Abstract
A chloride complex of a hexaprotonated azamacrocycle has been isolated, and its structure has been determined by X-ray crystallography showing two encapsulated chloride anions in the cavity. The two internal guests are coordinated at two binding sites on the opposite side of the macrocycle through trigonal recognition by hydrogen-bonding interactions. The other four chlorides are located outside the cavity, each with a single hydrogen bond from secondary amines. Ab initio calculations based on density functional theory (DFT) suggest that the encapsulation of two chlorides inside the cavity leads to a significant charge transfer from the anions to the protonated amines.
Because of the critical roles played by anions in biological and environmental systems, there is an immense interest in the scientific community to understand molecular recognition of anions with synthetic receptors.1 Among the various systems,2 synthetic azamacrocyles with multiple binding sites are particularly interesting since they mimic many biological polyamines3 and are attractive hosts for binding a diverse variety of inorganic and biological anions,4 as well as transition metal ions.5 A number of azamacrocycles with variable spacers have been reported to bind anionic guests in solution, and their interactions in the solid state have been characterized crystallographically showing the formation of both monotopic and ditopic complexes.6,7 Because of their structural flexibility, monocycles often undergo a conformational change in order to bind anions8; for instance, [24]N6O2 was found to adopt a cleft-like conformation in its hexachloride,8a or tetranitrate salt.8b A similar conformational change was observed in tetraamido macrocycle with perrhenate9 and pentaprotonated [26]phen2N4O2 with halide (chloride and bromide) complexes.10
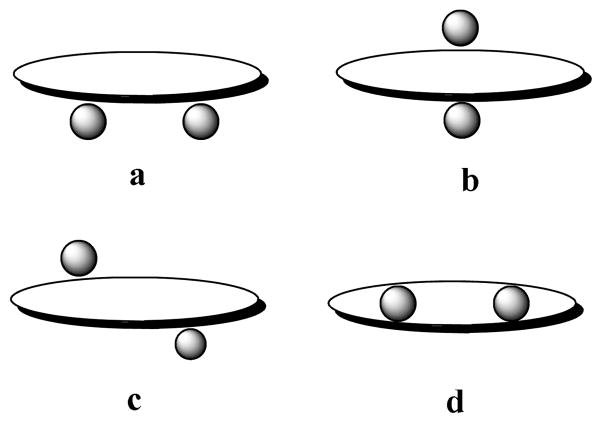
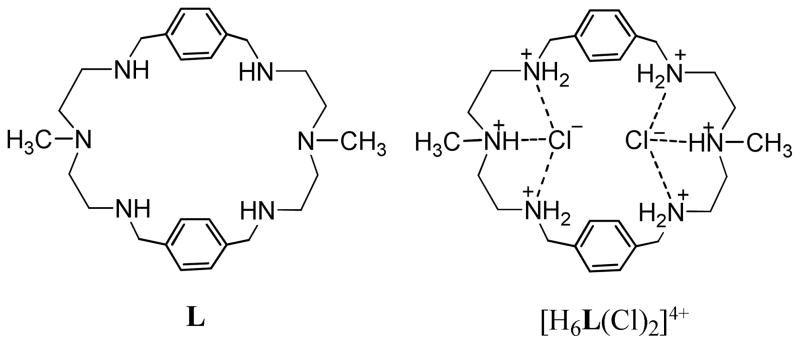
In the case of ditopic complexation, an azamacrocyclic ligand is found to interact with anions from one or both sides of the macrocycle in different fashions (Chart 1) such as face (a), bipyramidal (b) or cross binding (c), without complete encapsulation.11 Small macrocycles comprising multiple binding sites preferentially bind two anions from both sides in a binding fashion b, as observed earlier in the nitrate complex of [18]N4O2 reported by Bowman-James. A similar binding was also reported by Spiccia for [18]N611a and by Steed for metacyclophanes11b complexing chloride, bromide, or iodide. On the other hand, a p-xylyl-based macrocycle with a slightly larger cavity was reported to form ditopic complexes in a “cross binding” with 3,5-dinitrobenzoate,12 “bipyramidal binding” with tosylate13 and “face binding” with perchlorate.14 However, to the best of our knowledge, complete encapsulation of two anions (fashion d) in an azamacrocycle-based ligand has not been reported before, although such binding is present in neutral ligands coordinating two transition metal ions.5,15 In an attempt to explore selective binding partners for simple azamacrocycles, we synthesized a ligand L and isolated a crystal of its chloride salt (Figure 1). In this communication, we present the structural report of two encapsulated chlorides in H6[L]6+, the results of 1H NMR binding studies, and density functional theory (DFT) calculations.
