Abstract
Sleep disordered breathing (SDB) is a medical condition that has increasingly recognized adverse health effects. Obesity is the primary risk factor for the development of SDB and contributes to cardiovascular and metabolic abnormalities in this population. However, accumulating evidence suggests that SDB may be related to the development of these abnormalities independent of obesity. Periodic apneas and hypopneas during sleep result in intermittent hypoxemia, arousals and sleep disturbances. These pathophysiologic characteristics of SDB are likely mechanisms underlying cardiovascular and metabolic abnormalities including hypertension and other cardiovascular diseases, altered adipokines, inflammatory cytokines, insulin resistance, and glucose intolerance. It appears that treatment of SDB with continuous positive airway pressure reverses some but not all of these abnormalities; however, studies to date have demonstrated inconsistent findings. Weight loss strategies, including diet, exercise, medications and bariatric surgery have been evaluated as a treatment strategy for SDB. In preliminary studies, dietary intervention and exercise have reduced severity of SDB. One study demonstrated mild reductions in weight and subsequent improvements in SDB severity using the weight-reducing medication, sibutramine. In morbidly obese subjects, bariatric surgery effectively induces weight loss and improvement in SDB severity and symptoms, but long-term benefits remain uncertain. Large randomized trials are required to determine the utility of these strategies as long-term approaches to improving SDB and reducing associated complications.
Keywords: Sleep disordered breathing, obesity, glucose intolerance, weight loss, cytokines
Introduction
Sleep disordered breathing (SDB) is a term that encompasses obstructive apneas, central apneas, hypopneas and respiratory effort-related arousals that occur during sleep. Evidence increasingly indicates that SDB is associated with adverse effects on health and represents a growing public health concern (1). It is estimated that 18 millions Americans have SDB, many of whom remain undiagnosed (2). The prevalence of clinically significant SDB is estimated to be 2-4% in middle-aged adults (3). The therapy of choice for SDB is continuous positive airway pressure (CPAP), a device that splints open the upper airway by forcing positive air pressure in through the nose.
Obesity is one of the most significant risk factors for the development of SDB (4-6) and over 70 percent of patients with SDB are obese (7). Likewise, significant SDB is found in approximately 40 percent of obese persons (7). Patients with SDB appear to be predisposed to weight gain and have abnormalities in plasma leptin, ghrelin and other mediators that are involved in regulating body weight (8-12). Thus, the relationship between obesity and SDB is complex and may be bi-directional, such that SDB may also contribute to obesity. The purpose of this review is to provide a brief overview of the relationship between SDB, obesity, and associated cardiovascular and metabolic morbidity and complications. In addition, this review will highlight the effects of weight loss and other SDB treatments, and their influence on SDB and its consequences.
Pathophysiology of SDB
Respiratory perturbations during sleep including, obstructive apneas, central apneas, hypopneas, and respiratory effort-related arousals (RERAs) are collectively referred to as SDB. Obstructive apneas occur when the upper airway collapses during sleep, blocking airflow and oxygenation despite continued respiratory effort. Central apneas occur when loss of respiratory effort accompanies reductions in airflow. Hypopneas are described as reductions in airflow (partial airway closure) accompanied by arterial oxygen desaturation. RERAs are difficult breathing, snoring and throat narrowing that may not be enough to produce apneas or hypopneas but can awaken a person. Obesity, male gender, upper airway anatomical abnormalities, and increasing age are all risk factors for the development of SDB (13). The mechanisms of airway occlusion are heterogeneous and factors such as anatomical abnormalities, upper airway dilator muscle function, arousal threshold, and abnormalities in the control of breathing may all influence propensity for airway obstruction during sleep (14). As a result, the exact causes of airway obstruction and evolution of SDB vary between individual patients.
Clinical Symptoms and Diagnostic Criteria of SDB
SDB results in intermittent episodes of hypoxemia, hypercapnia, arousal, and subsequent fragmented sleep, leading to daytime sleepiness, increased risk of motor vehicle accidents, reduced quality of life and a variety of cardiovascular, psychiatric, and metabolic abnormalities (12,15,16). SDB severity is usually quantified by the number of apneas and hypopneas (AHI) or number of apneas, hypopneas and respiratory effort related arousals (RDI) per hour of sleep. Less than five respiratory events per hour of sleep is considered normal, five-15 respiratory events/hour sleep is mild SDB, 15-30 events/hour sleep is moderate SDB, and >30 events/hour sleep is severe SDB.
Obesity-related Contribution to SDB
Of all the risk factors for development of SDB, obesity may be the strongest and most studied (17-20). Increased body weight heightens the risk of prevalent SDB in population studies and weight gain over time increases the likelihood of developing or worsening SDB (21). Peppard and colleagues demonstrated in a longitudinal study that a 10% gain in body weight increased the odds of developing moderate-severe SDB by six-fold (21). In addition, each 1% change in body weight was associated with a 3% change in AHI (21) (Figure 1).
Figure 1.
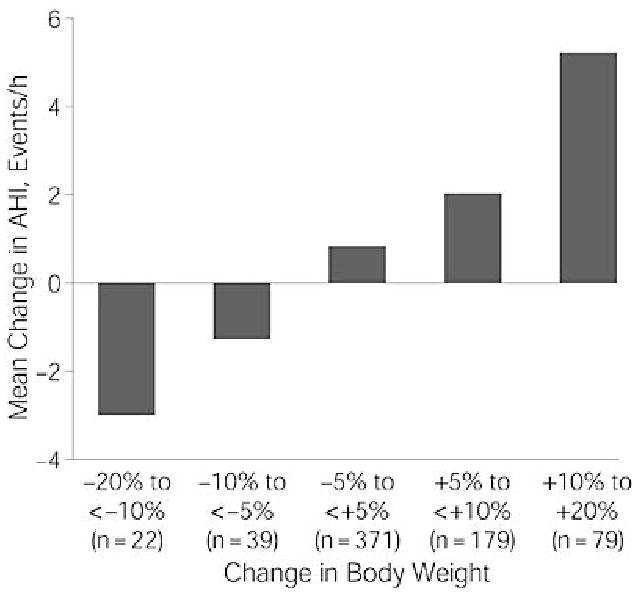
The mean change in apnea hypopnea index was related in a dose-response fashion to change in body weight during four years of follow-up. Used with permission (21).
The distribution and location of adipose tissue appears to influence the risk of developing SDB and contributes to the pathogenesis of SDB events. Increased fat and muscle tissue in the neck may narrow airway size and increase upper airway resistance (22,23). Men are at higher risk of SDB, and since women have less adipose tissue on the neck, trunk, and abdomen, it is theorized that sex-related differences in fat distribution may play a role in men's increased risk (24-26). Increases in central adiposity seen with aging and in postmenopausal women may explain the higher prevalence of SDB in these groups (27). Deposition of fat in tissues surrounding the upper airway produces a greater mechanical load on the upper airway that increases the likelihood of obstruction during sleep. Deposition of fat over the chest wall and abdomen reduces chest wall compliance and lowers lung volumes (28-30). Particularly important is the reduction in functional residual capacity caused by high intra-abdominal pressure. This decrease in end-expiratory lung volume reduces the “tug” on the trachea normally produced during inspiration by negative intrathoracic pressure. As a result, the lateral pharyngeal walls are thickened and the airway is narrowed. This obesity-related decrease in end-expiratory lung volume, combined with increased tissue oxygen consumption rates, results in faster depletion of lung oxygen stores during apnea (31). Thus, the amount of oxygen desaturation for a given apnea length is increased in obese vs. normal weight individuals.
Excess body weight and obesity clearly promote the development of SDB, but increasing evidence suggests that SDB may also promote weight gain and obesity (Figure 2). Repetitive episodes of SDB disrupt sleep and cause excessive sleepiness during the daytime. Sleepiness may reduce physical activity and promote weight gain over time. As a result, investigators have become interested in whether patients with SDB have reduced daily physical activity. A recent evaluation of daytime activity monitored by actigraphy was completed in patients with SDB. West et al monitored the effects of therapeutic and sham CPAP on daily activity, body weight and sleepiness in patients with SDB (32). Therapeutic CPAP reduced daytime sleepiness, but neither therapeutic nor sham CPAP altered activity levels or body weight over the 3 month study period (32). The authors postulate that habitual activity patterns or pre-existing reasons for lower physical activity may contribute to the findings (32). In contrast, analysis of physical activity levels in heart failure patients showed that patients with SDB were less active during the day compared to patients without SDB who had similar left ventricular function (33). Further research that monitors specific activities and patterns in the different patient groups with SDB may explain differences in these findings and the possible effect of CPAP on activity and on body weight.
Figure 2.
