp Neurol. 2001;170:48–62. doi: 10.1006/exnr.2001.7691. [DOI] [PubMed] [Google Scholar]
- 96.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 97.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 99.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 101.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, Berger MS. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007;9:424–429. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chaichana KL, McGirt MJ, Frazier J, Attenello F, Guerrero-Cazares H, Quinones-Hinojosa A. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol. 2008;89:219–224. doi: 10.1007/s11060-008-9609-2. [DOI] [PubMed] [Google Scholar]
- 104.Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 105.Kim SU. Genetically engineered human neural stem cells for brain repair in neurological diseases. Brain Dev. 2007;29:193–201. doi: 10.1016/j.braindev.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 106.Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology. 2004;24:159–171. doi: 10.1111/j.1440-1789.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 107.Cacci E, Villa A, Parmar M, Cavallaro M, Mandahl N, Lindvall O, Martinez-Serrano A, Kokaia Z. Generation of human cortical neurons from a new immortal fetal neural stem cell line. Exp Cell Res. 2007;313:588–601. doi: 10.1016/j.yexcr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 108.De Filippis L, Ferrari D, Rota Nodari L, Amati B, Snyder E, Vescovi AL. Immortalization of human neural stem cells with the c-myc mutant T58A. PLoS One. 2008;3:e3310. doi: 10.1371/journal.pone.0003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Filippis L, Lamorte G, Snyder EY, Malgaroli A, Vescovi AL. A novel, immortal, and multipotent human neural stem cell line generating functional neurons and oligodendrocytes. Stem Cells. 2007;25:2312–2321. doi: 10.1634/stemcells.2007-0040. [DOI] [PubMed] [Google Scholar]
- 110.Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, Ko Y, Jeong SW, Kim SU. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007;25:1204–1212. doi: 10.1634/stemcells.2006-0409. [DOI] [PubMed] [Google Scholar]
- 111.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 112.Counter CM, Hahn WC, Wei W, Caddle SD, Beijersbergen RL, Lansdorp PM, Sedivy JM, Weinberg RA. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc Natl Acad Sci U S A. 1998;95:14723–14728. doi: 10.1073/pnas.95.25.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Halvorsen TL, Leibowitz G, Levine F. Telomerase activity is sufficient to allow transformed cells to escape from crisis. Mol Cell Biol. 1999;19:1864–1870. doi: 10.1128/mcb.19.3.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harrington L, Zhou W, McPhail T, Oulton R, Yeung DS, Mar V, Bass MB, Robinson MO. Human telomerase contains evolutionarily conserved catalytic and structural subunits. Genes Dev. 1997;11:3109–3115. doi: 10.1101/gad.11.23.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kilian A, Bowtell DD, Abud HE, Hime GR, Venter DJ, Keese PK, Duncan EL, Reddel RR, Jefferson RA. Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types. Hum Mol Genet. 1997;6:2011–2019. doi: 10.1093/hmg/6.12.2011. [DOI] [PubMed] [Google Scholar]
- 116.Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 117.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 118.Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- 119.Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. [PubMed] [Google Scholar]
- 120.Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- 121.Phelps WC, Munger K, Yee CL, Barnes JA, Howley PM. Structure-function analysis of the human papillomavirus type 16 E7 oncoprotein. J Virol. 1992;66:2418–2427. doi: 10.1128/jvi.66.4.2418-2427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reznikoff CA, Belair C, Savelieva E, Zhai Y, Pfeifer K, Yeager T, Thompson KJ, DeVries S, Bindley C, Newton MA, et al. Long-term genome stability and minimal genotypic and phenotypic alterations in HPV16 E7-, but not E6-, immortalized human uroepithelial cells. Genes Dev. 1994;8:2227–2240. doi: 10.1101/gad.8.18.2227. [DOI] [PubMed] [Google Scholar]