Chart 1.
Classification of ditopic binding of anions by a macrocyclic ligand: (a) face binding, (b) bipyramidal binding, (c) cross binding, and (d) encapsulation.
Figure 1.
Hexaprotonated macrocyle (L) and its chloride salt ([H6L(Cl)2]4+).
The ligand L was prepared from the high-dilution condensation of an equimolar amount of N-methyl-2,2′-diaminodiethylamine and terephthalaldehyde in methanol followed by the reduction with NaBH4, as reported by us previously.16 The chloride complex was obtained as a white powder from the reaction of neutral L (50 mg) with a few drops of concentrated HCl in methanol. Crystals suitable for X-ray analysis were grown from the chloride salt dissolved in H2O/CH3OH (5:1, v/v) under the slow diffusion of Et2O.17
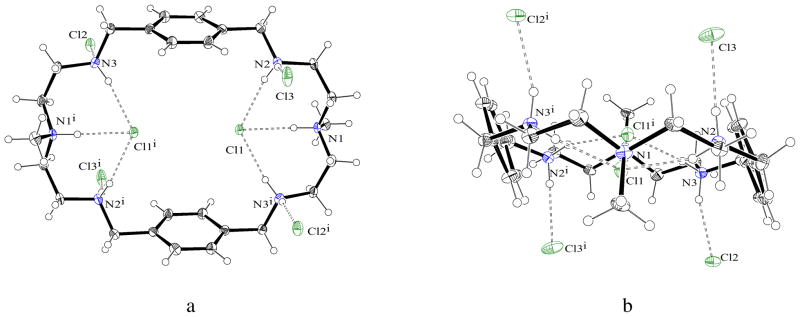
Structural analysis of the chloride complex reveals that the macrocycle in its hexaprotonated state crystallizes with 6 chloride anions and 2.34 water molecules, and lies on an inversion center. All charged nitrogen centers are involved in hydrogen-bonding interactions with chloride anions. As shown in Figure 2, two chlorides are completely encapsulated inside the cavity (Chart 1d) in close contact with two binding units on the opposite side of the macrocycle. The encapsulation occurs through trigonal recognition with three hydrogen bonds at distances ranging from 3.0674 (18) to 3.146 (2) Å (Table 1). These distances are comparable to 3.048(3) or 3.10 Å observed for the NH···Cl− distance in the thiophene-based azacryptand18a or tiny octaazacryptand,18b respectively.
Figure 2.
Crystal structure of the chloride complex L showing two chlorides bonded through trigonal recognition. (a) side view (b) perspective view down the two central amines (water molecules are omitted for clarity).
Table 1.
Hydrogen bonding interactions of symmetry-related encapsulated chlorides within the crystal and the DFT-optimized structure
| Atoms | Experimental bond distance (Å) | DFT-optimized bond distance (Å) |
|---|---|---|
| N1···Cl1 | 3.0674 (18) | 2.986 |
| N2···Cl1 | 3.126 (2) | 3.062 |
| N3···Cl1 | 3.146 (2) | 3.062 |
The two symmetry-related encapsulated chlorides are separated by 4.433 Å and lie at 0.588 Å from the axis of the two central nitrogen atoms. The position of two internal chlorides nearly on the macrocyclic plane is indicative of negligible chloride-chloride repulsion inside the cavity which can be attributed to the reduction of charges on the encapsulated chlorides caused by the formation of three hydrogen bonds. The remaining chlorides are outside the cavity, each with a single hydrogen bond from protonated secondary amines. The macrocyclic unit in the complex is essentially flat rather than a chair conformation observed in the p-xylyl macrocycle19 or in the chloride complex of the larger m-xylyl analogue.8b In the chloride complex of L, two aromatic units are parallel to each other at a distance of 7.842 Å (centroid-to-centroid), while the distance between the two central nitrogens is 10.338 Å, providing an oval-shaped cavity suitable for two chlorides (Figure 3). The six protons from the nitrogen centers at both sides are directed toward the center, making the ligand ideal to anchor the two chlorides inside the cavity.