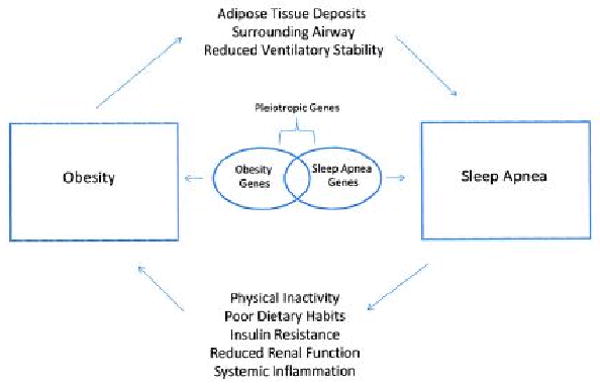
The potential mechanisms that mediate the relationship between sleep disordered breathing and obesity. Obesity imparts mechanical load on the upper airway predisposing it to collapse and repeated apneas during sleep. In turn, sleep apnea produces daytime sleepiness, and may promote physical inactivity and metabolic abnormalities that contribute to increases in body weight. Used with permission (178).
Since exercise programs may help patients lose weight and reduce the severity of SDB, increasing patients' physical activity appears to be an important treatment goal for patients with SDB. However, patients with SDB may be predisposed to weight gain and may have difficulty losing weight. Phillips et al showed that patients with SDB have greater weight gain in the year prior to being diagnosed with SDB compared to patients without SDB (12). This may reflect abnormalities in basal metabolic rate or metabolic regulators such as leptin and ghrelin.
Weight gain occurs when caloric intake exceeds energy expenditure (34). Energy expenditure includes resting energy expenditure (REE), dietary thermogenesis, energy expended during exercise, and energy expenditure in response to environmental stress (34). Characteristics of SDB, including arousals from sleep, and sympathetic activation may influence energy expenditure and risk of weight gain (35-37). Dysregulation of energy expenditure may contribute to the propensity for weight gain in patients with SDB. Unfortunately, studies investigating the effects of SDB on energy expenditure have been inconsistent (34,38-41). A recent study evaluated REE in adult patients with signs or symptoms of SDB (34). Resting energy expenditure was not different between patients free of SDB and patients with mild, moderate and severe SDB (34). However, in multiple regression analyses, AHI was positively correlated to REE after adjustment for age, sex, health status, and body mass index (BMI) in the models (31). Although alterations in energy expenditure would explain propensity for weight gain associated with SDB, energy expenditure is not decreased and may actually be increased in SDB. It is clear that other factors influence energy balance and weight gain in SDB.
Hormonal and Inflammatory Effects
Leptin is secreted by adipose tissue and normally acts to reduce appetite and food intake while ghrelin increases appetite and caloric intake. Patients with SDB have elevated plasma leptin levels, even when adiposity is accounted for (8-11). Since leptin functions to reduce serum insulin concentrations, hyperleptinemia may be a compensatory response to normalize insulin resistance and other metabolic abnormalities. Hyperleptinemia in the presence of obesity and SDB may also be indicative of “leptin resistance” whereby weight reducing and appetite regulating signals of leptin are not appropriately detected in the hypothalamus, resulting in persistent obesity and/or weight gain. Plasma ghrelin concentrations are also elevated in patients with SDB, which may increase caloric intake and facilitate weight gain (11,42). Although leptin and ghrelin are not used clinically as indicators of obesity, alterations in their expression indicate patients with SDB are at risk for weight gain and obesity. Therapy with CPAP reduces SDB-related elevations in plasma leptin and ghrelin, suggesting that these defects are somewhat reversible (42-44). Whether or not alleviation of SDB facilitates weight loss is unclear since studies evaluating weight before and after CPAP have reported inconsistent findings (43,45,46). Although CPAP has numerous benefits for patients with SDB, the current literature does not suggest that weight loss is one of them. Preliminary studies in patients with SDB that evaluated other adipose-related hormones such as adiponectin have produced inconsistent results (47-51). Adiponectin expression is inversely correlated with BMI, and adiponectin is involved in glucose and fatty acid regulation and may help ameliorate atherosclerosis, Type 2 diabetes, and obesity (47-51). Abnormalities in obesity-related hormones resulting from SDB may impair metabolic homeostasis, contribute to a positive energy balance that promotes weight gain, and increase cardiovascular risk.
Inflammation
Growing evidence suggests that inflammation plays an integral role in the development of vascular abnormalities and cardiovascular disease (52). Inflammation has also been proposed as a putative mechanism of cardiovascular disease in patients with SDB. In addition, increased activity of inflammatory pathways may impair insulin action in peripheral tissues (53). Inflammatory mediators such as C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF- α) and interleukin-8 (IL-8) have been studied in patients with SDB, leading to the hypothesis that dysregulation of inflammatory cytokines contributes to progression of cardiovascular disease and metabolic abnormalities in such individuals (54-58).
CRP is an inflammatory serum protein that is produced in adipose tissue and the liver (52,59). In population studies, CRP has been linked to increased risk of cerebrovascular and cardiovascular disease (60,61). A pilot study of newly diagnosed SDB patients matched for age and BMI with non-SDB controls demonstrated that serum CRP was significantly elevated in patients with SDB (57). Subsequent studies found that when obesity was accounted for, SDB severity and CRP were not independently related. Thus, obesity may mediate most of the relationship between SDB and CRP (62-65). In addition, the Wisconsin Sleep Cohort Study analyzed 907 patients with SDB and did not find a link between SDB and elevated CRP that was independent of obesity (65). CRP's relationship to obesity may be mediated by IL-6. IL-6 is a pro-inflammatory cytokine secreted from adipose tissue that stimulates CRP production in the liver. IL-6 concentrations are elevated in obese subjects (66) and initial studies demonstrated a significant increase in circulating IL-6 levels in patients with SDB (67,68). However, recent studies have shown no difference between SDB patients and non-SDB controls in levels of IL-6 (69). Although pilot studies initially suggested a relationship between SDB, CRP and IL-6, subsequent investigations suggest that CRP and IL-6 may not be independently related to SDB and their role in the genesis and progression of cardiovascular disease in patients with SDB is not entirely clear.
Alterations in TNF- α and IL-8 have also been observed in patients with SDB. TNF- α and IL-8 are also pro-inflammatory cytokines that may be involved in the pathogenesis of cardiovascular disease (70,71). Studies have shown that concentrations of TNF- α are significantly higher in patients with SDB, independent of obesity, and that treatment with CPAP decreases TNF- α levels (54,68,72). TNF- α also appears to mediate sleepiness in patients with SDB. Vgontzas et al demonstrated that three weeks of etanercept (a TNF- α inhibitor) reduced daytime sleepiness in patients with SDB (73). Increased levels of IL-8 have also been associated with SDB (69,74,75). Two of these studies also reported a decrease in IL-8 levels with CPAP therapy (69,75). TNF- α and IL-8 appear to be independently related to SDB and may contribute to inflammation and subsequent risk of cardiovascular disease.
Potential Mechanisms of Inflammation in SDB
Intermittent hypoxia and sleep disruption are pathogenic characteristics of SDB that are potential mechanisms of altered inflammatory mediators in patients with SDB (57). Intermittent hypoxia increases expression of the transcription factor nuclear factor kappa B (NF-κB) which activates production of TNF- α and IL-8 (72). Furthermore, hypoxic conditions at high altitude also increase levels of IL-6 and CRP (76), while sleep deprivation increases plasma levels of IL-6 (54,67). Studies in children with SDB have shown plasma CRP levels are positively correlated with both AHI and arousal index measures (77). Thus, both the intermittent hypoxia and sleep fragmentation associated with SDB may underlie abnormalities in inflammatory markers in patients with SDB. Elevations in inflammatory cytokines along with obesity may result in increased risk of cardiovascular disease, but determining the exact role obesity plays in this risk has been heavily scrutinized.
Clinical Consequences of SDB
Sleep Disordered Breathing, Obesity and Cardiovascular Comorbidities
Findings of early studies suggesting that SDB was associated with hypertension and other cardiovascular diseases were questioned since, in some cases, confounding variables such as obesity were not adequately controlled. This was understandable since patients with SDB and cardiovascular diseases had overlapping risk factors including obesity and obesity is independently correlated with hypertension. Subsequent investigations have accounted for known confounders and have still demonstrated significant associations between SDB, hypertension and other CVD. Epidemiologic studies have consistently demonstrated increased prevalence of hypertension, stroke, arrhythmias, and cardiovascular disease in patients with SDB. (78,79). Furthermore, the Wisconsin Sleep Cohort Study demonstrated that SDB is an independent risk factor for the development of hypertension. In this landmark study, investigators demonstrated that presence of SDB at baseline increased the risk of developing hypertension four years later (78). The risk of developing hypertension increased with severity of baseline SDB (78). In the most recent hypertension treatment guidelines, SDB is recognized as an identifiable cause of hypertension (80). However, recently published data from the prospective Sleep Heart Health population study found that in patients without hypertension, presence of SDB at baseline did not predict development of hypertension independent of obesity after five years of follow-up (81). Since subjects in the Wisconsin Sleep Cohort Study and Sleep Heart Health Study were very different in age and heterogeneity, the conflicting results need to be interpreted with caution.
