Abstract
We compared brain activations in response to acute noxious thermal stimuli in controls and chronic back pain (CBP) patients. Pain perception and related cortical activation patterns were similar in the two groups. However, nucleus accumbens (NAc) activity differentiated the groups at a very high accuracy, exhibiting phasic and tonic responses with distinct properties. Positive phasic NAc activations at stimulus onset and offset tracked stimulus salience and, in normal subjects predicted reward (pain relief) magnitude at stimulus offset. In CBP, NAc activity correlated with different cortical circuitry than normals and phasic activity at stimulus offset was negative in polarity, suggesting that the acute pain relieves the ongoing back pain. The relieving effect was confirmed in a separate psychophysical study in CBP. Therefore, in contrast to somatosensory pathways, which reflect sensory properties of acute noxious stimuli, NAc activity in humans encodes its predicted value and predicts its analgesic potential on chronic pain.
Introduction
While pain is typically defined by its subjective sensory qualities, it can also be understood by the behavioral responses it elicits, which include the motivation to escape, terminate and/or avoid tissue-damaging processes (Fields, 2006). In addition to its tissue protective role, pain provides a teaching signal that enables individuals to avoid future harm (Apkarian et al., 2009; Johansen and Fields, 2004). Thus pain is a primary punisher and its relief gives rise to negative reinforcement. Although there is now a large literature regarding brain areas encoding the subjective properties elicited by painful stimuli (Apkarian et al., 2005), the circuitry involved in translating nociceptive activity to motivated behavior remains unclear and minimally explored. The onset of a noxious stimulus typically occurs in the setting of competing motivations and therefore requires a decision process prior to a behavioral response. The decision process includes predictions of the probability and magnitude of anticipated pain and the anticipated utility of all competing goals (e.g. hunger, thirst, the presence of a predator). This implies that the motivational information provided by nociceptive input contributes to the activity of circuitry involved in predicting the utility and costs of competing goals and to behavioral decision in the presence of conflict (Fields, 2006; Glimcher, 2003; Glimcher et al., 2009; Rolls, 2005).
Our understanding of the neural mechanisms of reward valuation and appetitive motivation has advanced significantly. Early work identified brain regions in rodents that, when electrically stimulated, could elicit behavior analogous to that produced by natural rewards (Milner, 1991; Olds and Milner, 1954). These regions include the nucleus accumbens (NAc), ventral tegmental area (VTA), and prefrontal cortex (PFc) (Goeders and Smith, 1983). Furthermore, both dopaminergic projections from VTA to the NAc, and glutamatergic inputs to the NAc from the amygdala, hippocampus, and PFc participate in appetitive behaviors instructed by conditioned cues (Ambroggi et al., 2008; Carlezon and Thomas, 2009). Midbrain dopaminergic neurons respond in phasic bursts to unexpected reward, to sensory cues predictive of reward, and with phasic inhibition of firing when an expected reward is not received (Fields et al., 2007; Schultz, 2006; Schultz et al., 1997; Schultz and Romo, 1990), consistent with a role in signaling a reward expectancy error and in cue elicited approach behaviors (Fields et al., 2007; Montague and Berns, 2002; Montague et al., 1996). These studies on reward circuitry were seminal for human imaging studies indicating that neurons in the mesocorticolimbic system participate in decision making under uncertainty across diverse domains (Montague et al., 2006; O'Doherty, 2004; Platt and Huettel, 2008).
In contrast to the extensive work on reward-related activity, fewer studies have explicitly addressed the role of mesolimbic motivation/valuation circuitry for aversive events, and even less is known about how these systems operate in chronically painful conditions in humans. Although early animal studies indicated that VTA dopaminergic neurons are inhibited by aversive stimuli or unexpected costs (Maeda and Mogenson, 1982; Schultz and Romo, 1987; Tsai et al., 1980; Ungless et al., 2004), more recent studies demonstrate both excitatory and inhibitory responses of dopamine neurons to noxious or aversive conditions (Brischoux et al., 2009; Coizet et al., 2006; Joshua et al., 2008; Liu et al., 2008). Furthermore, some dopamine neurons increase their firing rate after a cue predicting an aversive outcome (Mirenowicz and Schultz, 1996). Consistent with the variability in midbrain dopamine neuron responses to aversive stimuli, animal studies report both decreases and increases in extracellular dopamine in NAc following aversive stimuli (Bassareo et al., 2002; Glimcher et al., 2009; Kalivas and Duffy, 1995; Young et al., 1993). Moreover, Salamone and colleagues have presented evidence in rodents that accumbens dopamine is required for exerting increased effort to obtain a larger reward (Salamone et al., 2009). Human functional imaging studies have demonstrated activity changes in NAc in response to both reward and pain predictive cues (Becerra and Borsook, 2008; Becerra et al., 2001; Delgado et al., 2008; Harris et al., 2007; Jensen et al., 2003; Jensen et al., 2007; Montague et al., 2006; O'Doherty, 2004; Platt and Huettel, 2008; Scott et al., 2006; Scott et al., 2007; Seymour et al., 2004; Seymour et al., 2005; Zubieta et al., 2005). A major confound in human imaging and animal dopamine release studies is that while the onset and maintenance of a noxious stimulus is aversive and acts as a punisher, its offset is potentially rewarding so that responses to transient noxious stimuli can have both early aversive and later appetitive components. This results in uncertainty regarding the motivational/hedonic valence of NAc signals generated by transient noxious stimuli. Resolving this temporal issue is critical to our understanding of how nociceptive information is processed by mesoaccumbens circuitry and how this circuit contributes to sensory valuation and behavioral decision.
How are we to interpret the activation patterns elicited at a given brain locus by the sequential aversive and appetitive components of transient noxious stimuli? One possibility is that there is a common neuronal population whose activity ranges from aversive/punishing to appetitive/rewarding. This model predicts signals of opposing valence for punishment and reward and that has not been consistently observed. We propose that in addition to the valuation of action outcome or unexpected aversive events, the mesolimbic circuitry is engaged in predictions of future outcomes and its activation in response to noxious stimuli is best understood in the context of conflicting motivations that have mutually exclusive behavioral goals. Here, in healthy human subjects and in chronic back pain patients, we examine brain activity in response to acute thermal painful stimuli. This analysis was addressed within the framework of the Motivation-Decision Model of pain (Fields, 2006), which posits that reward approach and escape from pain are typically competing and mutually inhibitory behaviors that demand a decision to engage one or the other. The circuitry underlying this action selection decision must have predictive information about imminent noxious stimuli. Previous human brain imaging studies have established that circuitry typically involved in valuation is activated by acute noxious stimuli (Becerra and Borsook, 2008; Becerra et al., 2001; Delgado et al., 2008; Harris et al., 2007; Jensen et al., 2003; Jensen et al., 2007; Scott et al., 2006; Scott et al., 2007; Seymour et al., 2004; Seymour et al., 2005; Zubieta et al., 2005). However, neither the potential role of phasic and tonic NAc activity in the evaluation and prediction of pain and its relief, nor possible changes in activity in the presence of chronic pain, have been explicitly addressed. Both of these issues are addressed in the current study.
Here we first use fMRI to address (1) what are the temporal characteristics of the motivation/valuation circuit during acute pain onset, plateau and offset; (2) how are these responses related to reported pain levels and to predictions of pain and pain relief; and (3) given that chronic pain can be viewed as an ongoing aversive signal (Apkarian, 2008), how does its magnitude interact with NAc responses to the onset, maintenance and offset of transient acute noxious thermal stimuli? In the second part of the study we use psychophysics in a different group of chronic pain patients to test predictions generated from the fMRI study, specifically we examine the effect of acute noxious thermal stimuli on chronic pain, and modulation of pleasantness/unpleasantness by this interaction.