Figure 3.
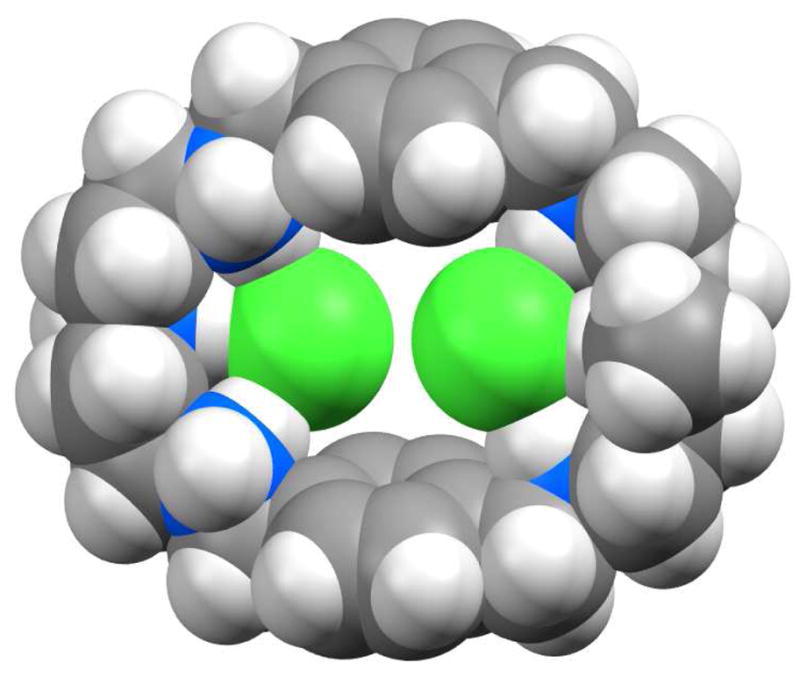
Space-filling representation of [H6L(Cl)2]4+ showing two encapsulated chlorides in the cavity. Color code: white = hydrogen; gray = carbon; blue = nitrogen; green = chloride.
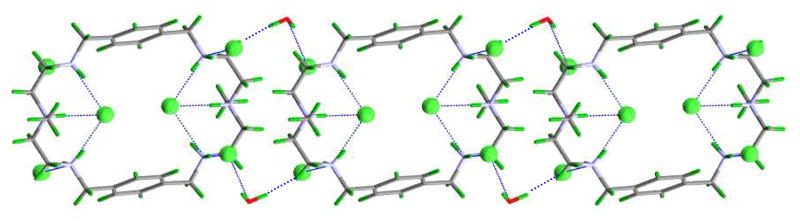
Water molecules in the crystal lattice are located outside the cavity and are not involved in hydrogen-bonding interactions with the macrocycle. However, they are coordinated with the two symmetry-related trans-chlorides located outside the cavity. In extending view, the water molecules play an important role in linking the neighboring macrocyclic units through chloride anions, forming an intermolecular chain viewed along the c axis (Figure 4).
Figure 4.
View of hydrogen-bonded chain of the chloride complex of L (viewed along the c axis). Water molecules are shown to link two macrocycles via chloride anions in the crystal lattice.