Direct evidence that SDB is a primary etiologic risk factor for development of stroke, coronary artery disease and heart failure is lacking. While pathophysiologic and cross-sectional studies suggest there is a relationship between SDB and these conditions, causation has not been established. In addition to cardiovascular abnormalities, increasing evidence suggests that SDB may alter insulin sensitivity and glucose metabolism.
Sleep Disordered Breathing, Glucose Handling and Type 2 diabetes
Type 2 diabetes and SDB frequently coexist in obese patients. However, there is emerging evidence that SDB and diabetes are related, and that the relationship between them is independent of obesity. Population studies suggest that patients with SDB frequently have diabetes, and conversely, that SDB is prevalent in patients with known diabetes (82-84). As a result, numerous population-based, clinic-based and mechanistic studies have been performed to determine if SDB leads to the development or worsening of Type 2 diabetes. To provide insight about the relationship, studies have focused on insulin sensitivity, glucose metabolism, development of diabetes, and treatment effects of continuous positive airway pressure in patients with SDB.
The majority of population-based studies have demonstrated a relationship between SDB or markers of SDB severity and Type 2 diabetes and have been cross-sectional in nature (85-92). These relationships have been significant even when accounting for adiposity (85,86,89,90). Abnormalities in fasting blood glucose, fasting insulin, hemoglobin A1c, oral glucose tolerance tests, and estimations of insulin resistance using the homeostasis model assessment (HOMA) are greater in SDB subjects or are related to severity of SDB (85-91). The only prospective analysis to diagnose SDB with polysomnography demonstrated greater prevalence of diabetes in SDB subjects (AHI≥15 events/hour), however, no independent relationship was found between SDB and incident diabetes after four years of follow-up (93).
Many clinic-based studies have consistently shown associations between abnormalities in glucose metabolism, insulin resistance and SDB (47,54,82, 94-100). As in the population-based studies, these associations have been shown to be independent of obesity (54,97-100). In these studies, various measures of obesity have been used, including BMI, wasit/hip ratio, and visceral fat measurements, however, these measures may be suboptimal measures of adiposity. To address this, Punjabi and Beamer measured insulin sensitivity, glucose disposition and beta-cell insulin output after an intravenous glucose tolerance test in patients with SDB (101). Adiposity was measured using dual energy x-ray absorptiometry, providing a precise measure of body fat. The study found that independent of adiposity, SDB subjects had impaired insulin sensitivity and glucose disposition index (an integrated measure of pancreatic beta-cell function), which was correlated with severity of SDB (101). Interestingly, they also demonstrated that even in the presence of reduced insulin sensitivity, patients with SDB did not increase pancreatic beta cell insulin output (101). Normally, as insulin sensitivity decreases, pancreatic beta cells increase insulin output in an attempt to maintain euglycemia. Interpreted collectively, this data suggests that subjects with SDB have multiple metabolic deficits that are key in the development of Type 2 diabetes.
Numerous studies have attempted to determine if treatment of SDB with CPAP reverses metabolic abnormalities and improves glycemic control in patients with and without existing Type 2 diabetes. Unfortunately, studies evaluating the effects of CPAP on glycemic measures and control of type 2 diabetes have demonstrated inconsistent results (102-109). While some studies have found improvements in insulin resistance and blood sugar control, others have demonstrated no change over the course of CPAP therapy. A recent study rigorously evaluated if therapeutic CPAP improved blood glucose control and insulin resistance compared to sham CPAP in patients with type 2 diabetes and SDB (110). Previous studies had been confounded by the lack of a control group that limited the interpretation of their results (102-106). After three months of treatment, glycemic control and insulin resistance did not significantly change in either sham or therapeutic CPAP groups. Use of CPAP was low (3.6±2.8 hrs/night) in the therapeutic CPAP group but was not correlated to measures of glycemic control (110). The conflicting results could be a result of differences in study design, study population characteristics, duration of CPAP therapy, adherence to CPAP therapy, and possible changes in body weight between studies (111). Future studies should evaluate the effects of CPAP over longer treatment periods (e.g. > 6 months) with objectively documented CPAP adherence. It is also important to evaluate the effects of CPAP on glycemia and insulin resistance in patients with SDB who are prediabetic to evaluate how the reversal of SDB influences glucose abnormalities before the onset of diagnosed diabetes. Current research does not provide evidence that treating SDB improves glucose control in patients with established diabetes, but current evidence does suggest that SDB does negatively influence glucose homeostasis.
Potential Mechanisms of Glucose and Insulin Disturbances in Sleep Disordered Breathing
SDB has numerous pathophysiologic effects that may influence insulin sensitivity, glucose metabolism and metabolic homeostasis. Studies in animals and healthy humans have demonstrated that intermittent hypoxia and sleep loss reduce insulin sensitivity and glucose tolerance (112-114). Interestingly, one of these studies found that intermittent hypoxia produced insulin resistance in conscious mice that had pharmacologic denervation of sympathetic and parasympathetic nervous systems (113). These findings suggest that sympathetic activation is not required for development of acute insulin resistance to intermittent hypoxia. Sympathetic activation may however contribute to the development of hypoxia-induced insulin resistance over time. Intermittent hypoxia and sleep fragmentation may elicit changes in glucose and insulin homeostasis through a number of mechanisms including increased sympathetic nervous system activity, abnormalities in adipokines and inflammatory cytokines, alterations in the HPA axis and oxidative stress (Figure 3). Animal and physiologic human studies have shown that sympathetic nerve activity, inflammatory cytokines, and HPA axis dysfunction contribute to insulin resistance and/or impaired glucose tolerance (115-119).
Figure 3.
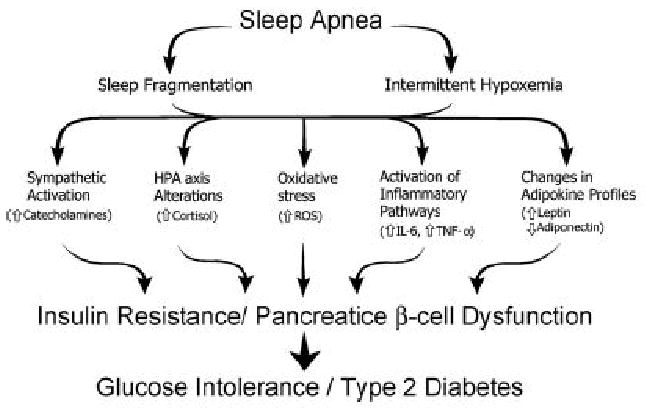
The potential mechanisms of glucose intolerance and insulin resistance in patients with sleep disordered breathing. Used with permission (179).
Treatment of SDB
General Approach and Treatment Options
Continuous positive airway pressure is the therapy of choice for SDB and produces numerous benefits for patients. First, CPAP improves nocturnal sleep disturbances and daytime sequelae of SDB, including daytime sleepiness, quality of life and cognitive function (120). Second, CPAP attenuates numerous cardiovascular and metabolic abnormalities and may reduce cardiovascular risk (121-125). Use of CPAP may improve survival compared to untreated SDB. Marin et al demonstrated greater incidence of fatal and nonfatal cardiovascular events in patients with severe SDB who were untreated compared to patients treated with CPAP (126). Although it is possible that patients who readily use CPAP may more willingly engage in healthful behaviors that influence cardiovascular risk, results of observational studies are consistent. Other observational studies have also demonstrated increased mortality in patients with SDB (127-131). Thus, a prospective, randomized controlled trial is needed to confirm the survival benefits of CPAP suggested by the observational studies to date. In the meantime, use of CPAP should be encouraged to improve patient symptoms and minimize exposure to the negative effects of untreated SDB.
Although CPAP is the therapy of choice for most patients with SDB, there are other treatment options that may be attempted. Patients who experience SDB events primarily in the supine position may be counseled on supine avoidance methods during sleep. Oral appliances (mandibular advancement devices and tongue-retaining devices) are an alternative therapy for patients who do not respond to, who are not appropriate candidates for, or who fail treatment with CPAP (132). Upper airway surgery is a treatment option that is recognized to be less effective than CPAP therapy but may be useful for patients with recognized anatomical or craniofacial abnormalities (133). Surgeries that may be performed include uvulopharyngoplasty (resection of the uvula and soft palate to open the upper airway), procedures to open nasal passages (septoplasty), and removal of the tonsils and adenoids (mostly performed in pediatric SDB). Uvulopharyngoplasty is the most common upper airway surgery More invasive and elaborate surgical procedures such as tracheostomy are effective but are usually reserved for emergencies. Patients who do not tolerate CPAP may be prescribed supplemental oxygen therapy. Supplemental oxygen prevents arterial oxygen desaturations but does not reduce the AHI or daytime sleepiness and is not recommended as first line therapy (134). The treatment of SDB is largely nonpharmacologic, but drug therapies may be appropriate for the management of daytime sleepiness and airway inflammation associated with SDB. The American Academy of Sleep Medicine (AASM) practice parameters for medical therapy of SDB advocate the use of nasal steroids for patients with SDB and concurrent rhinitis (135). AASM practice guidelines also recommend use of modafinil to treat residual daytime sleepiness in patients with SDB who use concurrent CPAP therapy (135). In overweight patients with SDB, weight loss is an important part of the treatment strategy.