- 123.Bai Y, Hu Q, Li X, Wang Y, Lin C, Shen L, Li L. Telomerase immortalization of human neural progenitor cells. Neuroreport. 2004;15:245–249. doi: 10.1097/00001756-200402090-00006. [DOI] [PubMed] [Google Scholar]
- 124.Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol. 2006;199:56–66. doi: 10.1016/j.expneurol.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Natesan S. Telomerase extends a helping hand to progenitor cells. Trends Biotechnol. 2005;23:1–3. doi: 10.1016/j.tibtech.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 126.Terai M, Uyama T, Sugiki T, Li XK, Umezawa A, Kiyono T. Immortalization of human fetal cells: the life span of umbilical cord blood-derived cells can be prolonged without manipulating p16INK4a/RB braking pathway. Mol Biol Cell. 2005;16:1491–1499. doi: 10.1091/mbc.E04-07-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu C, Jiang J, Sottile V, McWhir J, Lebkowski J, Carpenter MK. Immortalized fibroblast-like cells derived from human embryonic stem cells support undifferentiated cell growth. Stem Cells. 2004;22:972–980. doi: 10.1634/stemcells.22-6-972. [DOI] [PubMed] [Google Scholar]
- 128.Zhang X, Soda Y, Takahashi K, Bai Y, Mitsuru A, Igura K, Satoh H, Yamaguchi S, Tani K, Tojo A, Takahashi TA. Successful immortalization of mesenchymal progenitor cells derived from human placenta and the differentiation abilities of immortalized cells. Biochem Biophys Res Commun. 2006;351:853–859. doi: 10.1016/j.bbrc.2006.10.125. [DOI] [PubMed] [Google Scholar]
- 129.Takeuchi M, Takeuchi K, Kohara A, Satoh M, Shioda S, Ozawa Y, Ohtani A, Morita K, Hirano T, Terai M, Umezawa A, Mizusawa H. Chromosomal instability in human mesenchymal stem cells immortalized with human papilloma virus E6, E7, and hTERT genes. In Vitro Cell Dev Biol Anim. 2007;43:129–138. doi: 10.1007/s11626-007-9021-9. [DOI] [PubMed] [Google Scholar]
- 130.Truckenmiller ME, Vawter MP, Zhang P, Conejero-Goldberg C, Dillon-Carter O, Morales N, Cheadle C, Becker KG, Freed WJ. AF5, a CNS cell line immortalized with an N-terminal fragment of SV40 large T: growth, differentiation, genetic stability, and gene expression. Exp Neurol. 2002;175:318–337. doi: 10.1006/exnr.2002.7898. [DOI] [PubMed] [Google Scholar]
- 131.Bryan TM, Reddel RR. SV40-induced immortalization of human cells. Crit Rev Oncog. 1994;5:331–357. doi: 10.1615/critrevoncog.v5.i4.10. [DOI] [PubMed] [Google Scholar]
- 132.Jha KK, Banga S, Palejwala V, Ozer HL. SV40-Mediated immortalization. Exp Cell Res. 1998;245:1–7. doi: 10.1006/excr.1998.4272. [DOI] [PubMed] [Google Scholar]
- 133.Manfredi JJ, Prives C. The transforming activity of simian virus 40 large tumor antigen. Biochim Biophys Acta. 1994;1198:65–83. doi: 10.1016/0304-419x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 134.Yeager TR, Reddel RR. Constructing immortalized human cell lines. Curr Opin Biotechnol. 1999;10:465–469. doi: 10.1016/s0958-1669(99)00011-7. [DOI] [PubMed] [Google Scholar]
- 135.Morales CP, Holt SE, Ouellette M, Kaur KJ, Yan Y, Wilson KS, White MA, Wright WE, Shay JW. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat Genet. 1999;21:115–118. doi: 10.1038/5063. [DOI] [PubMed] [Google Scholar]
- 136.Vaziri H, Squire JA, Pandita TK, Bradley G, Kuba RM, Zhang H, Gulyas S, Hill RP, Nolan GP, Benchimol S. Analysis of genomic integrity and p53-dependent G1 checkpoint in telomerase-induced extended-life-span human fibroblasts. Mol Cell Biol. 1999;19:2373–2379. doi: 10.1128/mcb.19.3.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 138.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Z, Dullmann J, Schiedlmeier B, Schmidt M, von Kalle C, Meyer J, Forster M, Stocking C, Wahlers A, Frank O, Ostertag W, Kuhlcke K, Eckert HG, Fehse B, Baum C. Murine leukemia induced by retroviral gene marking. Science. 2002;296:497. doi: 10.1126/science.1068893. [DOI] [PubMed] [Google Scholar]
- 140.Sadelain M. Insertional oncogenesis in gene therapy: how much of a risk? Gene Ther. 2004;11:569–573. doi: 10.1038/sj.gt.3302243. [DOI] [PubMed] [Google Scholar]