Results
Brain activity for acute thermal pain does not differ between healthy and CBP patients, except for regions implicated in reward valuation
Sixteen CBP patients (Supplementary Table 1a) and sixteen healthy subjects participated in this study. During fMRI, subjects rated the magnitude of perceived intensity of pain (with visual feedback) for three different intensity levels and three durations of thermal stimuli applied to the skin of the lower back, using a finger-span device on a scale of 0 to 100, where 0 is no pain and 100 is the maximum imaginable pain (see methods, (Baliki et al., 2006; Baliki et al., 2009). All subjects performed this task twice. The first scan (scan 1) was used to compute group average brain activity maps using general linear modelling (GLM). The second scan data (scan 2) were utilized to validate the primary observations obtained from scan one (Kriegeskorte et al., 2009). Subjects’ mean pain ratings did not differ between the two groups for both scans (scan 1: CBP: 39.06 ± 18.62; healthy: 28.89 ± 18.7, t15=1.59, P = 0.72; scan 2: CBP: 33.90 ± 22.90; healthy: 36.34 ± 19.93, t15 = 0.83, P = 0.64). Individual subjective ratings from scan 1 were used to assess the fMRI BOLD signal associated with acute pain perception relative to rest, using GLM (see Methods and Figure 1a). We intentionally did not perform a voxel-wise brain mapping analysis for scan 2 data. Instead regions of interest (ROIs) derived from the first scan were used to extract activity and re-test in an independent data-set relationships derived from scan 1.
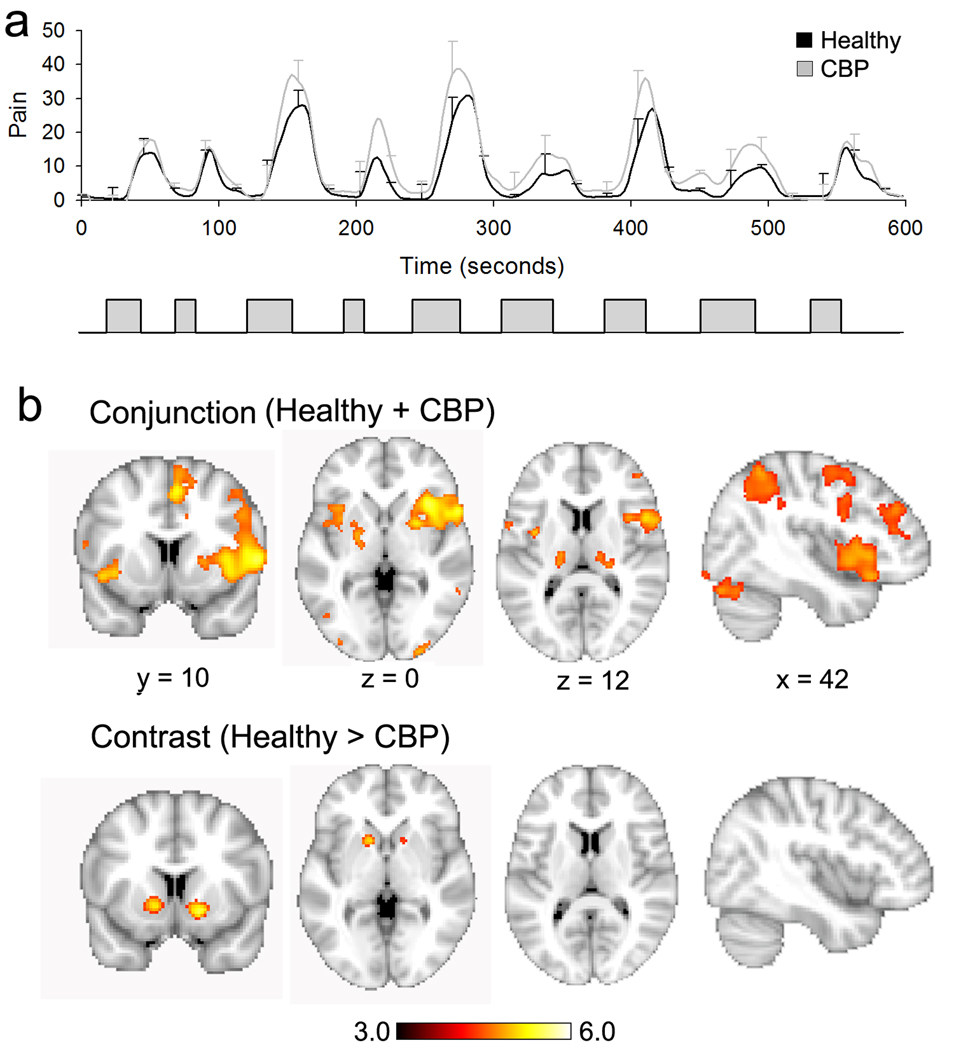
Figure 1. Brain activity maps for perception of thermal pain in healthy and CBP subjects.
(a) Top panel shows average pain ratings for painful heat in healthy (black trace) and CBP (gray trace), data presented as mean +/− S.E.M. Bottom panel shows the time course of the thermal stimulus applied to the lower back. (b) Random-effects analysis for pain rating tasks in healthy controls and CBP patients. Many cortical areas were commonly activated including bilateral thalamus, insula, S2. The conjunction is shown in the top row and represents the brain regions that were commonly significantly activated for both groups. The contrast map shows regions with higher activity in healthy in contrast to CBP. Only bilateral nucleus accumbens survived this contrast. Activity maps were generated using random effects contrasts with z-score > 3.0 and cluster threshold p<0.01 corrected for multiple comparisons.
When subjective ratings of pain were used to identify brain regions with significant activity changes, consistent with previous work (Apkarian et al., 2005), acute pain in healthy and CBP was associated with increased activity in brain regions previously established to encode acute pain intensity (Apkarian et al., 2005). To localize the brain regions commonly activated for perceived pain in CBP patients and healthy controls, we performed a whole brain voxel-wise conjunction analysis. The conjunction analysis included brain regions that were significantly activated for both patients and healthy subjects. Brain activity for the two groups exhibited nearly identical spatial patterns (94.3% overlap between group average statistical maps). Brain regions exhibiting significant activations for both groups are shown in Figure 1 (Supplementary Figure 1, Supplementary Table 2).
While activations throughout the previously established pain sensory circuitry were virtually identical in the two groups, when the pain rating related activity was contrasted for the whole brain (random effects analysis, unpaired t-test, z-score > 3.0 and cluster threshold P <0.01, corrected for multiple comparisons; as well as for age, sex and pain intensity) the difference in the two groups was restricted to bilateral NAc (Figure 1, Supplementary figure 2, Supplementary Table 2). As the NAc is part of the valuation circuitry and has been implicated in encoding salience, motivation and/or reward or punishment value (Montague et al., 2006; O'Doherty, 2004; Schultz, 2006), these results suggest that, although CBP and healthy subjects perceive and encode the sensory dimensions of an acute noxious thermal stimulus similarly, the circuitry implicated in valuation, motivation and action selection is differentially activated in the two groups by identical acute noxious thermal stimuli.
Activation of NAc for thermal pain has phasic and tonic responses, distinct for healthy and CBP patients
Previous studies demonstrated transient NAc activation by predictable repeated noxious thermal stimuli at the start and end of stimulus blocks (Becerra and Borsook, 2008; Becerra et al., 2001). Initially neutral sensory cues conditioned to predict an impending electrical shock were also shown to activate NAc (Jensen et al., 2003), but the detailed time course of NAc BOLD activity was not examined in those studies. To address these issues, we examined the temporal properties of NAc BOLD responses during a thermal pain rating task, where stimulus durations, intensities, and inter-stimulus intervals were presented in a pseudorandom sequence, rendering the time of stimulus onset, its intensity and duration unpredictable. We extracted BOLD time course for the peak contrast localized in NAc (ROI analysis, see Methods), and calculated the group-averaged temporal response pattern for the duration of the task.
Figure 2A shows the average fMRI BOLD signal in healthy controls and CBP. These time curves are overlaid on average pain rating and the derivative of the thermal stimulus (dstim/dt), after convolving the original curves with the hemodynamic response function. In both groups there was a positive phasic response at the onset of each stimulus concomitant with the time of the maximum rate of rise of stimulus intensity. Moreover, in the falling phase of each stimulus epoch there was a distinct second phasic NAc response, positive in the healthy subjects and negative in CBP, which peaked at the time of the maximal rate of decline of stimulus intensity. Thus, the phasic fMRI BOLD responses of NAc encode the rectified derivative of the stimulus (absolute value of the derivative of the stimulus, |dstim/dt|) in healthy subjects, and the derivative (dstim/dt) in CBP (group-averaged correlation in healthy subjects for <BOLDx|dstim/dt|> = 0.41 ± 0.11, mean ± s.d., t15 = 14.9, P < 10−3; and in CBP for <BOLDxdstim/dt > = 0.45 ± 0.12, t15 = 15.2 , P < 10−3).