Impact of Weight Loss on Reversal of SDB
Increased body weight elevates the risk of developing and/or worsening SDB (21). As a result, weight loss via dietary measures, physical activity, and a combination of the two have been studied as intervention strategies for the treatment of SDB (136-144). These interventions may improve SDB by relieving mechanical load on the upper airway, reducing propensity for its collapse (28).
Diet and Exercise
Studies that have investigated dietary interventions alone in SDB have found that significant weight loss (18.5 to 27.7 kg) can be induced by a low calorie diet (320 to 1,000 kcal/day) (141-143). Weight loss produced significant improvements in markers of SDB severity, including oxygen desaturation index (141), AHI (141-143), minimum oxygen saturation (141) and blood carbon dioxide concentrations during sleep (142). Although these results are encouraging, and suggest that low calorie diets alone may reduce SDB severity, subjects' amount of exercise was not monitored and subsequent studies should control for exercise since it also may have additive effects with diet on weight loss and SDB severity (145).
Population-based studies have examined the risk of prevalent SDB in patients who engage in regular exercise. A cohort study of 4,275 patients in the Sleep Heart Health Study found that a vigorous exercise regimen of 3 or more hours a week reduces the odds of having SDB (defined as an RDI >15 episodes/hr) (146). Peppard and Young demonstrated that individuals with more hours of exercise per week had a lower AHI, independent of age, sex, BMI and other covariates (147). In addition, two small studies found that physical activity in patients with SDB yielded significant improvement in symptoms and severity of SDB without major changes in body weight (148,149). These findings suggest that exercise alone may decrease the likelihood of developing SDB and may reduce SDB severity. Furthermore, exercise may have a protective effect that is independent of weight loss. Future studies are warranted to prospectively determine the ability of exercise to prevent the development of SDB and ability to reduce severity of existing SDB.
Interventions combining diet and exercise have been effective weight loss strategies in patients with SDB and are probably superior to either intervention alone (145). Studies have found that average weight loss of 9.2 kg to 19.1 kg resulted in improvements in oxygen desaturations during sleep (136-139,144), apnea frequency (140,144) and daytime sleepiness (138-140,144). Combined diet and physical activity reduce SDB severity even in patients with mild SDB (144). Tuomilehto et al showed that very low calorie diet (600-800 kcal/day) with physical activity led to significant weight loss and a corresponding decrease in AHI at three and 12 months in patients with mild SDB (144).
If initial weight loss can be achieved, maintaining weight loss over time is also a great challenge in obese patients (150). Sampol et al examined the effect of short and long term weight reduction (low calorie diet and exercise) on SDB (151). In this study 11% of the subjects were cured of their SDB (AHI<10) after a mean follow-up of 11.5 months. These subjects were reevaluated at 94 months and 13 of the 24 maintained their weight loss. Seven of the 13 who maintained weight loss remained cured of SDB (151). These finding suggest that long-term weight loss may alleviate SDB, but the relationship between SDB and weight loss is complex and maintenance of weight loss does not necessarily prevent recurrence of SDB. This latter idea is supported by a small study whose findings indicate that improvement in SDB may not be related to amount of weight lost (138). Consequently, it appears that the amount of weight loss required to improve SDB severity and symptoms varies between patients. This variability is not surprising given that not all SDB is obesity-related.
Although weight loss appears to hold promise as a treatment for SDB, the published studies are limited by small size, lack of randomization, confounding variables, or the absence of controls (136-143,148,149). A 2001 Cochrane review found zero prospective randomized controlled trials evaluating weight loss or exercise for treatment of SDB (152). Subsequently, there has only been one randomized controlled trial in this area (144). Large, randomized studies need to be conducted in this area to determine if preliminary findings demonstrating that weight loss improves SDB are confirmed in more robust investigations.
Medication-aided Weight Loss
Although preliminary findings suggest that weight loss may improve SDB (136-144), many obese patients have a difficult time losing weight and keeping weight off using diet and exercise alone. Sibutramine and orlistat are the only two FDA approved medications for treatment of obesity and in combination with lifestyle modifications, they have been shown to improve long term weight loss compared to diet alone (145). These medications combined with dietary and behavior modifications can result in significant weight loss and may help reduce SDB severity and associated symptoms (153).
Sibutramine is a derivative of an amphetamine precursor that works centrally to primarily inhibit the reuptake of norepinephrine and serotonin. It induces a feeling of satiety that can aid in weight loss when combined with a low calorie diet and an exercise program (153). Sibutramine is an effective treatment for weight loss (145,154,155) and it effectively reduces abdominal adiposity, a common risk factor for SDB (156). Two studies have evaluated the effects of sibutramine on weight loss and SDB. Yee et al examined 87 men with SDB who received 24 weeks of sibutramine in an open-label, uncontrolled cohort study (157). Sibutramine produced a mean weight loss of 8.3 ± 4.7 Kg (7.8 ± 4.2% of body weight), that significantly improved SDB severity, daytime sleepiness and reduced oxygen desaturations. Seventy of the subjects had a reduction in severity of SDB and 43% of subjects had a 50% or greater reduction in RDI. Four patients with prior SDB had an RDI <5 episodes/hr at the end of follow-up. Analysis revealed a strong correlation between amount of weight lost and % change in RDI (157). In contrast, a study of shorter duration evaluated subjects with SDB after one month of sibutramine treatment and found no significant impact on AHI, weight, sleep architecture or respiratory disturbance (158). The short one month treatment period limits the study's findings since weight loss is generally assessed after longer (3-6 month) sibutramine treatment periods. Since sibutramine can substantially increase blood pressure and heart rate in some patients, it should not be used in patients with a history of coronary artery disease, congestive heart failure, arrhythmias or stroke (159). Caution should be used in SDB since patients with SDB frequently have hypertension and cardiovascular disease.
Orlistat inhibits lipases in the intestinal tract, thus inhibiting the breakdown of dietary fats and decreasing the absorption of fat (160). Orlistat has been shown in multiple meta-analyses to produce approximately 3 kg greater weight loss than placebo (145,155). Currently there are no randomized controlled trials evaluating the effects of orlistat on SDB, but since orlistat facilitates weight loss, it may help reduce severity of SDB. Orlistat appears to be less effective than sibutramine at aiding weight loss. A meta-analysis of 8 studies comparing sibutramine and orlistat monotherapies found that sibutramine was associated with a 2.2 kg greater weight loss than orlistat (161). Adverse effects of orlistat include abdominal discomfort, liquid or oily stools, increased defecation and fecal incontinence. In addition, since orlistat decreases fat soluble vitamin absorption (most notable Vitamins D, E and beta-carotene), it is recommended to take a daily multivitamin during orlistat therapy (162). Further study evaluating the efficacy and safety with these two medications in patients with SDB is needed.
Bariatric Surgery
Weight loss can be difficult to maintain via diet and exercise alone, especially in the morbidly obese (163). Bariatric surgery is an effective method of weight loss, and consequently, may reduce the severity of SDB (164,165). Bariatric surgery has produced significant decreases in AHI (166,167), oxygen desaturation (168-170), sleep efficiency (169), daytime sleepiness (169), and respiratory disturbance index (169,170) over months of follow up. Not surprisingly, with effective weight loss, bariatric surgery also decreases the need for CPAP therapy, and 9-12 months after surgery patients may not require CPAP therapy at all (164,168,169).
Since non-randomized studies demonstrated encouraging effects on SDB severity and symptoms, a systematic review and meta-analysis of 136 studies reviewed the effect of bariatric surgery on SDB. The random effects model analyzed numerous surgical techniques and found that the mean percentage excess weight loss was 61.2% (165). Furthermore, SDB was resolved in 85.7% of morbidly obese patients undergoing bariatric surgery (165). Combined data from the various surgical techniques showed that bariatric surgery reduced mean AHI by 34 events per hour. A later meta-analysis was performed including only investigations that used polysomnography to document AHI before and after bariatric surgery and found similar effects of surgery on SDB severity (171). The analysis found that bariatric surgery significantly reduced AHI in the 12 studies included, and the mean AHI after surgery in the included studies was still 15.8 events per hour. However, follow-up investigations are necessary to determine if reductions in AHI are maintained during long-term follow-up. A preliminary study indicated that reductions in AHI seen four months after bariatric surgery were not maintained after 7.5 years of follow-up (172). This small study found that changes in AHI after 7.5 years were independent of changes in body weight. Recurrences of SDB and mechanisms of airway collapse are complex and influenced by variables other than weight. It is important to note that bariatric surgery has potential complications; about 10% of patients receiving this treatment have a significant adverse event (173), so application of this intervention requires careful risk/benefit analysis. Complications can be acute (hemhorrage, obstruction, anastomotic leak, infection, pulmonary emboli) or chronic (neuropathies resulting from nutritional deficiencies, hernias, and anastomotic stenoses) in nature (174).