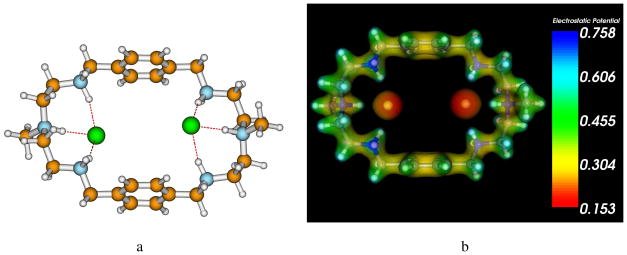
In order to understand the binding interactions of L with chloride anions, a series of DFT calculations were carried out using the Gaussian 09 software codes.20 Since the macrocycle and chlorides are involved in hydrogen bonding interactions, it was necessary to choose a functional which accurately captures these effects on the potential energy surface. To this end, all DFT calculations were performed using the M06-2X21 hybrid functional which incorporates an improved description of dispersion energies, an effect which was previously found to be necessary (compared to other hybrid functionals) for describing noncovalent interactions.22 The initial equilibrium geometry for the free protonated macrocycle with six positive charges was first optimized at the M06-2X/6-31G(d,p) level of theory. From this equilibrium geometry, two chloride anions were added to the center of the macrocycle, and the geometry of the complex was fully re-optimized at the same M06-2X/6-31G(d,p) level of theory. Harmonic frequencies were also computed to verify all structures were true minima. Single-point properties with the 6-311G(d,p) basis set (a diffuse 6-311+G(d,p) basis set was used for the chloride anions) were subsequently performed for each of the relaxed geometries. The calculated binding energy was found to be −756.5 kcal/mol for the two chlorides encapsulated within the protonated macrocycle, while the binding energy for a single anion was significantly less (−394.6 kcal/mol), confirming the experimental observation of two chloride anions internally bound to the macrocycle. The resulting binding energies obtained from the DFT calculations are large and do not take into account solvent effects. However, incorporation of explicit solvent molecules to understand their effect on binding energies would be extremely computationally demanding, especially for this large macrocycle. Our DFT results should thus be considered as upper bounds for both the binding energy and charge transfer between the chloride ions and the macrocycle. In a related work, the group of Bowman-James calculated binding energies of −364 and −668 kcal/mol for 1:1 and 1:2 nitrate binding in hexaprotonated m-xylyl-based cryptand.23 In the optimized structure of [H6L(Cl)2]4+ shown in Figure 5a, each encapsulated chloride is coordinated with three NH···Cl− bonds at each binding unit of the macrocycle in good agreement with the experimental crystal structure (Table 1). From the DFT-optimized geometry, we also computed the total electrostatic potential from the self-consistent density matrix. As shown in Figure 5(b), the chloride ions have the most (negatively-charged) electron density of the entire complex and, therefore, have the lowest electrostatic potential. However, since the protonated macrocycle has a very large positive charge, there is significant charge transfer between the chlorides and the protonated amines, and the resulting potential on the chlorides is slightly positive.
Figure 5.
(a) Optimized geometry of [H6L(Cl)2]4+ at the M06-2X/6-31G(d,p) level of theory. (b) Electrostatic potential of [H6L(Cl)2]4+.
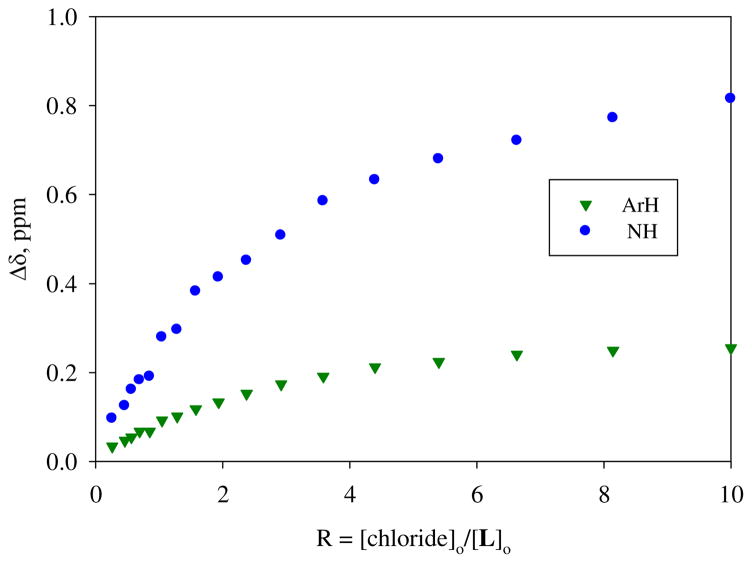