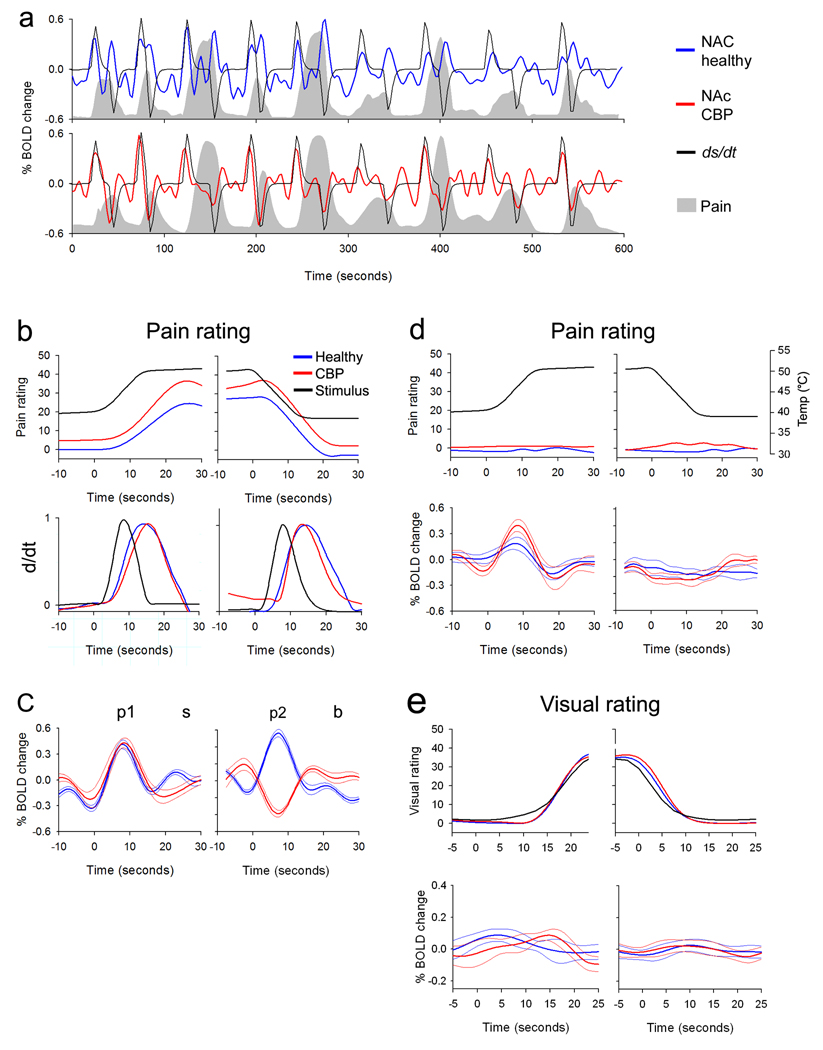
Figure 2. Differences in time course of NAc BOLD signal between CBP patients and healthy controls.
(a) Group-averaged BOLD signals from NAc in healthy (blue) and CBP patients (red) are shown superimposed on the respective group-averaged pain ratings (grey area). The black trace represents the derivative of the stimulus (stimulus and pain ratings are convolved with hemodynamic function). The BOLD signal closely follows the derivative or rectified derivative of the stimulus, in CBP and healthy controls, respectively. Moreover, the baseline activity in the interval between thermal stimuli tends to be more negative in the healthy subjects. (b) Top panels show the average time course of the stimulus (black trace) and pain rating (convolved with hemodynamic function) for healthy (blue) and CBP (red) during start (left) and end (right panel) of thermal stimulus. The time courses were averaged across all stimulation epochs where subjects reported pain (> 5 on a scale of 0–100). Bottom panels show the absolute value of the derivative, |d/dt|, for the stimulus and pain ratings. (c) Top panel shows the time course of average BOLD responses for NAc in healthy (blue) and CBP (red) for the same time periods depicted in (b). (d) shows the same data as in (b–c) for time periods where subjects did not report any significant pain in response to the thermal stimulus. (e) shows NAc activity when both groups rated the length of a visual bar. Top panels are averaged stimulus and ratings, lower panels are averaged NAc BOLD signal. (c–e thin lines are +/− S.E.M.)
To better characterize the temporal relationship between phasic responses of NAc and both the noxious thermal stimulus and subjective pain ratings, we averaged the BOLD signal, for the stimulus onset and stimulus offset, across all stimulation epochs where subjects reported a pain rating change greater than 5%. In healthy and CBP subjects, the NAc signal exhibited two prominent transient peaks (Figure 2b and 2c). The first peak (p1) preceded pain perception and coincided with a positive dstim/dt maximum (which is a predictive cue for impending pain of uncertain magnitude), and similarly the second peak (p2) coincided with a negative dstim/dt at the stimulus offset (predicting the decrease in pain intensity, which corresponds to the reward of pain relief). In CBP patients, the polarity of the second peak was inverted.
In order to assess the specificity of the observed NAc signal, we performed the same stimulus-epoch related BOLD signal averaging analysis for the time periods when the stimulus was delivered but the subjects reported it as non-painful (pain rating < 5%) (Figure 2d). We observed that p1 is sustained in both groups. That this peak is independent of perceived pain intensity as well as independent of whether subjects perform the rating task implies that it does not represent encoding of either a psychophysical attribute of pain nor of the motor or cognitive performance of rating. For the second peak (p2), there was a significant attenuation in the amplitude for both groups when it was not preceded by subjectively experienced pain. This is consistent with the interpretation that p2, at least in healthy subjects, is related to the predicted value of imminent pain offset (i.e. it is a reward prediction cue that encodes the magnitude of the positive value of anticipated pain relief).
As a control for the action of magnitude rating we examined the NAc signal when subjects performed a visual magnitude-rating task. During the visual rating task, no significant phasic change was observed in the NAc signal in either group (Figure 2e), again implying that the observed NAc activity for pain is independent of motor performance, and attention (the visual ratings require vigilance as the start, end, and overall variability of the visual bars are as unpredictable as the thermal stimuli), but is task or stimulus modality specific (pain is more salient, has an implied hedonic value, than rating the size of a visual bar in the absence of reward or punishment).
NAc activity predicting pain relief distinguishes between CBP and controls with 100% sensitivity
We compared peak activity of NAc signal between the two groups for four different time-widows: 1) Peak 1 (p1): the period where dstim/dt is positive; 2) Peak 2 (p2): the period where dstim/dt is negative; 3) During painful stimulation (s): the time-window between p1 and p2 (stimulus reaches its highest level and the subject is rating perceived pain); 4) Baseline (b): the time-window between stimulus presentations (duration between p2 and p1, where the subject is at rest and stimulus temperature is at baseline). Peak percent BOLD response for each of these time periods was extracted for each epoch relative to time = 0 (start of thermal stimulus in Figure 2b), averaged across scans and subjects, for each group (Figure 3a). There was no group difference in activity for p1 (healthy: 0.41 ± 019; CBP: 0.42 ± 0.21; mean ± sd., t15 = −0.12, P = 0.91) and s (healthy: 0.03 ± 0.13; CBP: −0.01 ± 0.07; t15 = 1.11, p = 0.28). Healthy subjects exhibited a significantly higher activity for p2 (healthy: 0.49 ± 0.14; CBP: −0.39 ± 0.16; t15 = 16.21, p<10−5), while CBP had a higher post-stimulus baseline activity, b (healthy: −0.21 ± 0.08; CBP: 0.01 ± 0.12; t15 = −5.43, p<10−5). Therefore, NAc activity during p2 and b differentiate between healthy and CBP patients. When we examined the activity for the different NAc peaks for scan 2 we observed similar results. There was no group difference in activity for p1 (healthy: 0.23 ± 0.14; CBP: 0.27 ± 0.29, t15 = −0.51, P = 0.61) and s (healthy: 0.02 ± 0.13; CBP: −0.02 ± 0.14, t15 = 1.09, P = 0.28). On the other hand p2 showed higher activity in healthy (healthy: 0.32 ± 0.17; CBP: −0.25 ± 0.21, t15 = 8.34, P <10−5), whereas b was higher in patients (healthy: −0.149 ± 0.09; CBP: 0.04 ± 0.12, t15 = −4.97, P <10−4).