Summary and Conclusions
Obesity is the most significant risk factor for the development of SDB, and as the prevalence of obesity increases, the prevalence of SDB is also likely to increase. Since many patients with SDB remain undiagnosed, identification of SDB in patients at risk will help to reduce the individual and societal burden. Patients with SDB have abnormalities in obesity hormones, inflammatory cytokines and insulin and glucose regulation that increase cardiovascular risk. Current therapies effectively treat SDB in many patients but are not perfect. CPAP does not cure SDB, it may be cumbersome, and long-term CPAP adherence is approximately 60-70 percent (175-177). Weight loss and exercise have shown promise in preliminary studies but require patient commitment and dedication over time. Future investigations should address whether the various therapies for SDB truly correct underlying metabolic and cardiovascular abnormalities and if long-term therapy improves survival. In addition, mechanistic investigations will hopefully identify new potential therapies for SDB that target putative mechanisms for these metabolic and cardiovascular abnormalities.
Acknowledgments
This project was supported by UW Institutional Clinical and Translational Science Award (UW-Madison) (KL2) RR025012-01(JMD) and by NIH grant HL-074072 (BJM).
Footnotes
Conflicts of Interest: NONE
References
- 1.Wolk R, Shamsuzzaman ASM, Somers VK. Obesity, sleep apnea, and hypertension. Hypertension. 2003;42:1067. doi: 10.1161/01.HYP.0000101686.98973.A3. [DOI] [PubMed] [Google Scholar]
- 2.National Commission on Sleep Disorders Research. Wake up America: a national sleep alert. Washington, DC: Government Printing Office; 1993. [Google Scholar]
- 3.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 4.Strohl KP, Redline S. Recognition of obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154:279–289. doi: 10.1164/ajrccm.154.2.8756795. [DOI] [PubMed] [Google Scholar]
- 5.Levinson PD, McGarvey ST, Carlisle CC, et al. Adiposity and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 1993;103:1336–1342. doi: 10.1378/chest.103.5.1336. [DOI] [PubMed] [Google Scholar]
- 6.Phillips B, Cook Y, Schmitt F, et al. Sleep apnea: prevalence of risk factors in the general population. South Med J. 1989;82:1090–1092. [PubMed] [Google Scholar]
- 7.Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154:1705–1711. [PubMed] [Google Scholar]
- 8.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–586. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 9.Ozturk L, Unal M, Tamer L, Celikoglu F. The association of the severity of obstructive sleep apnea with plasma leptin levels. Arch Otolaryngol Head Neck Surg. 2003;129:538–540. doi: 10.1001/archotol.129.5.538. [DOI] [PubMed] [Google Scholar]
- 10.Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279:H234–237. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 11.Ulukavak Ciftci T, Kokturk O, Bukan N, Bilgihan A. Leptin and ghrelin levels in patients with obstructive sleep apnea syndrome. Respiration. 2005;72:395–401. doi: 10.1159/000086254. [DOI] [PubMed] [Google Scholar]
- 12.Phillips BG, Hisel TM, Kato M, et al. Recent weight gain in patients with newly diagnosed obstructive sleep apnea. J Hypertens. 1999;17:1297–300. doi: 10.1097/00004872-199917090-00009. [DOI] [PubMed] [Google Scholar]
- 13.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–153. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owens RL, Eckert DJ, Yeh SY, Malhotra A. Upper airway function in the pathogenesis of obstructive sleep apnea: a review of the current literature. Curr Opin Pulm Med. 2008;14:519–524. doi: 10.1097/MCP.0b013e3283130f66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaa T. Impairments, Diseases, Age and Their Relative Risks of Accident Involvement: Results from a Meta-Analysis. Oslo: Institute of Transport Economics; 2003. [Google Scholar]
- 16.Sharafkhaneh N, Giray P, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28:1405–1411. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]
- 17.Davies RJ, Stradling JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur Respir J. 1990;3:509–514. [PubMed] [Google Scholar]
- 18.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 19.Shinohara E, Kihara S, Yamashita S, Yamane M, Nishida M, Arai T, et al. Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med. 1997;241:11–18. doi: 10.1046/j.1365-2796.1997.63889000.x. [DOI] [PubMed] [Google Scholar]
- 20.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;15:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 21.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 22.Davies RJ, Ali NJ, Stradling JR. Neck circumference and other clinical features in the diagnosis of obstructive sleep apnoea syndrome. Thorax. 1992;47:101–105. doi: 10.1136/thx.47.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Series F, Cote C, Simoneau JA, et al. Physiologic, metabolic, and muscle fiber type characteristics of musculus uvulae in sleep apnea hypopnea syndrome and in snorers. J Clin Invest. 1995;95:20–25. doi: 10.1172/JCI117640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 25.Millman RP, Carlisle CC, McGarvey ST, Eveloff SE, Levinson PD. Body fat distribution and sleep apnea severity in women. Chest. 1995;107:362–366. doi: 10.1378/chest.107.2.362. [DOI] [PubMed] [Google Scholar]
- 26.Dancey DR, Hanly PJ, Soong C, Lee B, Shepard J, Jr, Hoffstein V. Gender differences in sleep apnea: the role of neck circumference. Chest. 2003;123:1544–1550. doi: 10.1378/chest.123.5.1544. [DOI] [PubMed] [Google Scholar]
- 27.Guilleminault C, Stoohs S, Kim YD, Chervin R, Black J, Clerk A. Upper airway sleep-disordered breathing in women. Ann Intern Med. 1995;122:493–501. doi: 10.7326/0003-4819-122-7-199504010-00003. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz AR, Gold AR, Schubert N, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144:494–498. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 29.Thut DC, Schwartz AR, Roach D, Wise RA, Permutt S, Smith PL. Tracheal and neck position influence upper airway airflow dynamics by altering airway length. J Appl Physiol. 1993;75:2084–2090. doi: 10.1152/jappl.1993.75.5.2084. [DOI] [PubMed] [Google Scholar]
- 30.Sharp JT, Henry JP, Sweany SK, Meadows WR, Pietras RJ. Effects of mass loading the respiratory system in man. J Appl Physiol. 1964;19:959–966. doi: 10.1152/jappl.1964.19.5.959. [DOI] [PubMed] [Google Scholar]
- 31.Findley LJ, Ries AL, Tisi GM, Wagner PD. Hypoxemia during apnea in normal subjects: mechanisms and impact of lung volume. J Appl Physiol. 1983;55:1777–1783. doi: 10.1152/jappl.1983.55.6.1777. [DOI] [PubMed] [Google Scholar]
- 32.West SD, Kohler M, Nicoll DJ, Stradling JR. The effect of continuous positive airway pressure treatment on physical activity in patients with obstructive sleep apnoea: a randomized controlled trial. Sleep Med. 2009 doi: 10.1016/j.sleep.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Hastings PC, Vazir A, O'Driscoll DM, Morrell MJ, Simonds AK. Symptom burden of sleep-disordered breathing in mild-to-moderate congestive heart failure patients. Eur Respir J. 2006;27:748–755. doi: 10.1183/09031936.06.00063005. [DOI] [PubMed] [Google Scholar]
- 34.Kezirian EJ, Kirisoglu CE, Riley RW, Chang E, Guilleminault C, Powell NB. Resting energy expenditure in adults with sleep disordered breathing. Arch Otolaryngol Head Neck Surg. 2008;134:1270–1275. doi: 10.1001/archotol.134.12.1270. [DOI] [PubMed] [Google Scholar]
- 35.Bergmann BM, Everson CA, Kushida CA, et al. Sleep deprivation in the rat: V. Energy use and meditation. Sleep. 1989;12:31–41. doi: 10.1093/sleep/12.1.31. [DOI] [PubMed] [Google Scholar]
- 36.Bonnet MH, Berry RB, Arand DL. Metabolism during normal, fragmented, and recovery sleep. J Appl Physiol. 1991;71:1112–1118. doi: 10.1152/jappl.1991.71.3.1112. [DOI] [PubMed] [Google Scholar]
- 37.Landsberg L, Yeung JB. The role of sympathetic nervous system and catecholamines in the regulation of energy metabolism. Am J Clin Nutri. 1983;38:1018–1024. doi: 10.1093/ajcn/38.6.1018. [DOI] [PubMed] [Google Scholar]
- 38.Stenlof K, Grunstein R, Hedner J, Sjostrom L. Energy expenditure in obstructive sleep apnea: effects of treatment with continuous positive airway pressure. Am J Physiol. 1996;271:E1036–E1043. doi: 10.1152/ajpendo.1996.271.6.E1036. [DOI] [PubMed] [Google Scholar]
- 39.Hins J, Series F, Almeras N, Tremblay A. Relationship between severity of nocturnal desaturation and adaptive thermogenesis: preliminary data of apneic patients tested in whole-body indirect calorimetry chamber. Int J Obes (Lond) 2006;30:574–577. doi: 10.1038/sj.ijo.0803159. [DOI] [PubMed] [Google Scholar]