In solution, the binding affinity of L for chloride was evaluated by 1H NMR titration studies using H6L(OTs)6 in DMSO-d6. Tetrabutyl ammonium chloride was used as a source for chloride ions. The titration of H6L(OTs)6 (5 mM) with chloride anion (50 mM) resulted in a downfield shift of macrocyclic protons (Figure 6 and supporting materials). The largest shift was observed for NH protons, demonstrating the participation of NH in chloride binding, as seen in the solid state. The change in the chemical shift with an increasing amount of chloride solution at room temperature gave the best fit for a 1:1 complex, yielding a binding constant K = 70 M−1. The obtained 1:1 binding mode, however, is not consistent with the results observed in crystal structure. This inconsistency is most likely due to the effect of polar solvent used in the titration which tends to highly solvate the ligand, resulting in reduced populations of binding sites.14,16 We were, therefore, interested in examining the binding studies in a non-polar solvent like CDCl3. However, in CDCl3, the titration of H6L(OTs)6 (5 mM) with tetrabutyl ammonium chloride was hampered due to precipitation that occurred after an addition of guest solution, resulting in the gradual disappearance of ligand resonances. Further dilution (up to 2 mM) of the initial concentration of the ligand solution also did not allow us to complete the full titration. However, this observation indicates that the ligand interacts strongly with chloride in chloroform forming a complex that is poorly soluble in non-polar solvent, as expected.
Figure 6.
1H NMR titration curves of H6L(OTs)6 with chloride in DMSO-d6 at room temperature.
In summary, we have demonstrated an elegant example of an azamacrocycle capable of encapsulating two chlorides inside its cavity, a phenomenon commonly observed in macrocyclic systems which bind two transition metal ions.5.15 All nitrogens in the anion complex are protonated, and the two chlorides remain within the cavity in close contact with binding units at opposite sides. The six protons on the nitrogen centers point towards the cavity, making an attractive cavity for encapsulating two chlorides within the macrocyclic ring. Each of the remaining four chlorides binds outside the cavity with a single hydrogen bond from four secondary amines. In the complex, all ten protons (eight from secondary and two from tertiary nitrogens) on the charged nitrogen centers are effectively utilized in coordinating chloride anions. The water molecules that are present in the lattice play an important role in linking the neighboring macrocyclic frameworks through the external chlorides. Ab initio calculations performed on the ligand-chloride complex not only support the stabilization of two chlorides inside the highly charged cavity, but also suggest that the complex with two encapsulated chlorides is energetically more favorable than that with one chloride. While there are many examples of ditopic binding of anions in different fashions (Chart 1a–c),6,7,11–14 the complete encapsulation of two anionic guests has not been previously shown in an azamacrocycle-based ligand. The presence of two methyl groups coupled with fully-charged nitrogens makes the ligand ideal for fitting two compatible chlorides inside the cavity through trigonal recognition with three hydrogen bonds. We are currently pursuing further work on related ligands for designing other charge-assisted encapsulated complexes.
Supplementary Material
Acknowledgments
The project described was supported by Grant Number G12RR013459 from the National Center for Research Resources. This material is based upon work supported by the National Science Foundation under CHE-0821357. Purchase of the diffractometer was made possible by grant No. LEQSF (1999-2000)-ENH-TR-13, administered by the Louisiana Board of Regents.