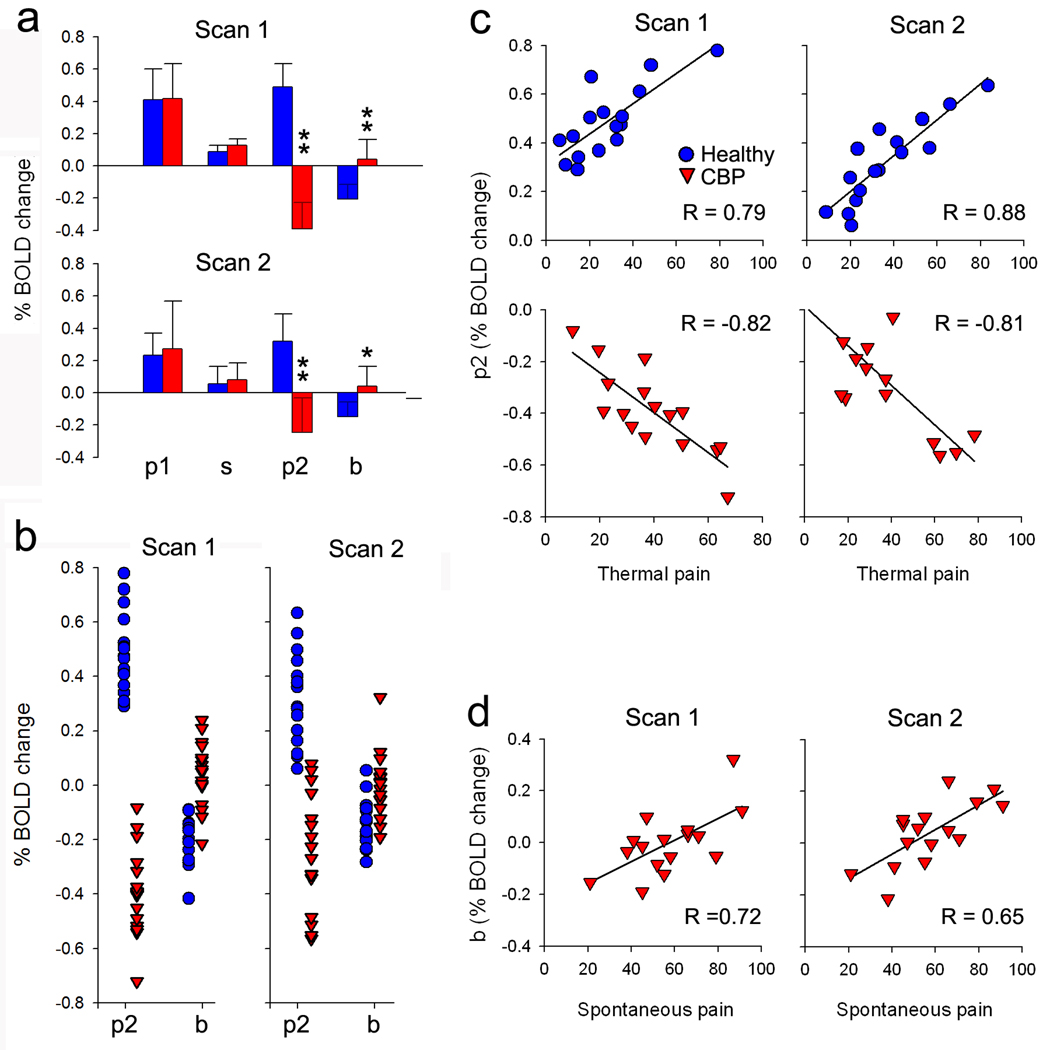
Figure 3. Phasic and tonic NAC activity distinguish between the groups and depend on stimulus and chronic pain parameters.
(a) Average NAc signal differences between the two groups for four time windows: p1, phasic response when thermal pain is increasing; s, tonic response during thermal pain; p2, phasic response when thermal pain is decreasing; b, tonic baseline activity between stimuli. Healthy and CBP exhibited similar activity for p1 and s. Healthy subjects exhibited higher activity for p2 and lower activity for b, when compared to CBP. Similar results are seen for scan 1 and scan 2. (b) Panel shows individual mean BOLD responses for p2 and b, in CBP (red) and healthy controls (blue) for scan1 (left column) and scan2 (right column). The ordinate is the BOLD signal for each subject for p2 and b, averaged across all stimuli. (c) Activity for p2 is significantly correlated with ratings of thermal pain in healthy subjects, for both scan 1 and scan 2 (blue). This relationship is reversed in CBP (red). (d) Activity for b shows a positive correlation with individual scores for magnitude of back pain (visual analogue scale, VAS) in CBP in scan 1 and scan 2. (In a, error bars are +/− S.E.M. In b–d, each symbol is a subject’s value)
Figure 3b is a scattergram showing the individual subject values for p2 and b for each group for both scans. In scan 1, using a threshold cutoff value of zero, for p2 we obtain a sensitivity of 100% and specificity of 100% for distinguishing between CBP and controls. For b with the same zero cutoff, we obtain a sensitivity of 60% and specificity of 66.7%. When we perform the same analysis on data from the second scan, where BOLD activity is derived from a 10-mm-diameter ROI with center coordinates identified from the contrast performed for scan 1, we obtain a sensitivity of 100% and a specificity of 87.5% for p2, while for b we obtain a sensitivity of 50% and specificity of 63%.
Phasic and tonic NAc activity differentially relate to magnitudes of acute pain and chronic pain
We investigated the correlation of NAc activity with perceived magnitude of acute pain and magnitude of chronic pain during the four time periods (p1, s, p2, b, using within subject averaged activity for each period). For scan 1 we found that p2 was positively correlated with the magnitude of acute pain in healthy subjects (R = 0.78, P < 10−5), indicating that phasic NAc response to the prediction of pain relief is significantly correlated with the magnitude of stimulus evoked pain experienced in the immediately preceding period. In contrast in CBP patients, p2 was negatively correlated with the magnitude of acute pain perceived during the stimulation epoch (R = − 0.82, P < 10−5) (Figure 3c).
Activity of NAc for post-stimulus baseline, b, positively correlated with the CBP subject’s magnitude rating of spontaneous back pain (visual analog scale, VAS, rating for back pain; a measure of the intensity of their spontaneous back pain, R = 0.72, P = 0.002) (Figure 3d). This indicates that NAc tonic activity increases with magnitude of ongoing back pain.
Similar results were observed for scan 2: p2 showed a significant positive correlation with thermal pain in healthy subjects (R = 0.88, P < 10−5), and a negative correlation in CBP (R = − 0.81, P < 10−5). Similarly b exhibited a negative correlation with stimulus pain in healthy subjects (R = −0.50, P = 0.047), and a positive correlation with spontaneous pain in CBP (R = 0.65, P = 0.007).
We also examined the interaction between phasic and tonic NAc activity and compared the results between the groups. We observe a complex interaction between tonic and phasic responses within and across epochs for both groups (see supplementary material).
Functional connectivity of NAc changes with CBP
To identify the brain circuitry that contributes/participates in the NAc activations we performed a whole brain correlation analysis (Fox et al., 2005). NAc activity was used as a seed to compute its linear correlation coefficients against all other brain voxels’ time series for each subject. The individual correlation maps were transferred into standard space and averaged for healthy and CBP groups, using random effects statistical thresholds (see methods for details). The resultant correlation maps summarize functionally co-activated areas of the brain with NAc throughout the pain-rating task.
In both groups, NAc exhibited significant positive correlations with brain regions that have been implicated in valuation, action selection and pain modulation, including the basal ganglia, amygdala, ACC, medial and orbital prefrontal cortex, medial thalamus, and anterior insula. Of particular interest in view of their relation to reward and pain modulation, positive correlations were found with the rostral portions of the midbrain periaqueductal gray (PAG) and the ventral tegmental area (VTA) (Figure 4a). In CBP subjects this network was more extensive and showed a significantly stronger connectivity with mPFc (whole brain t-test P < 0.01, Supplementary Figure 3). We also examined the NAc connectivity in CBP patients while they rated their spontaneous CBP pain in the absence of thermal stimulation. Results showed that the NAc was significantly connected with mPFc and amygdala (data not shown). Given that rating thermal pain by CBP subjects can be viewed as a combination of rating the stimulus and also subjectively feeling ongoing back pain, we can conclude that the increased mPFc-NAc connectivity that we observe in CBP for the acute thermal pain rating task is at least partially a reflection of the enhanced mPFc-NAc connectivity due to spontaneous back pain.