- 141.Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, Thrasher AJ, Wulffraat N, Sorensen R, Dupuis-Girod S, Fischer A, Davies EG, Kuis W, Leiva L, Cavazzana-Calvo M. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 142.Pike-Overzet K, van der Burg M, Wagemaker G, van Dongen JJ, Staal FJ. New insights and unresolved issues regarding insertional mutagenesis in X-linked SCID gene therapy. Mol Ther. 2007;15:1910–1916. doi: 10.1038/sj.mt.6300297. [DOI] [PubMed] [Google Scholar]
- 143.Hacein-Bey-Abina S, Fischer A, Cavazzana-Calvo M. Gene therapy of X-linked severe combined immunodeficiency. Int J Hematol. 2002;76:295–298. doi: 10.1007/BF02982686. [DOI] [PubMed] [Google Scholar]
- 144.Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, Radford I, Villeval JL, Fraser CC, Cavazzana-Calvo M, Fischer A. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 145.Check E. Gene therapy put on hold as third child develops cancer. Nature. 2005;433:561. doi: 10.1038/433561a. [DOI] [PubMed] [Google Scholar]
- 146.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, de Saint Basile G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Stoppa-Lyonnet D, Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre E, Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Le Deist F, Fischer A, Cavazzana-Calvo M. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 147.Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K, Asnafi V, MacIntyre E, Dal Cortivo L, Radford I, Brousse N, Sigaux F, Moshous D, Hauer J, Borkhardt A, Belohradsky BH, Wintergerst U, Velez MC, Leiva L, Sorensen R, Wulffraat N, Blanche S, Bushman FD, Fischer A, Cavazzana-Calvo M. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marshall E. Gene therapy. Second child in French trial is found to have leukemia. Science. 2003;299:320. doi: 10.1126/science.299.5605.320. [DOI] [PubMed] [Google Scholar]
- 149.Gan Y, Dai K, Zhang P, Tang T, Zhu Z, Lu J. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29:3973–3982. doi: 10.1016/j.biomaterials.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 150.Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343–353. [PubMed] [Google Scholar]
- 151.Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell based bone tissue engineering in jaw defects. Biomaterials. 2008;29:3053–3061. doi: 10.1016/j.biomaterials.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 152.Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, Chung JH, Bae JW, Oh BH, Park YB, Kim HS. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 153.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 154.Mazzini L, Mareschi K, Ferrero I, Vassallo E, Oliveri G, Nasuelli N, Oggioni GD, Testa L, Fagioli F. Stem cell treatment in Amyotrophic Lateral Sclerosis. J Neurol Sci. 2008;265:78–83. doi: 10.1016/j.jns.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 155.Mohyeddin Bonab M, Yazdanbakhsh S, Lotfi J, Alimoghaddom K, Talebian F, Hooshmand F, Ghavamzadeh A, Nikbin B. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol. 2007;4:50–57. [PubMed] [Google Scholar]
- 156.Muller I, Kordowich S, Holzwarth C, Isensee G, Lang P, Neunhoeffer F, Dominici M, Greil J, Handgretinger R. Application of multipotent mesenchymal stromal cells in pediatric patients following allogeneic stem cell transplantation. Blood Cells Mol Dis. 2008;40:25–32. doi: 10.1016/j.bcmd.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 157.Mohyeddin-Bonab M, Mohamad-Hassani MR, Alimoghaddam K, Sanatkar M, Gasemi M, Mirkhani H, Radmehr H, Salehi M, Eslami M, Farhig-Parsa A, Emami-Razavi H, Alemohammad MG, Solimani AA, Ghavamzadeh A, Nikbin B. Autologous in vitro expanded mesenchymal stem cell therapy for human old myocardial infarction. Arch Iran Med. 2007;10:467–473. [PubMed] [Google Scholar]
- 158.Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, Baharvand H, Ghavamzadeh A, Malekzadeh R. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10:459–466. [PubMed] [Google Scholar]