- 40.Ryan CF, Love LL, Buckley PA. Energy expenditure in obstructive sleep apnea. Sleep. 1995;18:180–187. doi: 10.1093/sleep/18.3.180. [DOI] [PubMed] [Google Scholar]
- 41.Lin CC, Chang KC, Lee KS. Effects of treatment by laser-assisted uvuloplasty on sleep energy expenditure in obstructive sleep apnea patients. Metabolism. 2002;51:622–627. doi: 10.1053/meta.2002.31969. [DOI] [PubMed] [Google Scholar]
- 42.Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour Schahin S, Wiest GH, Hahn EG, Lohmann T, Ficker JH. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J. 2003;22:251–257. doi: 10.1183/09031936.03.00010103. [DOI] [PubMed] [Google Scholar]
- 43.Chin K, Shimizu K, Nakamura T, Narai N, Masuzaki H, Ogawa Y, Mishima M, Nakao K, Ohi M. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706–712. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 44.Sanner BM, Kollhosser P, Buechner N, Zidek W, Tepel M. Influence of treatment on leptin levels in patients with obstructive sleep apnoea. Eur Respir J. 2004;23:601–604. doi: 10.1183/09031936.04.00067804. [DOI] [PubMed] [Google Scholar]
- 45.Redenius R, Murphy C, O'Neill EO, Al-Hamwi M, Zallek SN. Does CPAP lead to change in BMI? J Clin Sleep Med. 2008;4:205–209. [PMC free article] [PubMed] [Google Scholar]
- 46.Loube DI, Loube AA, Erman MK. Continuous positive airway pressure treatment results in weight loss in obese and overweight patients with obstructive sleep apnea. J Am Diet Assoc. 1997;97:896–897. doi: 10.1016/s0002-8223(97)00220-4. [DOI] [PubMed] [Google Scholar]
- 47.Makino S, Handa H, Suzukawa K, et al. Obstructive sleep apnoea syndrome, plasma adiponectin levels, and insulin resistance. Clin Endocrinol. 2006;64:12–19. doi: 10.1111/j.1365-2265.2005.02407.x. [DOI] [PubMed] [Google Scholar]
- 48.Sharma SK, Kumpawat S, Gael A, Banga A, Ramakrishnam L, Chatnrvedi P. Obesity, not obstructive sleep apnea, is responsible for metabolic abnormalities in a cohort with sleep disordered breathing. Sleep Med. 2007;8:12–17. doi: 10.1016/j.sleep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 49.Tauman R, Serpero LD, Capdevilla OS, et al. Adipokines in children with sleep disordered breathing. Sleep. 2007;30:443–449. doi: 10.1093/sleep/30.4.443. [DOI] [PubMed] [Google Scholar]
- 50.Wolk R, Svatikova A, Nelson CA, et al. Plasma levels of adiponectin, a novel adipocyte-derived hormone, in sleep apnea. Obesity Res. 2005;13:186–90. doi: 10.1038/oby.2005.24. [DOI] [PubMed] [Google Scholar]
- 51.Lam JCM, Xu A, Tam S, et al. Hypoadiponectinemia is related to sympathetic activation and severity of obstructive sleep apnea. Sleep. 2008;31:1721–1727. doi: 10.1093/sleep/31.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 53.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of IKKβ. Science. 2001;31:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 54.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: Relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 55.Ryan S, McNicholas WT. Inflammatory cardiovascular risk markers in obstructive sleep apnoea syndrome. Cardiovasc Hematol Agents Med Chem. 2009;7:76–81. doi: 10.2174/187152509787047685. [DOI] [PubMed] [Google Scholar]
- 56.Kapsimalis F, Varouchakis G, Manousaki A, et al. Association of sleep apnea severity and obesity with insulin resistance, C-reactive protein, and leptin levels in male patients with obstructive sleep apnea. Lung. 2008;186:209–217. doi: 10.1007/s00408-008-9082-x. [DOI] [PubMed] [Google Scholar]
- 57.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–2464. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 58.Kasasbeh E, Chi DS, Krishnaswamy G. Inflammatory aspects of sleep apnea and their cardiovascular consequences. South Med J. 2006;99:58–67. doi: 10.1097/01.smj.0000197705.99639.50. [DOI] [PubMed] [Google Scholar]
- 59.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: Molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–41. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 60.Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 62.Ryan S, Nolan GM, Hannigan E, Cunningham S, Taylor C, McNicholas WT. Cardiovascular risk markers in obstructive sleep apnoea syndrome and correlation with obesity. Thorax. 2007;62:509–514. doi: 10.1136/thx.2006.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guilleminault C, Kirisoglu C, Ohayon MM. C-reactive protein and sleep-disordered breathing. Sleep. 2004;27:1507–1511. doi: 10.1093/sleep/27.8.1507. [DOI] [PubMed] [Google Scholar]
- 64.Barcelo A, Barbe F, Llompart E, et al. Effects of obesity on C-reactive protein level and metabolic disturbances in male patients with obstructive sleep apnea. Am J Med. 2004;117:118–121. doi: 10.1016/j.amjmed.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 65.Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E. Correlates of serum C-reactive protein (CRP)--no association with sleep duration or sleep disordered breathing. Sleep. 2007;30:991–996. doi: 10.1093/sleep/30.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 67.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 68.Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 69.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174:824–830. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- 70.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: Results from the health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 71.Boekholdt SM, Peters RJ, Hack CE, et al. IL-8 plasma concentrations and the risk of future coronary artery disease in apparently healthy men and women: The EPIC-norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2004;24:1503–1508. doi: 10.1161/01.ATV.0000134294.54422.2e. [DOI] [PubMed] [Google Scholar]
- 72.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 73.Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab. 2004;89:4409–4413. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 74.Alzoghaibi MA, Bahammam AS. Lipid peroxides, superoxide dismutase and circulating IL-8 and GCP-2 in patients with severe obstructive sleep apnea: A pilot study. Sleep Breath. 2005;9:119–126. doi: 10.1007/s11325-005-0022-1. [DOI] [PubMed] [Google Scholar]
- 75.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol. 2003;94:179–184. doi: 10.1152/japplphysiol.00177.2002. [DOI] [PubMed] [Google Scholar]
- 76.Hartmann G, Tschop M, Fischer R, et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12:246–252. doi: 10.1006/cyto.1999.0533. [DOI] [PubMed] [Google Scholar]
- 77.Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma C-reactive protein levels among children with sleep-disordered breathing. Pediatrics. 2004;113:e564–9. doi: 10.1542/peds.113.6.e564. [DOI] [PubMed] [Google Scholar]
- 78.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 79.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 80.Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 81.O'Connor GT, Caffo B, Newman AB, et al. Prospective study of sleep-disordered breathing and hypertension: the sleep heart health study. Published ahead of print on March 5, 2009. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meslier N, Gagnadoux F, Giraud P, et al. Impaired glucose-insulin metabolism in males with obstructive sleep apnoea syndrome. Eur Respir J. 2003;22:156–160. doi: 10.1183/09031936.03.00089902. [DOI] [PubMed] [Google Scholar]
- 83.Elmasry A, Lindberg E, Berne C, et al. Sleep-disordered breathing and glucose metabolism in hypertensive men: a population-based study. J Intern Med. 2001;249:153–161. doi: 10.1046/j.1365-2796.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- 84.Resnick HE, Redline S, Shahar E, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26:702–709. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 85.Elmasry A, Lindberg E, Berne C, et al. Sleep-disordered breathing and glucose metabolism in hypertensive men: a population-based study. J Intern Med. 2001;249:153–161. doi: 10.1046/j.1365-2796.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- 86.Punjabi NM, Sorkin JD, Katzel LI, et al. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 87.Ip S, Lam B, Ng M, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 88.Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 89.Lam JC, Lam B, Lam CL, et al. Obstructive sleep apnea and the metabolic syndrome in community-based Chinese adults in Hong Kong. Respir Med. 2006;100:980–987. doi: 10.1016/j.rmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 90.Okada M, Takamizawa A, Tsushima K, et al. Relationship between sleep-disordered breathing and lifestyle-related illnesses in subjects who have undergone health-screening. Intern Med. 2006;45:891–896. doi: 10.2169/internalmedicine.45.1592. [DOI] [PubMed] [Google Scholar]