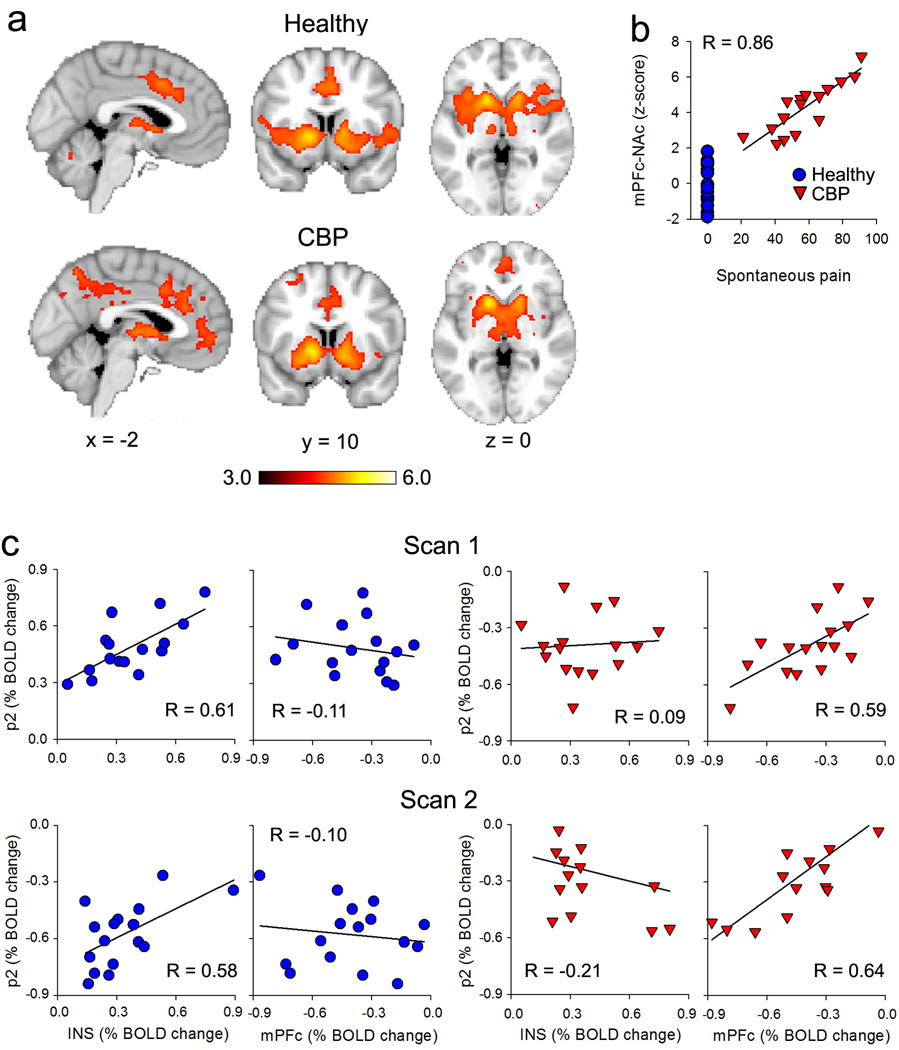
Figure 4. Functional connectivity of NAC and its differential dependence on specific cortical regions.
(a) Group average connectivity maps when NAc activity is used as seed, in healthy subjects (top panel) and CBP (lower panel). The NAc exhibited significant connectivity to bilateral amygdala, caudate, putamen, medial thalamus, PAG, ventral striatum and ACC in both groups. However, NAc connectivity to mPFc was stronger in CBP (unpaired t-test, random effects z-score > 3.0 and cluster threshold p<0.01 corrected for multiple comparisons, see Supplementary Figure 3). (b) Scatter plot shows a strong correlation between the strength of NAc-mPFc connectivity (z-score is standardized correlation coefficient for each subject) and intensity of back pain (VAS) at the day of the scan in CBP. (c) The relationship between activity in NAc at p2 with magINS (portion of insula related to magnitude perception) and mPFc (computed as % change in BOLD signal) in healthy (blue) and CBP (red), shows a double dissociation between the two groups. In healthy subjects the NAc p2 exhibits a strong positive correlation with magINS activity and no correlation with mPFc, this relationship was reversed in CBP. Similar results are seen for data derived from scan 1 and scan 2.
Since in CBP spontaneous fluctuations of ongoing back pain activates mPFc and this activity correlates with individual subject’s rating of the intensity of their back pain (Baliki et al., 2006), and since NAc-mPFc connectivity is stronger in CBP in the current study, we determined whether mPFc connectivity is dependent on the magnitude of patients ongoing back pain. We found that the strength of NAc-mPFc connectivity is significantly correlated to VAS ratings of magnitude of back pain (R = 0.86, P <10−5; Figure 4b). This indicates that changes in connectivity between mPFc and NAc in CBP patients, due to the presence of spontaneous ongoing fluctuations of back pain, modulates the activation of NAc for acute noxious thermal stimuli. Using an ROI analysis, we show that p2 exhibits a double dissociation between magINS (a region of the insula that best represented the magnitude of thermal pain perception in healthy subjects, (Baliki et al., 2009) and mPFc between healthy and CBP subjects (Figure 4c, supplementary material). Therefore, at least for the p2 response, NAc activity is influenced from different cortical sources in healthy and CBP subjects.
Psychophysics of interaction between painful thermal stimuli and chronic pain
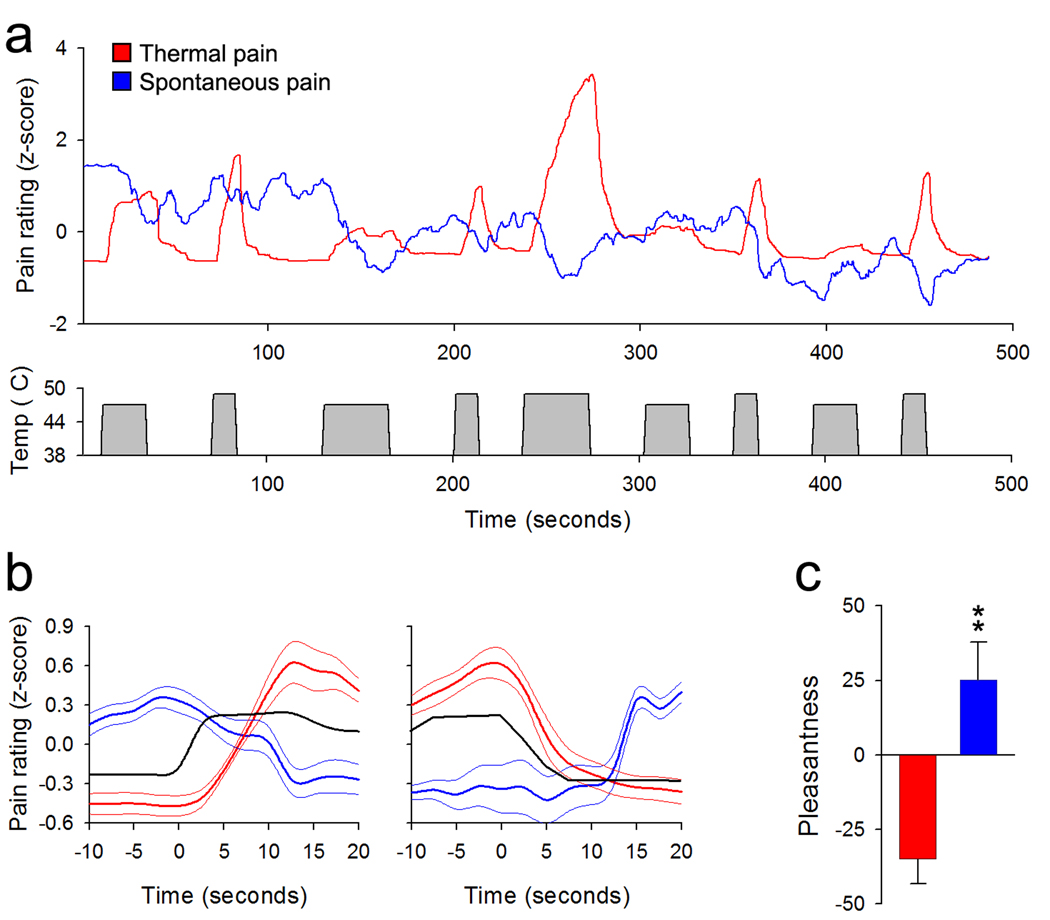
NAc activity (during b) and NAc-mPFc connectivity are related to the intensity of ongoing chronic pain, and specific phases of NAc activity (p2, b) distinguish between patients and controls. Furthermore, NAc activity at the initiation of the offset of the noxious stimulus (p2) shows deactivation in CBP while acute pain-related cortical activity is similar between the two groups. One possible explanation of the opposing sign of the NAc response to predicted offset of the acute noxious stimulus in CBP versus normal subjects is that in the back pain subjects the acute noxious stimulus produces relief of a more salient on-going aversive signal reflecting their clinical pain. In this case, the initiation of the acute stimulus offset would reflect a predicted punishment (increased clinical pain) as opposed to a reward in normal subjects (pain offset). To test this hypothesis directly we performed a psychophysical study in eight CBP patients. These patients first continuously rated the fluctuations of their spontaneous pain (Baliki et al., 2006). Next they continuously rated the magnitude for a thermal painful stimulus (similar in stimulus parameters to that used in the fMRI study) applied to their back, at the termination of which they were asked to rate the pleasantness/unpleasantness of the experience (on a +100 to −100 scale). In the third trial the thermal stimulus was applied again but now participants were instructed to continuously rate the fluctuations of their spontaneous pain. At the termination of the third run participants again rated pleasantness/unpleasantness of the experience.