Footnotes
Supporting Information available: Crystallographic file in CIF format, 1H NMR titration spectra, hydrogen bonding interactions, and full list of the authors in Ref 20. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Lehn J-M. Supramolecular Chemistry: Concepts and Perspectives. VCH; Weinheim, Germany: 1995. [Google Scholar]; (b) Beer PD, Wheeler JW, Moore C. In: Supramolecular Chemistry. Balzani V, De Cola L, editors. Kluwer Academic Publishers; Dordrecht: 1992. p. 105. [Google Scholar]; (c) Seel C, Galan A, de Mendoza J. Top Curr Chem. 1995;175:101. [Google Scholar]; (d) García-España E, Díaz P, Llinares JM, Bianchi A. Coord Chem Rev. 2006;250:2952. [Google Scholar]; (e) Gale PA. Acc Chem Res. 2006;39:465. doi: 10.1021/ar040237q. [DOI] [PubMed] [Google Scholar]; (g) Bowman-James K. Acc Chem Res. 2005;38:671. doi: 10.1021/ar040071t. [DOI] [PubMed] [Google Scholar]; (f) Sessler L, Gale PA, Cho WS. In: Anion Receptor Chemistry, (Monographs in Supramolecular Chemistry) Stoddart JF, editor. Royal Society of Chemistry; Cambridge: 2006. [Google Scholar]
- 2.(a) Bianchi A, Bowman-James K, García-España E, editors. Supramolecular Chemistry of Anions. Wiley-VCH; New York: 1997. [Google Scholar]; (b) Gale PA, editor. Coord Chem Rev. 2003. 35 years of Synthetic Anion Receptor Chemistry; p. 240. [Google Scholar]; (c) Hossain MA. Curr Org Chem. 2008;12:1231. [Google Scholar]
- 3.Nagarajan S, Ganem B. J Org Chem. 1987;52:5044. [Google Scholar]
- 4.(a) Motekaitis RJ, Martell AE, Dietrich B, Lehn JM. Inorg Chem. 1984;23:1588. [Google Scholar]; (b) Bazzicaluppi C, Bencini A, Bianchi A, Fusi V, Giorgi C, Paoletti P, Stefani A, Valtancoli B. J Chem Soc, Perkin Trans 2. 1995:275. [Google Scholar]; (c) Wiórkiewicz-Kuczera J, Kuczera K, Bazzicalupi C, Bencini A, Valtancoli B, Bianchi A, Bowman-James K. New J Chem. 1999;23:1007. [Google Scholar]; (d) Clifford T, Danby A, Llinares JM, Mason S, Alcock NW, Powell D, Aguilar JA, Garcia-Espana E, Bowman-James K. Inorg Chem. 2001;40:4710. doi: 10.1021/ic010135l. [DOI] [PubMed] [Google Scholar]
- 5.(a) Wang Z, Reibenspies J, Martell AE. Inorg Chem. 1997;36:629. [Google Scholar]; (b) Nation DA, Martell AE, Carroll RI, Clearfield A. Inorg Chem. 1996;35:7246. doi: 10.1021/ic960661q. [DOI] [PubMed] [Google Scholar]; (c) Hosseini MW, Lehn JM, Duff SR, Gu K, Mertes MP. J Org Chem. 1987;52:1662. [Google Scholar]; (d) Tomat E, Cuesta L, Lynch VM, Sessler JL. Inorg Chem. 2007;46:6224. doi: 10.1021/ic700933p. [DOI] [PubMed] [Google Scholar]; (e) Andrés A, Aragó J, Bencini A, Bianchi A, Domenech A, Fusi V, García-España E, Paoletti PJM, Ramírez JA. Inorg Chem. 1993;32:3418. [Google Scholar]; (f) Bencini A, Bianchi A, Garcia-Espana E, Scott EC, Morales L, Wang B, Deffo T, Takusagawa F, Mertes MP, Mertes KB, Paoletti P. Bioorg Chem. 1992;20:8. [Google Scholar]; (g) Hosseini MW, Lehn JM. Helv Chim Acta. 1987;70:1312. [Google Scholar]; (h) Hosseini MW, Lehn JM. Chem Commun. 1991:451. [Google Scholar]; (i) Martinez-Sanchez J-M, Bastida de la Calle R, Macias A, Perez-Lourido P, Matarranz LV. Polyhedron. 2006;25:3495. [Google Scholar]
- 6.Llinares JM, Powell D, Bowman-James K. Coord Chem Rev. 2003;240:57. [Google Scholar]
