Abstract
Study Objective
The purpose of this study was to determine if intermittent hypoxia that mimics obstructive sleep apnea would upregulate myocardial and hepatic p-glycoprotein protein and Abcb1a/Abcb1b mRNA expression.
Design
Prospective, randomized, blinded, parallel designed animal study
Setting
University Research Laboratory
Participants
Thirty adult, male Sprague-Dawley rats
Intervention
We assigned rats to either two weeks of intermittent hypoxia similar to sleep apnea (N=12) or control treatment (N=18) (no hypoxia).
Measurements and Main Results
After intermittent hypoxia or normoxia exposure, rats were anesthetized and the heart and liver were harvested and small samples were taken from the left ventricle (heart) and the liver for analysis. P-glycoprotein protein expression was measured by Western blotting, while Abcb1a/Abcb1b mRNA expression (genes that code for P-glycoprotein) was assessed by real-time polymerase chain reaction. Band density of myocardial (but not hepatic) p-glycoprotein protein expression (standardized by β-actin) was higher in hypoxic compared to control rats (p=0.03). Quantitative polymerase chain reaction revealed that myocardial and hepatic Abcb1a and myocardial Abcb1b mRNA expression were increased in hypoxic rats compared to controls.
Conclusions
Myocardial P-glycoprotein expression and myocardial and hepatic Abcb1a mRNA expression are significantly increased by two weeks of intermittent hypoxia. Hypoxia-induced increases in p-glycoprotein expression may partially explain drug resistant cardiovascular disease in OSA.
Keywords: intermittent hypoxia, drug resistance, p-glycoprotein, sleep apnea
Introduction
Sleep apnea is a medical condition characterized by recurrent airway obstruction, reductions in blood oxygen saturation, and disrupted sleep. Apnea events may occur hundreds of times each night and trigger increases in sympathetic nerve activity and blood pressure. Much research completed over the past two decades has established that obstructive sleep apnea (OSA) increases the risk of developing high blood pressure and cardiovascular disease. Intermittent hypoxia and subsequent sympathetic activation, oxidative stress, and inflammation are thought to play key roles in the development of hypertension and cardiovascular abnormalities in individuals with OSA.1-3 OSA appears to worsen the severity of concurrent cardiovascular conditions. For example, sleep apnea is present in approximately 50 percent of patients with heart failure and increases heart failure mortality.4,5 OSA increases the likelihood of atrial fibrillation recurrence while on drug therapy,6 and patients with OSA have worse atherosclerotic burden compared to patients without OSA.7 In addition, OSA appears to make hypertension more difficult to control with drug therapy in certain patients. Hypertensive patients with OSA whose blood pressure responds to treatment had less severe OSA than patients whose blood pressure remains elevated.8 In one study, over three quarters of patients with resistant hypertension had undiagnosed OSA.9 OSA is also an identifiable cause of hypertension and a potential cause of resistant hypertension according to the Joint National Commission on Blood Pressure Control.10
P-glycoprotein (Pgp) is an ATP binding cassette transporter and is a product of the multidrug resistance gene (Abcb1). In rodents Pgp is encoded by Abcb1a and Abcb1b (Abcb1 is another name for the Mdr1 gene). Although it is most famous for increasing drug resistance in tumor cells, Pgp is found in normal tissues, including the heart, liver, kidney, intestine, adrenal cortex, and at the blood brain barrier. Pgp functions as a drug efflux transporter and is thought to protect cells from potentially toxic substances, including medications. As a result, by transporting medications out of cells, Pgp may function as an obstacle to achieving optimal cellular drug concentrations and desired therapeutic effect. Hepatic Pgp is located at the canicular membrane of the bile duct and increases drug elimination into the bile. It is believed that myocardial Pgp is localized in the endothelium of both arterioles and capillaries in heart samples. Pgp mediated drug resistance has been observed in the treatment of cancer, epilepsy, hypercholesterolemia, and certain infectious pathogens.11-14 Pgp expression is increased by stress stimuli, including hypoxia. In fact, chemotherapeutic resistance in cancer cells is increased under hypoxic conditions.15 In vitro studies and animal studies of rats and rabbits indicate that transcription of Abcb1 is induced by hypoxia.16-18 Cell studies indicate this may be mediated via the c-Jun NH2-terminal kinase (JNK) pathway, although transcription of Pgp is a complex process that involves interplay between numerous repressors and inducers.19 It is conceivable that Pgp expression may be upregulated in medical conditions characterized by hypoxia, such as OSA.