The results indicate that these CBP patients rate the thermal pain similarly to the CBP patients and healthy controls whose ratings were collected during fMRI scanning (Figure 5a), and this experience is rated as quite unpleasant (−35.0 ± 8.2, mean ± s.e.m.). When the subjects rate their own spontaneous pain during the thermal painful stimulation, we observe a robust decrease in their back pain (spontaneous pain rating was negatively correlated with stimulus time course: −0.30 ± 0.19, P < 0.004) (Figure 5b), and rate this experience significantly more pleasant than the rating of the stimulus pain when not attending to their back pain (25.00 ± 12.72, paired t-test t7 = −9.23, P < 10−5) (Figure 5c). Besides the stimulus locked decreases in ongoing pain, group averaged spontaneous back pain ratings also indicate a sustained slow overall decrease (from 1.39 ± 0.52, mean ± s.e.m., of normalized spontaneous pain at start of rating session to −0.54 ± 0.67 at the end of the stimulation session, paired t-test, t7 = 4.8, P = 0.002). Thus, these psychophysical observations support the hypothesis that the reversal of NAc signal in CBP patients compared to normals is due to the net decrease in their ongoing spontaneous pain in CBP during the acute noxious thermal stimulation, despite their report of the stimulus evoked pain. In CBP, acute stimulus offset thus is paradoxically predictive of increased pain (punishment). In the absence of ongoing pain, the NAc signal is positive at the initiation of acute stimulus offset reflecting predicted pain relief.
Figure 5. Reduction in perceived magnitude of back pain by thermal painful stimuli.
(a) Top panel shows average ratings (n = 8 CBP patients) of the magnitude of the stimulus (red trace) and spontaneous fluctuations of back pain (blue trace) during the application of a thermal stimulus to the lower back. Bottom panel shows the time course of the thermal stimulus. (b) Average time course of the stimulus (black trace), rating the stimulus pain (red) and spontaneous back pain (blue) during start (left) and end (right panel) of thermal stimuli. The time curves were averaged across all stimulation epochs and all 8 CBP patients. (c) Bar graph shows the group averaged pleasantness (on a +100 to −100 pleasantness to unpleasantness scale) evaluation of the experience of rating the thermal pain (red) and the spontaneous pain (blue), indicating that attending to the back pain during the thermal painful stimulus reveals that the back pain is reduced and this is accompanied by increased pleasantness. (b – c error bars and thin lines are +/− S.E.M.).
Discussion
Our results demonstrate that identical acute noxious thermal stimuli that produce similar patterns of sensory activations in chronic back pain patients and in healthy controls elicit distinct patterns of NAc activity in the two groups. This NAc activity could be divided into temporally separate phasic (p1 and p2) and tonic (s and b) responses, which correlated with the perceived magnitudes of acute pain, duration of acute pain, and, in CBP, with the intensity of ongoing chronic pain. Furthermore, in a given thermal stimulation epoch, phasic responses were significantly correlated with tonic responses, and tonic responses predicted phasic and tonic responses in subsequent epochs. The phasic responses are characterized as predictive: 1) by their correlation with the derivative of the stimulus (or the absolute value of the derivative in CBP), as anticipated in computational models for reward valuation circuitry (Sutton and Barto, 1998), 2) at stimulus onset predicting salience or arousal for imminent pain, 3) at stimulus offset predicting reward value for expected pain relief in healthy subjects and, 4) at stimulus offset predicting punishment/cost in CBP. These observations extend current theories regarding the role of NAc in reward valuation (Montague and Berns, 2002; Montague et al., 1996) (Schultz, 2007) suggesting a more complex multi-component role for NAc. They are consistent with the Motivation-Decision Model of pain (Fields, 2006) in demonstrating activation of valuation circuitry, including the NAc at the onset of a stimulus predicted to be painful.
Despite indistinguishable central nervous system sensory activation patterns and sensory reports produced by acute noxious thermal stimuli, we show that CBP and healthy subjects differ in the dominant pattern of connectivity with NAc. In healthy subjects, NAc activity is correlated with magINS activity but not with mPFc activity, the reverse is true in CBP subjects, and NAc-mPFc connectivity in CBP is stronger in patients with more severe back pain. These connectivity differences may contribute to the NAc activity that distinguishes the two groups. The psychophysical study supports our hypothesis that the NAc signal difference between the two groups (at p2 and b) reflects differences in the predicted valuation of the offset of the acute painful stimulus; in CBP it reflects the prediction of worsening of the ongoing back pain, while in normals it reflects the prediction of relief.
In addition, our psychophysical studies have uncovered an uncoupling between NAc valuation and conscious states of pleasantness/unpleasantness: even though the valuation of the thermal stimulus offset relative to the subject’s own pain is apparently encoded by NAc activity, CBP patients become subjectively aware of it only when their attention is specifically directed to their own ongoing pain. This results in a shift in the reported evaluation of the overall experience of acute thermal noxious stimuli from unpleasant (when attending to the acute stimulus) to pleasant when attending to the back pain.
Thermal stimuli were presented in a pseudo-random design. This unpredictable pattern parallels the circumstances of pain perception outside the laboratory, as it requires an individual to select and execute actions in the face of uncertainty (fight or flight, approach or escape). Under these circumstances, phasic activation of NAc was observed at the onset of the rise and fall of noxious thermal stimuli in both groups. The early response (p1) preceded perception and was independent of reported pain intensity. Similarly, in the early declining phase of the thermal stimulus a second phasic NAc response (p2) occurred prior to the perception of a decrease in pain. These results demonstrate that NAc, and its correlated valuation/decision circuitry, phasically respond to a sensory cue (rate of change in skin temperature, within a range where primarily nociceptors are activated, (Meyer et al., 2006) that signals the increased probability of an impending but uncertain painful event/punishment (p1) and/or certain impending pain relief/reward (p2) in normal subjects. P1, but not p2, was present even in the absence of subsequent pain perception. Importantly, NAc showed no responses when a similar but hedonically neutral magnitude-rating task was used (i.e. rating the length of a visual bar, rather than pain), even though the task design is formally identical to the pain task. These observations demonstrate that the anticipated aversiveness or motivational salience of pain is critical for NAc responses to noxious stimuli and suggests that p1 reflects the salience and/or uncertainty about imminent potential pain. In contrast, the magnitude of impending pain relief at p2 is certain because it is predicted by the known magnitude of the immediately preceding pain intensity and this reward prediction (a direct function of pain level during s) is captured by the amplitude of p2. Thus, at least in healthy subjects, p2 reflects the predicted reward value of pain relief and perhaps its salience.