- 91.Sulit L, Storfer-Isser A, Kirchner HL, et al. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep. 2006;29:777–783. doi: 10.1093/sleep/29.6.777. [DOI] [PubMed] [Google Scholar]
- 92.Stoohs R, Facchini F, Guilleminault C. Insulin resistance and sleep-disordered breathing in healthy humans. Am J Respir Crit Care Med. 1996;154:170–174. doi: 10.1164/ajrccm.154.1.8680675. [DOI] [PubMed] [Google Scholar]
- 93.Reichmuth KJ, Austin D, Skatrud JB, et al. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tiihonen M, Partinen M, Närvänen S. The severity of obstructive sleep apnoea is associated with insulin resistance. J Sleep Res. 1993;2:56–61. doi: 10.1111/j.1365-2869.1993.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 95.Strohl KP, Novak RD, Singer W, et al. Insulin levels, blood pressure and sleep apnea. Sleep. 1994;17:614–618. doi: 10.1093/sleep/17.7.614. [DOI] [PubMed] [Google Scholar]
- 96.Peltier AC, Consens FB, Sheikh K, et al. Autonomic dysfunction in obstructive sleep apnea is associated with impaired glucose regulation. Sleep Med. 2007;8:149–155. doi: 10.1016/j.sleep.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 97.Kono M, Tatsumi K, Saibara T, et al. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest. 2007;131:1387–1392. doi: 10.1378/chest.06-1807. [DOI] [PubMed] [Google Scholar]
- 98.McArdle N, Hillman D, Beilin L, et al. Metabolic risk factors for vascular disease in obstructive sleep apnea: a matched controlled study. Am J Respir Crit Care Med. 2007;175:190–195. doi: 10.1164/rccm.200602-270OC. [DOI] [PubMed] [Google Scholar]
- 99.Tassone F, Lanfranco F, Gianotti L, et al. Obstructive sleep apnoea syndrome impairs insulin sensitivity independently of anthropometric variables. Clin Endocrinol (Oxf) 2003;59:374–379. doi: 10.1046/j.1365-2265.2003.01859.x. [DOI] [PubMed] [Google Scholar]
- 100.Coughlin SR, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 101.Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179:235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harsch IA, Schahin SP, Radespiel-Troger M, et al. Am J Respir Crit Care Med. 2004;169:156–162. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 103.Harsch IA, Schahin SP, Bruckner K, et al. The effect of continuous positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration. 2004;71:252–259. doi: 10.1159/000077423. [DOI] [PubMed] [Google Scholar]
- 104.Brooks B, Cistulli PA, Borkman M, et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effects of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79:1681–1685. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 105.Saarelainen S, Lahtela J, Kallonen E. Effect of nasal CPAP treatment on insulin sensitivity and plasma leptin. J Sleep Res. 1997;6:146–147. doi: 10.1046/j.1365-2869.1997.00034.x. [DOI] [PubMed] [Google Scholar]
- 106.Smurra M, Philip P, Taillard J, et al. CPAP treatment does not affect glucose-insulin metabolism in sleep apneic patients. Sleep Med. 2001;2:207–213. doi: 10.1016/s1389-9457(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 107.Babu AR, Herdegen J, Fogelfeld L, et al. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–452. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 108.Hassaballa HA, Tulaimat A, Herdegen JJ, et al. The effect of continuous positive airway pressure on glucose control in diabetic patients with severe obstructive sleep apnea. Sleep Breath. 2005;9:176–180. doi: 10.1007/s11325-005-0033-y. [DOI] [PubMed] [Google Scholar]
- 109.Lindberg E, Berne C, Elmasry A, et al. CPAP treatment of a population-based sample: what are the benefits and the treatment compliance? Sleep Med. 2006;7:553–560. doi: 10.1016/j.sleep.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 110.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–974. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tasali E, Mokhlesi B, Cauter EV. Obstructive sleep apnea and type 2 diabetes. Interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 112.Polotsky VY, Li J, Punjabi NM, et al. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol. 2003;552:253–264. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Iiyori N, Alonso LC, Li J, et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. 2007;175:851–857. doi: 10.1164/rccm.200610-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 115.Deibert DC, DeFronzo RA. Epinephrine-induced insulin resistance in man. J Clin Invest. 1980;65:717–721. doi: 10.1172/JCI109718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martin IK, Weber KM, Boston RC, Alford FP, Best JD. Effects of epinephrine infusion on determinants of intravenous glucose tolerance in dogs. Am J Physiol. 1988;255:E668–E673. doi: 10.1152/ajpendo.1988.255.5.E668. [DOI] [PubMed] [Google Scholar]
- 117.Lembo G, Capaldo B, Rendina V, et al. Acute noradrenergic activation induces insulin resistance in human skeletal muscle. Am J Physiol. 1994;266:E242–E247. doi: 10.1152/ajpendo.1994.266.2.E242. [DOI] [PubMed] [Google Scholar]
- 118.Dinneen S, alzaid A, Miles J, Rizza R. Metabolic effects of the nocturnal rise in cortisol on carbohydrate metabolism in normal humans. J Clin Invest. 1993;92:2283–2290. doi: 10.1172/JCI116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci (Lond) 1999;96:513–523. doi: 10.1042/cs0960513. [DOI] [PubMed] [Google Scholar]
- 120.Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest. 2007;132:1057–1072. doi: 10.1378/chest.06-2432. [DOI] [PubMed] [Google Scholar]
- 121.Narkiewicz K, Kato M, Phillips BG, et al. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–2335. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- 122.Kato M, Roberts-Thompson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 123.Chin K, Ohi M, Kita H, et al. Effects of NCPAP therapy on fibrinogen levels in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1996;153:1972–1976. doi: 10.1164/ajrccm.153.6.8665063. [DOI] [PubMed] [Google Scholar]
- 124.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–939. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 125.Bokinsky G, Miller M, Ault K, et al. Spontaneous platelet activation and aggregation during obstructive sleep apnea and its response to therapy with nasal continuous positive airway pressure: a preliminary investigation. Chest. 1995;108:625–630. doi: 10.1378/chest.108.3.625. [DOI] [PubMed] [Google Scholar]
- 126.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 127.He J, Kryger MH, Zorick FJ, et al. Mortality and apnea index in obstructive sleep apnea: experience in 385 male patients. Chest. 1988;94:373–376. [PubMed] [Google Scholar]
- 128.Doherty LS, Kiely JL, Swan V, et al. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127:2076–2084. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 129.Peker Y, Hedner J, Norum J, et al. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–165. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 130.Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with continuous positive airway pressure. Chest. 2005;128:624–633. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 131.Marti S, Sampol G, Munoz X, et al. Mortality in severe sleep apnnoea/hypopnoea syndrome patients: impact of treatment. Eur Respir J. 2002;20:1511–1518. doi: 10.1183/09031936.02.00306502. [DOI] [PubMed] [Google Scholar]
- 132.Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240–243. doi: 10.1093/sleep/29.2.240. [DOI] [PubMed] [Google Scholar]
- 133.Sher AE. Upper airway surgery for obstructive sleep apnea. Sleep Med Rev. 2002;6:195–212. doi: 10.1053/smrv.2002.0242. [DOI] [PubMed] [Google Scholar]
- 134.Phillips BA, Schmitt FA, Berry DT, et al. Treatment of obstructive sleep apnea. A preliminary report comparing nasal CPAP to nasal oxygen in patients with mild OSA. Chest. 1990;98:325–330. doi: 10.1378/chest.98.2.325. [DOI] [PubMed] [Google Scholar]
- 135.Morgenthaler TI, Kapen S, Lee-Chiong T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29:1031–1035. [PubMed] [Google Scholar]
- 136.Kansanen M, Vanninen E, Tuunainen A, et al. The effect of a very low-calorie diet-induced weight loss on the severity of obstructive sleep apnoea and autonomic nervous function in obese patients with obstructive sleep apnoea syndrome. Clin Physiol. 1998;18:377–385. doi: 10.1046/j.1365-2281.1998.00114.x. [DOI] [PubMed] [Google Scholar]