Many drugs used to treat hypertension, arrhythmias, and cardiovascular diseases are substrates for Pgp transporters. Losartan, HMG coA reductase inhibitors, beta-blockers, anti-arrhythmics, and calcium channel blockers have all been shown to be substrates for Pgp.20-23 Failure to achieve adequate plasma and cellular concentrations of cardiovascular agents may clinically result in suboptimal therapeutic outcomes and drug resistance. Use of multiple drug therapy in drug-resistant hypertension and heart disease may expose patients to increased risk of adverse events and increased costs. One could postulate that upregulated Pgp expression may contribute to resistant hypertension, arrhythmias, and more severe heart failure seen in many patients with OSA. Whether or not intermittent hypoxia, that simulates OSA, increases myocardial Pgp expression has not been evaluated. Therefore, we measured Pgp protein expression and Abcb1a and Abcb1b mRNA expression in hearts and livers of rats exposed to two weeks of intermittent hypoxia that simulates OSA.
Methods
Animals
Thirty adult male Sprague Dawley rats (250-300g upon arrival) (Harlan Sprague Dawley Indianapolis, IN) were randomly assigned to two weeks of hypoxia (N=12) or control treatment (N=18). Animal experiments were performed according to the US National National Institutes of Health Guide for Care and Use of Laboratory Animals, and the animal protocol was approved by the Research Animal Resource Center at the University of Wisconsin-Madison. There were no observed differences in animal grooming, behavior, or diet during the exposure to intermittent hypoxia or room air. Ad libitum access was provided to standard rat chow and water.
Hypoxic Exposure
Intermittent hypoxia rats were housed in a Plexiglass chamber (1-2 rats per cage) in accordance with recommendations outlined in the National Institutes of Health Guide for the Care of Laboratory Animals (NIH Pub. No. 85-23, Revised 1985). Oxygen concentrations in the chamber were assessed with a heated zirconium sensor (Fujikura America, Pittsburgh, PA) connected to valves controlling the flow of oxygen and nitrogen into the chamber. A microprocessor-controlled timer operated the valves and delivery of gas into the chamber to achieve hypoxic exposures at four-minute intervals. For the first minute of each cycle, nitrogen was flushed into the chamber at a rate sufficient to achieve a fraction of inspired oxygen (FIO2) of 0.10 within the first 60 seconds. This level of FIO2 was maintained for an additional 60 seconds. After this, oxygen was infused into the chamber at a rate to achieve a FIO2 of 0.209 within 30 seconds and this FIO2 was maintained for the duration of the four-minute cycle.24 This exposure to chronic intermittent hypoxia was maintained for 12 hours a day for 14 days. The oxygen concentrations were checked daily using a TED60T sensor (Teledyne, City of Industry, CA). The chamber temperature was maintained at 76±1° F. The relative humidity in the chamber was monitored continuously and was maintained between 20% and 70%. Control rats that received no intermittent hypoxia were placed in standard plastic cages on top of the Plexiglass chamber so that they were exposed to similar environmental sounds and surroundings.
Surgery
Rats were anesthetized with isoflurane (5% induction followed by 2.5% maintenance) during the surgery. A left thoracotomy was performed approximately 10 mm from the sternum to expose the heart at the fifth intercostal space. The pericardium was removed and the heart was immediately excised, snap frozen in liquid nitrogen, and stored at −80 C until the day of analysis. After the heart was removed, an incision was made down to the abdomen and the left lower liver lobe was excised, snap frozen in liquid nitrogen, and stored at -80 C until analysis.