To our knowledge this is the first human brain imaging study to distinguish between phasic and tonic NAc activity. The tonic responses during painful stimulation correlated negatively with stimulus duration and, following stimulus cessation, correlated negatively with stimulus pain in healthy subjects and positively with chronic pain in CBP. Moreover, post-stimulus tonic responses were negatively influenced by phasic and topic responses within the painful stimulus epoch, and in turn negatively affected phasic and tonic responses across stimulus epochs. Recently Niv (Niv, 2007) has argued that tonic NAc activity due to changes in baseline dopamine levels (partially determined by spillover of dopamine from phasic dopamine) should mediate the effects of motivation on response vigor, or response rate, mediated by a change in expected net rate of rewards. The model predicts that tonic NAc activity will be higher when performing a more rewarding or less costly task and during enhanced motivational states, and lower when working harder or for fewer rewards resulting in slothful behavior. We observe that stimulus features modulate tonic activity reflecting their aversiveness. Cessation of the stimulus results in decreased tonic activity during b in healthy subjects. Thus, post-stimulus tonic activity in healthy subjects may reflect both the withdrawal of an aversive condition as well as the markedly reduced probability of receiving another noxious stimulus. Post-stimulus tonic activity is higher in CBP than in healthy subjects and this is consistent with the psychophysical evidence that back pain intensity increases in this phase in CBP. Therefore, similar to the phasic NAc responses, the tonic activity seems to reflect a combination of parameters including a preceding outcome (at least reflecting reward/punishment) and a prediction (motivation/expectation) that should influence future decisions.
In CBP, NAc activity was different from healthy subjects for phasic activity at stimulus offset (p2), and for tonic activity after stimulus cessation (b). P2 activity changed in proportion to p1 in both groups, and was positively related to perceived magnitude of acute pain in healthy subjects but negatively correlated in CBP. This suggests that early NAc activity encodes the predicted reward value of its relief. Consistent with our psychophysical studies, the reversal of valence of p2 in back pain patients appears to reflect an inversion of the predicted valuation of acute noxious stimulus offset due to the relieving effect of the acute thermal stimulus on ongoing back pain. Given the relationship between baseline tonic activity and spontaneous pain in CBP, the influence of chronic back pain on NAc responses for subsequent stimuli, and the modulation of the back pain by phasic and tonic activity during the stimulus, we propose that the overall motivational state of CBP patients is distinct from control subjects. CBP patients appear to continuously evaluate/predict future outcomes in relation to their ongoing back pain, even when they are not consciously attending to this pain. The psychophysical study demonstrates that the relief from ongoing back pain by the thermal stimulus is proportional to the rating of the magnitude of stimulus evoked pain. This is consistent with the idea that the NAc response differences between the two groups is related to the opposing valuation predictions in the two groups produced by the presence of chronic pain. This idea is further supported by the fact that CBP patients reverse unpleasantness ratings during the acute stimulus when attention is directed to their spontaneous pain.
The properties of NAc activity observed for pain are consistent with the critical features of the Motivation-Decision Model of pain (Fields, 2006): an early phasic NAc activation at the onset of a sensory stimulus predicting imminent pain; a network of brain structures correlated with NAc activity including the VTA which contains dopaminergic neurons projecting to the NAc and the amygdala and PAG which give rise to descending bidirectional pain modulatory circuits (Fields, 2004); modulation of reward related NAc activity by ongoing back pain; and tonic NAc activity that correlates with motivation/aversiveness (p1). Thus, the present study is consistent with a role for NAc in the prediction of the value of a noxious thermal stimulus and its offset, and in the consequent changes in motivational state.
The phasic NAc activations we report are consistent with an earlier thermal noxious stimulus study (Becerra and Borsook, 2008). They are also consistent with a previous report using a conditioning task for monetary reward and for aversive electrical stimuli which resulted in ventral striatal activity reflecting salience, rather than valence or reward prediction error (Jensen et al., 2007), as well as with other earlier observations (Jensen et al., 2003; Seymour et al., 2004; Seymour et al., 2005). In general, p1 and p2 responses better fit with partially signed prediction error model, and NAc activity during s is consistent with an aversive prediction error because it tracks stimulus duration, yet separate aversive representation for pain also seems to exist in other brain areas (Seymour et al., 2005). On the other hand, according to most models based on the assumption that the NAc participates in a unidirectional reward generating circuit (Montague and Berns, 2002; Montague et al., 1996) p1 and p2 should be of opposite sign in the healthy subjects. Thus, our data indicate that the role of phasic NAc activity in valuation is more complex and may be related to activity in functionally distinct subsets of neurons with different inputs and outputs (see e.g. Fields et al 2007 for review). In fact, similar to a more nuanced view of midbrain dopaminergic function; in addition to reward prediction error, NAc phasic activity can signal an impending salient and uncertain decision and may contribute to arousal/motivation and action selection (Berridge, 2007; Horvitz, 2000). The timing, valence and magnitude of the later p2 is consistent with it being a reward prediction signal in healthy subjects, and with impending punishment (cessation of reward) in CBP, in agreement with reward valuation studies (Glimcher et al., 2009; Montague et al., 2006).
Recent investigators have commented on the circular analysis practices in functional brain imaging studies and on potential inflation of correlations between brain activity and behavioral parameters of interest by ‘double-dipping’ (Kriegeskorte et al., 2009; Poldrack and Mumford, 2009). To circumvent such issues we used a second data set for validating the primary outcomes of the first fMRI data from which regions of interest were derived and used to examine temporal properties of NAc in the second set. We observe a close match between results from both sources strongly validating these outcomes. In this study we also derive a novel hypothesis from fMRI activity, which we then confirm by a psychophysical study. Moreover, we show that the NAc phasic activity at stimulus offset distinguishes the two groups at a very high rate of sensitivity and specificity implying that this signal can be used as an objective marker of chronic pain (Miller, 2009).
Abnormal brain chemistry, regional gray matter atrophy, cognitive changes, and unique patterns of brain activity have been demonstrated in CBP - engaging mainly mPFc with no overlap with activity for acute thermal pain (Apkarian et al., 2009). Here we show that for the CNS response to an uncertain acute transient noxious thermal stimulus, only NAc activity distinguishes CBP from healthy subjects. We further demonstrate that these activity differences correlate with changes in functional connectivity between NAc and other limbic forebrain areas. Given that NAc activity is fundamental for guiding the direction and vigor of motivated behavior, contributes to learned associations in animals (Fields et al., 2007), and reflects valuation differences representing individual CBP subject’s ongoing chronic pain, it is likely that the transition from acute to chronic back pain represents a dysfunctional associative learning process (Apkarian, 2008) emanating in part from predictions, valuations, and related motivations and mediated by distinct circuitry involving connections with the NAc. Moreover, although NAc activity is modulated by the properties of perceived stimulus pain and its interaction with chronic pain, valuations and motivational outcomes emanating from NAc and related circuitry in CBP do not modulate sensory perception of acute pain (rating of external inputs) and instead favor decisions relative to their own chronic pain (emphasizing internal states), this switch may be an integral component of the pathophysiology of chronic pain.
Methods
Participants
Sixteen healthy subjects (8 males, 8 females; average age: mean = 38.77, s.d. = 12.50 years) and 16 CBP patients (8 males, 8 females; average age: mean = 45.06, s.d. = 11.98 years) participated in the functional brain imaging portion of this study. An additional eight CBP patients (4 males, 4 females; average age: mean = 49.75, s.d. = 7.79 years) participated in the psychophysical portion of the study. All participants were right-handed, and gave informed consent to procedures approved by Northwestern University IRB committee. All patients, recruited by newspaper ads in Chicago area, were clinically diagnosed with CBP by a clinician and had to fulfill a specific list of inclusion/exclusion criteria. Their clinical and demographic data, as well as pain-related parameters are presented in supplementary Tables 1a and 1b.
Procedures and stimuli
Subjects were scanned twice while rating their pain in response to thermal stimuli applied to their back (pain rating task scan 1 and scan 2). Participants underwent an initial training phase prior to scanning, in which they learned to use the finger-span device, comprised of a potentiometer the voltage of which was digitized and time-stamped in reference to fMRI image acquisition and connected to a computer providing visual feedback of the ratings (Baliki et al., 2006). A purpose built, fMRI compatible thermal stimulator delivered fast ramping (20 °C per second) painful thermal stimuli (baseline 38 °C, peak temperatures 47 °C, 49 °C and 51 °C) via a contact probe (1 × 1.5 cm peltier). Durations and intensities of thermal stimuli as well as inter-stimulus intervals were presented in a pseudorandom fashion. During a given functional imaging session, 9 noxious thermal stimuli ranging in duration from 12 to 30 seconds were applied to the lower back just off midline in healthy subjects. For the patients the thermal stimulus was applied on the back at a location where the participant indicated best approximated the site where back pain was experienced.