- 137.Kajaste S, Brander PE, Telakivi T, Partinen M, Mustajoki P. A cognitive-behavioral weight reduction program in the treatment of obstructive sleep apnea syndrome with or without initial nasal CPAP: A randomized study. Sleep Med. 2004;5:125–131. doi: 10.1016/j.sleep.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 138.Lojander J, Mustajoki P, Ronka S, Mecklin P, Maasilta P. A nurse-managed weight reduction programme for obstructive sleep apnoea syndrome. J Intern Med. 1998;244:251–255. doi: 10.1046/j.1365-2796.1998.00387.x. [DOI] [PubMed] [Google Scholar]
- 139.Hakala K, Maasilta P, Sovijarvi AR. Upright body position and weight loss improve respiratory mechanics and daytime oxygenation in obese patients with obstructive sleep apnoea. Clin Physiol. 2000;20:50–55. doi: 10.1046/j.1365-2281.2000.00223.x. [DOI] [PubMed] [Google Scholar]
- 140.Smith PL, Gold AR, Meyers DA, Haponik EF, Bleecker ER. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med. 1985;103:850–855. doi: 10.7326/0003-4819-103-6-850. [DOI] [PubMed] [Google Scholar]
- 141.Pasquali R, Colella P, Cirignotta F, et al. Treatment of obese patients with obstructive sleep apnea syndrome (OSAS): Effect of weight loss and interference of otorhinolaryngoiatric pathology. Int J Obes. 1990;14:207–217. [PubMed] [Google Scholar]
- 142.Suratt PM, McTier RF, Findley LJ, Pohl SL, Wilhoit SC. Effect of very-low-calorie diets with weight loss on obstructive sleep apnea. Am J Clin Nutr. 1992;56(1 Suppl):182S–184S. doi: 10.1093/ajcn/56.1.182S. [DOI] [PubMed] [Google Scholar]
- 143.Nahmias J, Kirschner M, Karetzky MS. Weight loss and OSA and pulmonary function in obesity. N J Med. 1993;90:48–53. [PubMed] [Google Scholar]
- 144.Tuomilehto HP, Seppa JM, Partinen MM, et al. Lifestyle intervention with weight reduction-first line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:320–327. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 145.Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107:1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 146.Quan SF, O'Connor GT, Quan JS, et al. Association of physical activity with sleep-disordered breathing. Sleep Breath. 2007;11:149–157. doi: 10.1007/s11325-006-0095-5. [DOI] [PubMed] [Google Scholar]
- 147.Peppard PE, Young T. Exercise and sleep-disordered breathing: An association independent of body habitus. Sleep. 2004;27:480–484. doi: 10.1093/sleep/27.3.480. [DOI] [PubMed] [Google Scholar]
- 148.Giebelhaus V, Strohl KP, Lormes W, Lehmann M, Netzer N. Physical exercise as an adjunct therapy in sleep apnea-an open trial. Sleep Breath. 2000;4:173–176. doi: 10.1007/s11325-000-0173-z. [DOI] [PubMed] [Google Scholar]
- 149.Norman JF, Von Essen SG, Fuchs RH, McElligott M. Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online. 2000;3:121–129. [PubMed] [Google Scholar]
- 150.Brownell KD, Rodin J. The dieting maelstrom. is it possible and advisable to lose weight? Am Psychol. 1994;49:781–791. doi: 10.1037//0003-066x.49.9.781. [DOI] [PubMed] [Google Scholar]
- 151.Sampol G, Munoz X, Sagales MT, et al. Long-term efficacy of dietary weight loss in sleep apnoea/hypopnoea syndrome. Eur Respir J. 1998;12(5):1156–1159. doi: 10.1183/09031936.98.12051156. [DOI] [PubMed] [Google Scholar]
- 152.Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database Syst Rev. 2001;(1):CD002875. doi: 10.1002/14651858.CD002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Klein S. Long-term pharmacotherapy for obesity. Obes Res. 2004;12(Suppl):163S–6S. doi: 10.1038/oby.2004.283. [DOI] [PubMed] [Google Scholar]
- 154.Horvath K, Jeitler K, Siering U, et al. Long-term effects of weight-reducing interventions in hypertensive patients: Systematic review and meta-analysis. Arch Intern Med. 2008;168:571–580. doi: 10.1001/archinte.168.6.571. [DOI] [PubMed] [Google Scholar]
- 155.Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: Updated meta-analysis. BMJ. 2007;335:1194–1199. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kamel EG, McNeill G, Van Wijk MC. Change in intra-abdominal adipose tissue volume during weight loss in obese men and women: Correlation between magnetic resonance imaging and anthropometric measurements. Int J Obes Relat Metab Disord. 2000;24:607–613. doi: 10.1038/sj.ijo.0801204. [DOI] [PubMed] [Google Scholar]
- 157.Yee BJ, Phillips CL, Banerjee D, Caterson I, Hedner JA, Grunstein RR. The effect of sibutramine-assisted weight loss in men with obstructive sleep apnoea. Int J Obes (Lond) 2007;31:161–168. doi: 10.1038/sj.ijo.0803363. [DOI] [PubMed] [Google Scholar]
- 158.Martinez D, Basile BR. Sibutramine does not worsen sleep apnea syndrome: A randomized double-blind placebo-controlled study. Sleep Med. 2005;6:467–470. doi: 10.1016/j.sleep.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 159.Product Information: Meridia™, sibutramine. Abbott Laboratories; North Chicago, IL: 2007. [Google Scholar]
- 160.Hadvary P, Lengsfeld H, Wolfer H. Inhibition of pancreatic lipase in vitro by the covalent inhibitor tetrahydrolipstatin. Biochem J. 1988;256:357–361. doi: 10.1042/bj2560357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Neovius M, Johansson K, Rossner S. Head-to-head studies evaluating efficacy of pharmaco-therapy for obesity: a systematic review and meta-analysis. Obes Rev. 2008;9:420–427. doi: 10.1111/j.1467-789X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 162.Product Information: Xenical™, orlistat. Roche Laboratories; Nutley, NJ: 2008. [Google Scholar]
- 163.Fritscher LG, Mottin CC, Canani S, Chatkin JM. Obesity and obstructive sleep apnea-hypopnea syndrome: The impact of bariatric surgery. Obes Surg. 2007;17:95–99. doi: 10.1007/s11695-007-9012-7. [DOI] [PubMed] [Google Scholar]
- 164.Varela JE, Hinojosa MW, Nguyen NT. Resolution of obstructive sleep apnea after laparoscopic gastric bypass. Obes Surg. 2007;17:1279–1282. doi: 10.1007/s11695-007-9228-6. [DOI] [PubMed] [Google Scholar]
- 165.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: A systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 166.Charuzi I, Fraser D, Peiser J, Ovnat A, Lavie P. Sleep apnea syndrome in the morbidly obese undergoing bariatric surgery. Gastroenterol Clin North Am. 1987;16:517–519. [PubMed] [Google Scholar]
- 167.Fritscher LG, Canani S, Mottin CC, et al. Bariatric surgery in the treatment of obstructive sleep apnea in morbidly obese patients. Respiration. 2007;74:647–652. doi: 10.1159/000107736. [DOI] [PubMed] [Google Scholar]
- 168.Marti-Valeri C, Sabate A, Masdevall C, Dalmau A. Improvement of associated respiratory problems in morbidly obese patients after open roux-en-Y gastric bypass. Obes Surg. 2007;17:1102–1110. doi: 10.1007/s11695-007-9186-z. [DOI] [PubMed] [Google Scholar]
- 169.Haines KL, Nelson LG, Gonzalez R, et al. Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea. Surgery. 2007;141:354–358. doi: 10.1016/j.surg.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 170.Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg. 2003;13:58–61. doi: 10.1381/096089203321136593. [DOI] [PubMed] [Google Scholar]
- 171.Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535–542. doi: 10.1016/j.amjmed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 172.Pillar G, Peled R, Lavie P. Recurrence of sleep apnea without concomitant weight increase 7.5 years after weight reduction surgery. Chest. 1994;106:1702–1704. doi: 10.1378/chest.106.6.1702. [DOI] [PubMed] [Google Scholar]
- 173.Livingston EH. Procedure incidence and in-hospital complication rates of bariatric surgery in the united states. Am J Surg. 2004;188:105–110. doi: 10.1016/j.amjsurg.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 174.Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab. 2008;93:S89–S96. doi: 10.1210/jc.2008-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Weaver T, Kribbs N, Pack A, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20:278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 176.Fitzpatrick MF, Alloway CE, Wakeford TM, MacLean AW, Munt PW, Day AG. Can patients with obstructive sleep apnea titrate their own continuous positive airway pressure? Am J Respir Crit Care Med. 2003;167:716–722. doi: 10.1164/rccm.200204-360OC. [DOI] [PubMed] [Google Scholar]
- 177.Grote L, Hedner J, Grunstein R, Kraiczi H. Therapy with CPAP: incomplete elimination of sleep related breathing disorder. Eur Respir J. 2000;16:921–927. doi: 10.1183/09031936.00.16592100. [DOI] [PubMed] [Google Scholar]
- 178.Carter RC, III, Watenpaugh DE. Obesity and obstructive sleep apnea: or is it OSA and obesity? Pathophysiology. 2008;15:71–77. doi: 10.1016/j.pathophys.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 179.Shaw JE, Punjabi NM, Wilding JP, Alberti KGMM, Zimmet PZ. Sleep-disordered breathing and type 2 diabetes. A report from the international diabetes federation taskforce on epidemiology and prevention. Diabetes Res Clin Prac. 2008;81:2–12. doi: 10.1016/j.diabres.2008.04.025. [DOI] [PubMed] [Google Scholar]