- 159.Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, Locatelli F, Fibbe WE. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764–2767. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- 160.Ripa RS, Jorgensen E, Wang Y, Thune JJ, Nilsson JC, Sondergaard L, Johnsen HE, Kober L, Grande P, Kastrup J. Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction: result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (STEMMI) trial. Circulation. 2006;113:1983–1992. doi: 10.1161/CIRCULATIONAHA.105.610469. [DOI] [PubMed] [Google Scholar]
- 161.Liu L, Sun Z, Chen B, Han Q, Liao L, Jia M, Cao Y, Ma J, Sun Q, Guo M, Liu Z, Ai H, Zhao RC. Ex vivo expansion and in vivo infusion of bone marrow-derived Flk-1+CD31-CD34- mesenchymal stem cells: feasibility and safety from monkey to human. Stem Cells Dev. 2006;15:349–357. doi: 10.1089/scd.2006.15.349. [DOI] [PubMed] [Google Scholar]
- 162.Numaguchi Y, Sone T, Okumura K, Ishii M, Morita Y, Kubota R, Yokouchi K, Imai H, Harada M, Osanai H, Kondo T, Murohara T. The impact of the capability of circulating progenitor cell to differentiate on myocardial salvage in patients with primary acute myocardial infarction. Circulation. 2006;114:I114–I119. doi: 10.1161/CIRCULATIONAHA.105.000588. [DOI] [PubMed] [Google Scholar]
- 163.Ripa RS, Wang Y, Goetze JP, Jorgensen E, Johnsen HE, Tagil K, Hesse B, Kastrup J. Circulating angiogenic cytokines and stem cells in patients with severe chronic ischemic heart disease--indicators of myocardial ischemic burden? Int J Cardiol. 2007;120:181–187. doi: 10.1016/j.ijcard.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 164.Le Blanc K, Ringden O. Use of mesenchymal stem cells for the prevention of immune complications of hematopoietic stem cell transplantation. Haematologica. 2005;90:438. [PubMed] [Google Scholar]
- 165.Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, Shpall EJ, McCarthy P, Atkinson K, Cooper BW, Gerson SL, Laughlin MJ, Loberiza FR, Jr, Moseley AB, Bacigalupo A. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 166.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 167.Cogliano VJ. Current criteria to establish human carcinogens. Semin Cancer Biol. 2004;14:407–412. doi: 10.1016/j.semcancer.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 168.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G, Figueiredo JL, Martuza RL, Weissleder R, Shah K. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci U S A. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Maestroni GJ, Hertens E, Galli P. Factor(s) from nonmacrophage bone marrow stromal cells inhibit Lewis lung carcinoma and B16 melanoma growth in mice. Cell Mol Life Sci. 1999;55:663–667. doi: 10.1007/s000180050322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 171.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, Zhang J, Raffeld M, Rogers TB, Stetler-Stevenson W, Frank JA, Reitz M, Finkel T. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 173.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 174.Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y, Hamada H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 175.Elzaouk L, Moelling K, Pavlovic J. Anti-tumor activity of mesenchymal stem cells producing IL-12 in a mouse melanoma model. Exp Dermatol. 2006;15:865–874. doi: 10.1111/j.1600-0625.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 176.Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 177.Kyriakou CA, Yong KL, Benjamin R, Pizzey A, Dogan A, Singh N, Davidoff AM, Nathwani AC. Human mesenchymal stem cells (hMSCs) expressing truncated soluble vascular endothelial growth factor receptor (tsFlk-1) following lentiviral-mediated gene transfer inhibit growth of Burkitt's lymphoma in a murine model. J Gene Med. 2006;8:253–264. doi: 10.1002/jgm.840. [DOI] [PubMed] [Google Scholar]
- 178.Miletic H, Fischer Y, Litwak S, Giroglou T, Waerzeggers Y, Winkeler A, Li H, Himmelreich U, Lange C, Stenzel W, Deckert M, Neumann H, Jacobs AH, von Laer D. Bystander killing of malignant glioma by bone marrow-derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol Ther. 2007;15:1373–1381. doi: 10.1038/sj.mt.6300155. [DOI] [PubMed] [Google Scholar]