- 7.García-España E, Díaz P, Llinares JM, Bianchi A. Coord Chem Rev. 2006;250:2952. [Google Scholar]
- 8.(a) Boudon S, Decian A, Fischer J, Hosseini MW, Lehn JM, Wipff GJ. Coord Chem. 1991;23:113. [Google Scholar]; (b) Papoyan G, Gu K, Wiórkiewicz-Kuczera J, Kuczera K, Bowman-James K. J Am Chem Soc. 1996;118:1354. [Google Scholar]; (c) Liu HY, Wei GH, Ma JF. Acta Crystallogr. 2008;E64:o126. doi: 10.1107/S1600536807063064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roehm B, Ghosh S, Begum R, Kut J, Hossain MA, Day V, Bowman-James K. Inorg Chem. 2007;46:9519. doi: 10.1021/ic701371f. [DOI] [PubMed] [Google Scholar]
- 10.Drew GB, Félix V. J Org Chem. 2007;72:4023. doi: 10.1021/jo062653p. [DOI] [PubMed] [Google Scholar]
- 11.(a) Warden AC, Warren M, Hearn MTW, Spiccia L. New J Chem. 2004;28:1160. [Google Scholar]; (b) Illioudis CA, Steed JW. Organ Biomol Chem. 2005;3:2935. doi: 10.1039/b506828b. [DOI] [PubMed] [Google Scholar]
- 12.Burchell CJ, Ferguson G, Lough AJ, Glidewell C. Acta Cryst. 2001;C57:88. doi: 10.1107/s0108270100013792. [DOI] [PubMed] [Google Scholar]
- 13.Saeed MA, Thompson JJ, Fronczek FR, Hossain MA. Acta Cryst. 2009;E65:o2405. doi: 10.1107/S1600536809035648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saeed MA, Thompson JJ, Fronczek FR, Hossain MA. Cryst Eng Comm. 2010;12:674. doi: 10.1039/b918152k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao J, Reibenspies JH, Martell AE. Inorg Chim Acta. 2002;335:125. [Google Scholar]
- 16.Gibson D, Dey KR, Fronczek FR, Hossain MA. Tetrahedron Lett. 2009;50:6537. doi: 10.1016/j.tetlet.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crystallographic data: C26H48N6Cl6·2.34H2O, M = 699.47, crystal size 0.25 × 0.18 × 0.17 mm3, Monoclinic, P21/c, a = 10.925 (3), b = 12.736 (2), c = 12.453 (3) Å, β = 98.413 (9)°, V = 1714.1 (7) Å3, Z = 2, dcalc = 1.355 Mg m−3, T = 90 K, F(000) = 743, , μ(Mo-Kα) = 50.54 mm−1, 4083 independent reflections (18451 measured), Rint = 0.066, R[F2 > 2σ(F2)] = 0.054, wR2 = 0.122. Structural data were collected on a Nonius KappaCCD (with Oxford Cryostream) diffractometer. The structure was solved by direct methods and refined with the full-matrix least-squares technique. Selected bond lengths and angles for hydrogen bonding interactions in the complex are listed in Table S1.
- 18.(a) Saeed MA, Fronczek FR, Hossain MA. Chem Commun. 2009:6409. doi: 10.1039/b916099j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hossain MA, Llinares JM, Miller C, Seib L, Bowman-James K. Chem Commun. 2000:2269. [Google Scholar]
- 19.He WJ, Ye ZF, Xu Y, Guo ZJ, Zhu LG. Acta Cryst. 2000;C56:1019. doi: 10.1107/s0108270100007095. [DOI] [PubMed] [Google Scholar]
- 20.Frisch MJ, et al. Gaussian 09, Revision A.02. Gaussian, Inc; Wallingford, CT: 2009. [Google Scholar]
- 21.Zhao Y, Truhlar DG. Theor Chem Acc. 2008;120:215. [Google Scholar]
- 22.(a) Wong BM. J Comput Chem. 2009;20:51. doi: 10.1002/jcc.21022. [DOI] [PubMed] [Google Scholar]; (b) Dey KR, Wong BM, Hossain MA. Tetrahedron Lett. 2010;51:1329. doi: 10.1016/j.tetlet.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason S, Clifford T, Seib L, Kuczera K, Bowman-James K. J Am Chem Soc. 1998;120:8899. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.