Western Blot Analysis
Small (3mm × 3mm) sections of tissue were excised from the left ventricle of the heart near the apex and from the bottom of the left lobe of the liver. Tissue sections were homogenized in a 10 mM Tris HCl solution containing 1 mM phenylmethanesulfonylfluoride and 0.0002 mM aprotinin. The homogenate was centrifuged and the supernatant was kept for analysis as tissue fractions. The protein concentrations in tissue fractions were determined with a Bradford Assay, using bovine serum albumin as standards (Pierce, Rockford, IL) and Coomassie blue dye (Pierce, Rockford, IL). Equal amounts of tissue protein (25 micrograms) were separated on an 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (Pierce, Rockford, IL). Samples were stained with pyronin Y solution to visualize that electrophoresis was complete. Protein was transferred to a 0.45 micron polyvinylidene difluoride (PVDF) membrane (Osmonics Inc, Gloucester, MA) prior to immunoblotting. The membrane was washed with 1X phosphate buffered saline with tween-20 (PBST) and nonspecific binding sites were blocked with 5% instant dry nonfat milk (Nestle, Solon, Ohio) at 4° C overnight. Western membranes were cut and incubated separately with the appropriate antibodies. PVDF membranes were not stripped. The blots were washed in 1X PBST twice (five minutes per wash) prior to being incubated with Pgp mouse monoclonal C494 antibody (Signet Laboratories, Dedham, MA) (dilution 1:7500) for 90 minutes. Subsequently, the membranes were washed twice (five minute per wash) in 1X PBST and incubated with a goat anti-mouse antibody (dilution 1:5000) (Santa Cruz Biotech, Santa Cruz, CA) for 45 minutes. For beta-actin, membranes were incubated with 1:1000 mouse antibody (Santa Cruz Biotech, Santa Cruz, CA) for 45 minutes, washed and subsequently incubated with goat anti-mouse secondary antibody (1:1000) (Santa Cruz Biotech, Santa Cruz, CA) for 60 minutes. The blots were developed using Pierce SuperSignal Chemiluminescent Substrate Luminol/Enhancer Solution (Pierce Biotechnology Inc, Rockford, IL). Densitometry analysis was performed using Doc-It software (UVP, Upland, CA). Pgp western blots were standardized to β-actin for each sample analyzed.
Quantitative Real-Time Polymerase Chain Reaction
Total cellular RNA was isolated from treated tissue or control samples using TRIzol® reagent (Invitrogen, Carlsbad, CA). Complementary DNA (cDNA) was prepared using the Promega reverse transcription system (Promega, Madison, WI). Two micrograms of total cellular RNA were incubated in an optimized buffered solution containing oligo(dt)15 primers, dNTPs, and AMV reverse transcriptase for 15 min. at 42 C. All steps were performed in accordance with the manufacturer's instructions. In preparation for real-time PCR, primers specific for Abcb1a, Abcb1b, and 18S rRNA (Table 1) were designed and ordered through Integrated DNA Technologies Primer Quest software (IDT, Coralville, IA). Real-time PCR was performed using the AB 7500 Real-time System.(Applied Biosystems, Foster City, CA). Each sample was run in triplicate for both genes of interest and the housekeeping gene. A calibrator sample was included on each run to ensure consistency between plates. Each well contained 12.5μl SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), 50nM sense primer, 50nM anti-sense primer, 2μl template, and nuclease free water to 25μl final volume. Gel electrophoresis and thermal denaturation (melt curve analysis) were used to confirm specific product formation. mRNA levels of analyzed genes were normalized to that of 18S RNA, and relative gene expression was determined using the ΔΔCT method.