In addition to the pain-rating task, subjects performed a visual rating task (for details see (Baliki et al., 2009). During the visual rating scan, subjects rated the length of the bar projected on a screen using the finger span device in the absence of painful stimuli. Thus this task serves a control for task related activations such as magnitude estimation, attention, and anticipation.
fMRI data and acquisition
fMRI data was acquired with a 3T Siemens Trio whole-body scanner with echo-planar imaging (EPI) capability using the standard radio-frequency head coil. The anatomical and three separate fMRI scans (pain scans 1, 2, and visual rating scan) were collected during a single brain imaging session with scan sequence randomized across subjects. Image analysis to reveal significant brain activity based on changes in BOLD signal was performed on each subject's data and averaged activity across subjects were determined using FMRIB Expert Analysis Tool (FEAT, (Smith et al., 2004), www.fmrib.ox.ac.uk/fsl, see supplementary methods).
Brain intrinsic correlational networks
Brain networks correlated to specific regional activity (seed) were identified using a well-validated method, see (Baliki et al., 2008; Fox et al., 2005). Correlational network maps were produced by first extracting the BOLD time course from a seed region and then, computing correlation coefficient between its time course and the time variability of all other brain voxels, in first level analysis in FSL. Group mean maps were generated using a random-effects analysis corrected for multiple comparisons at a significance level z > 3.0, P < 0.01. A two-sample paired-t test was used to compare connectivity maps across different seeds. This seed based analysis was done for scan 1 and scan 2 data.
Region of interest (ROI) and BOLD analysis
The ROIs were a priori determined fixed size 10-mm-diameter spheres centered at peak coordinates defined from contrast, conjunction, or brain correlation analysis maps. One ROI was defined from the contrast map NAc (16, 10, −8), and 2 ROIs were defined from the intrinsic brain correlation maps: magINS (40, 8, −2) and mPFc (0, 52, −14). The ROIs were reverse-normalized and projected back into the unnormalized individual brain space. The BOLD signal for the total trial duration was obtained by averaging the raw data for all voxels across a given ROI. BOLD time course was measured first by calculating percent BOLD change (deviation from the mean for voxels within the ROI). The BOLD was band-pass filtered (0.009 < f < 0.08 Hz) and corrected for motion artifacts and noise through linear regression with the six parameters obtained by rigid body correction of head motion and signal from a ventricular region of interest. The ROI based analysis was performed for scan 1 and scan 2 data.
Thermal and chronic pain ratings
In two separate stimulation runs, CBP patients rated their online thermal and spontaneous pain in the presence of a thermal stimulus applied to their lower back using the same thermal stimulator described above. Thermal stimuli sequence resembled the one used in the fMRI study. In brief patients received 9 noxious thermal stimuli ranging in duration from 12 to 30 seconds and intensities of 47, 49, and 51 degrees Celsius applied to the lower back. Stimuli and inter-stimulus intervals were presented in a pseudo random fashion. During a given stimulation session patients were instructed to either rate the thermal pain or spontaneous pain in response to the stimuli or their spontaneous pain. After each stimulation trial, patients were asked to report the pleasantness of the stimuli applied on a scale of −100 to 100, where the extremes represented the most unpleasant and pleasant experience. In a third run subjects rated their spontaneous in the absence of any stimulation.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV. Pain perception in relation to emotional learning. Curr.Opin.Neurobiol. 2008;18:464–468. doi: 10.1016/j.conb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog.Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV. Parsing pain perception between nociceptive representation and magnitude estimation. J.Neurophysiol. 2009;101:875–887. doi: 10.1152/jn.91100.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di CG. Differential Expression of Motivational Stimulus Properties by Dopamine in Nucleus Accumbens Shell versus Core and Prefrontal Cortex. J.Neurosci. 2002;22:4709–4719. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Borsook D. Signal valence in the nucleus accumbens to pain onset and offset. Eur.J.Pain. 2008;12:866–869. doi: 10.1016/j.ejpain.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc.Natl.Acad.Sci.U.S.A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coizet V, Dommett EJ, Redgrave P, Overton PG. Nociceptive responses of midbrain dopaminergic neurones are modulated by the superior colliculus in the rat. Neuroscience. 2006;139:1479–1493. doi: 10.1016/j.neuroscience.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Li J, Schiller D, Phelps EA. The role of the striatum in aversive learning and aversive prediction errors. Philos.Trans.R.Soc.Lond B Biol.Sci. 2008;363:3787–3800. doi: 10.1098/rstb.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL. State-dependent opioid control of pain. Nat.Rev.Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Fields HL. A motivation-decision model of pain: the role of opioids; Proceedings of the 11th world congress on pain; Seattle: IASP press; 2006. pp. 449–459. [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu.Rev.Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc.Natl.Acad.Sci.U.S.A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW. Decisions, uncertainty, and the brain: the science of neuroeconomics. Cambridge: MIT press; 2003. [Google Scholar]
- Glimcher PW, Camerer CF, Fehr E, Poldrack RA. Neuroeconomics. London: Elsevier; 2009. [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Jensen J, Smith AJ, Willeit M, Crawley AP, Mikulis DJ, Vitcu I, Kapur S. Separate brain regions code for salience vs. valence during reward prediction in humans. Hum.Brain Mapp. 2007;28:294–302. doi: 10.1002/hbm.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat.Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- Joshua M, Adler A, Mitelman R, Vaadia E, Bergman H. Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J.Neurosci. 2008;28:11673–11684. doi: 10.1523/JNEUROSCI.3839-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZH, Shin R, Ikemoto S. Dual role of medial A10 dopamine neurons in affective encoding. Neuropsychopharmacology. 2008;33:3010–3020. doi: 10.1038/npp.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Mogenson GJ. Effects of peripheral stimulation on the activity of neurons in the ventral tegmental area, substantia nigra and midbrain reticular formation of rats. Brain Res.Bull. 1982;8:7–14. doi: 10.1016/0361-9230(82)90021-1. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanisms of cutaneous nociception. London: Elsevier; 2006. [Google Scholar]
- Miller G. Neuroscience. Brain scans of pain raise questions for the law. Science. 2009;323:195. doi: 10.1126/science.323.5911.195. [DOI] [PubMed] [Google Scholar]
- Milner PM. Brain-stimulation reward: a review. Can.J.Psychol. 1991;45:1–36. doi: 10.1037/h0084275. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J.Neurosci. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, King-Casas B, Cohen JD. Imaging valuation models in human choice. Annu.Rev.Neurosci. 2006;29:417–448. doi: 10.1146/annurev.neuro.29.051605.112903. [DOI] [PubMed] [Google Scholar]
- Niv Y. Cost, benefit, tonic, phasic: what do response rates tell us about dopamine and motivation? Ann.N.Y.Acad.Sci. 2007;1104:357–376. doi: 10.1196/annals.1390.018. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr.Opin.Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J.Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Platt ML, Huettel SA. Risky business: the neuroeconomics of decision making under uncertainty. Nat.Neurosci. 2008;11:398–403. doi: 10.1038/nn2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA. Independence in ROI analysis: where is the voodoo? Soc Cogn Affect Neurosci. 2009;4:208–213. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Emotion explained. Oxford: Oxford university press; 2005. [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Front Behav Neurosci. 2009;3:13. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu.Rev.Neurosci. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu.Rev.Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Romo R. Responses of nigrostriatal dopamine neurons to high-intensity somatosensory stimulation in the anesthetized monkey. J.Neurophysiol. 1987;57:201–217. doi: 10.1152/jn.1987.57.1.201. [DOI] [PubMed] [Google Scholar]
- Schultz W, Romo R. Dopamine neurons of the monkey midbrain: contingencies of responses to stimuli eliciting immediate behavioral reactions. J.Neurophysiol. 1990;63:607–624. doi: 10.1152/jn.1990.63.3.607. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26:10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Dayan P, Koltzenburg M, Jones AK, Dolan RJ, Friston KJ, Frackowiak RS. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K, Dolan R. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat.Neurosci. 2005;8:1234–1240. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister P, De Luca CJ, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(S1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement learning: an introduction. IEEE Trans Neural Netw. 1998;9:1054. [Google Scholar]
- Tsai CT, Nakamura S, Iwama K. Inhibition of neuronal activity of the substantia nigra by noxious stimuli and its modification by the caudate nucleus. Brain Res. 1980;195:299–311. doi: 10.1016/0006-8993(80)90066-9. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Young AM, Joseph MH, Gray JA. Latent inhibition of conditioned dopamine release in rat nucleus accumbens. Neuroscience. 1993;54:5–9. doi: 10.1016/0306-4522(93)90378-s. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.