Table 1. Abcb1 Primer Sequences.
| Sense Primer | |
| Abcb1a | 5′-TCTCCTATGCTGCTTGTTTCCGGT-3′ |
| Abcb1b | 5′-TGCCCAGAGCTTGCAGATACCATA-3′ |
| 18S rRNA | 5′-TCAACTTTCGATGGTAGTCGCCGT-3′ |
| Antisense Primer | |
| Abcb1a | 5′-TGATGATGTGGGATGCCGAGACTT-3′ |
| Abcb1b | 5′-ACCGGAAACAAGCAGCATAGGAGA-3′ |
| 18S rRNA | 5′-TCCTTGGATGTGGTAGCCGTTTCT-3′ |
Data Analysis
Rat demographic and PCR data are expressed as mean±SEM. For protein expression, median and interquartile ranges are shown along with means. Western blots were analyzed using densitometry and pixel numbers were compared between the two groups using a Wilcoxon rank sum test due to its statistical robustness.25 The Wilcoxon rank sum test is preferable if variances are different between comparison groups and it is less sensitive to outliers. During quantitative PCR, the change in fluorescence of SYBR Green I dye in every cycle was monitored by the system software, and the cycle threshold (CT) above background for each reaction was calculated. The CT value of 18S rRNA was subtracted from that of the gene of interest to obtain a ΔCT value. ΔCT values were compared between hypoxia and control rats using a Wilcoxon rank sum test. The average ΔCT value for control rats was subtracted from the ΔCT value for hypoxia rats to obtain a ΔΔCT value. The gene expression for hypoxic rats compared to control rats was expressed as 2 - ΔΔCT.26 In order to detect a 30% difference in myocardial Pgp expression between the hypoxia and control rats, including 12 rats in the hypoxia group and 15 rats in the control group would give us power of 0.8 at the 0.05 significance level (assuming a standard deviation of 0.25). We enrolled more control rats to allow for difficulties with the assays and to bolster our power. Statistical significance was defined as p<0.05.
Results
Animal Characteristics
On average, control rats tended to weigh more (357±5 g) (Mean±SEM) than rats exposed to intermittent hypoxia (345±5 g) (p=0.06). No rats in either group died during intermittent hypoxia or normoxia exposure.
Western Blot of P-glycoprotein
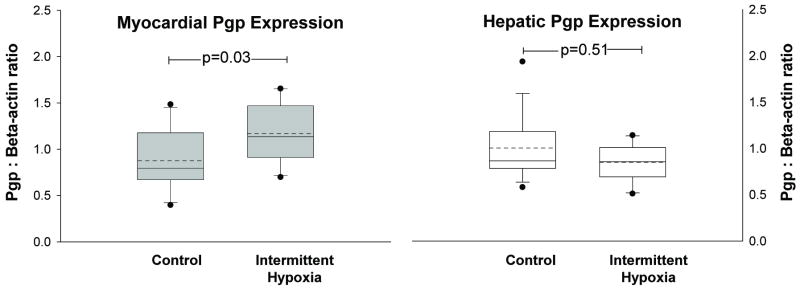
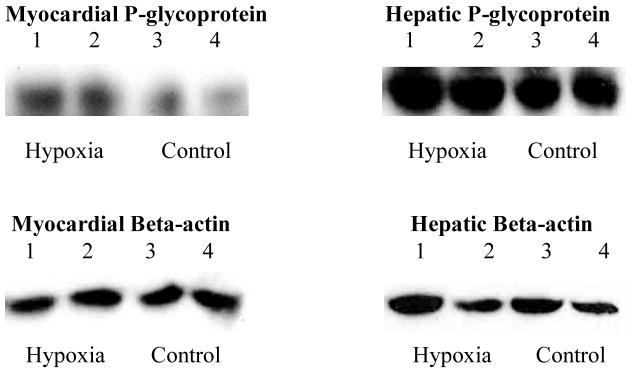
Densitometry analysis of myocardial Pgp (normalized to β-actin) demonstrated significantly more myocardial Pgp in rats exposed to hypoxia compared to controls (Figure 1). Expression of hepatic Pgp was not significantly different between hypoxia and control rats (Figure 1). Images of the western blots for myocardial and hepatic Pgp and beta-actin are shown in Figure 2.
Figure 1.
Mean (dashed horizontal line) and median (solid horizontal line) band density (pixels) of P-glycoprotein in homogenized heart (left) and liver (right) samples from rats exposed to two weeks of intermittent hypoxia and normoxia (control) are depicted in the boxplots. Myocardial Pgp protein expression was significantly increased (p=0.03) in rats exposed to hypoxia compared to rats exposed to room air. Hepatic Pgp expression was similar between hypoxia rats and controls. Outliers are depicted as dots above and below each boxplot.
Figure 2. Western Blots of Myocardial and Hepatic P-glycoprotein Expression.
Top - Western blots of P-glycoprotein in homogenized heart (left) and liver (right) in rats exposed to intermittent hypoxia and control conditions. Myocardial P-glycoprotein was significantly more abundant (darker bands) in hypoxic rats compared to control rats. Hepatic P-glycoprotein was similar between hypoxic and control rats. Bottom – Western blots of beta-actin in heart (left) and liver (right) for hypoxic and control rats. Beta-actin expression was similar between hypoxia and control rats in both heart and liver samples.
Quantitative Polymerase Chain Reaction of ABCB1 mRNA
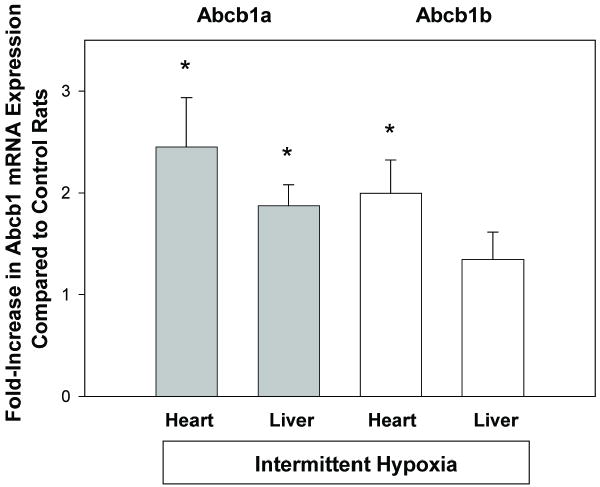
Relative expression of Abcb1a mRNA was significantly increased (> two-fold) in hearts of rats exposed to intermittent hypoxia compared to control rats (Figure 3). Likewise, relative expression of Abcb1a mRNA was significantly increased in livers of hypoxic rats compared to control rats (Figure 3). Abcb1b mRNA was significantly increased in heart but not liver tissue of rats exposed to intermittent hypoxia compared to control rats (Figure 3).
Figure 3.
Quantitative real time reverse-transcriptase PCR analysis of Abcb1a (gray bars) and Abcb1b (white bars) mRNA expression in heart and liver tissue of rats exposed to intermittent hypoxia compared to control rats. Data are shown as mean±SEM. Abcb1a was significantly upregulated in both heart and liver tissue, while Abcb1b was significantly upregulated in heart tissue of rats exposed to intermittent hypoxia. * p<0.05 compared to control
Discussion
The key findings to this study are that intermittent hypoxia increased myocardial Pgp expression and myocardial and hepatic Abcb1a mRNA expression. Liver Pgp was not significantly increased by intermittent hypoxia. It is conceivable that there were small changes in hepatic Pgp protein, since Western blots are mostly qualitative. However, visual inspection of the blots did not reveal noticeable differences in hepatic Pgp expression between control and hypoxic rats. There are potential reasons that we did not observe increased hepatic protein expression. There was only a modest, yet significant increase in the hepatic Abcb1a mRNA of hypoxic rats compared to controls. Hepatic Abcb1b mRNA was not significantly increased in hypoxic rats compared to controls. Thus, protein expression mostly mirrors the mRNA results. It is important to note, however, that changes in mRNA expression do not always correlate with changes in expression of protein.27-32 This may be a result of differences between the transcription and translation processes, differential mRNA and protein turnover, or posttranslational protein modifications.32 Future studies are needed to determine if there are functional changes that result in differential drug disposition and efficacy. Increased activity of Pgp in myocardial cells may decrease intracellular drug concentrations by increasing drug efflux and reducing therapeutic effects in myocardial tissue.
Previous studies investigating Pgp expression have focused on continuous hypoxia or a hypoxic tumor environment. To our knowledge, this is the first study investigating the effects of intermittent hypoxia on myocardial Pgp expression. Hypoxia has been shown to significantly increase expression of Abcb1, the gene that encodes Pgp expression.17,18 We hypothesized that intermittent hypoxia would also upregulate Abcb1 expression, but we were curious to determine what effect intermittent reoxygenations would have on expression. Abcb1 expression is mediated by the binding of hypoxia-inducible factor 1 (HIF1) to the hypoxia response element (HRE) in the Abcb1 promoter.17 Hypoxia-inducible factor 1 has alpha and beta subunits, and under hypoxic conditions, HIF1alpha moves to the nucleus, dimerizes with HIF1-beta and binds to the HRE.33 Since HIF1-alpha is rapidly degraded in normoxic conditions, we were unsure if intermittent periods of normoxia between hypoxic episodes would blunt the effect of hypoxia on Abcb1 expression. It appears that the c-Jun NH2-terminal kinase (JNK) pathway positively regulates Abcb1 gene transcription during hypoxia (mediated by increased HIF1 binding to the HRE in the Abcb1 gene promoter).19 During normoxia, increased c-Jun binding to the AP1 site in the Abcb1 gene promoter decreases Abcb1 gene expression.19 How the JNK pathway mediates both upregulation during hypoxia and downregulation of Abcb1 during normoxia is especially relevant during intermittent periods of hypoxia and reoxygenation. Future research is needed to provide mechanistic insights about the net change in Abcb1 expression during intermittent hypoxia.
Intermittent exposure to continuous hypoxia can stimulate angiogenesis and adaptive myocardial changes resulting in increased capillary and vessel density. However, we are not aware of any research investigating myocardial capillary density in a model of intermittent hypoxia that mimics sleep apnea. Intermittent hypoxia that mimics sleep apnea does increase capillary density in the hippocampus and cortex of neonatal mice.34 Immunohistochemical and gene expression studies have demonstrated that Pgp is localized in the endothelium of both arterioles and capillaries in heart samples.35 We did not evaluate capillary density in our study, but it is conceivable that increased Pgp expression after intermittent hypoxia may be partially explained by increased vascular density. Regardless of the mechanism, increased Pgp expression in itself is an important finding.
The presence of Pgp and Abcb1 mRNA in the rat heart has been confirmed in previous studies.36,37 Pgp is expressed in the human heart, but studies have demonstrated relatively low expression. Myocardial expression of Pgp is increased by ischemia and stress.38 OSA may also cause stress and intermittent hypoxia may increase myocardial Pgp expression resulting in cardiovascular conditions that respond poorly to drug therapy. Numerous medications used to treat heart disease and cardiac arrhythmias are known substrates for Pgp, and cardiovascular disorders in OSA patients are not easily controlled by drug therapy. For example, patients with OSA are twice as likely as patients without OSA to have recurrence of atrial fibrillation despite similar use of antiarrhythmic medications.6 Potential mechanisms responsible for recurrent atrial fibrillation in OSA patients include hypoxic modulation of cardiac electrical stability, chemoreflex activation, increased transmural pressure gradients, and distortion of cardiac configuration.6 Decreased availability of medications in the myocardium due to upregulated Pgp expression may also contribute to recurrent atrial fibrillation and other cardiovascular abnormalities. Since most antihypertensives (except beta-blockers) work in the vasculature, increases in myocardial but not hepatic Pgp would indicate that Pgp changes are unlikely to explain resistant hypertension in patients with OSA.
Important strengths of our study are, first, that all rats were randomly assigned to treatment with intermittent hypoxia and the analysis was blinded. Second, the use of an animal model eliminates the potential influence of medication use that may influence Pgp expression. Third, we used a model of intermittent hypoxia that mimics OSA instead of intermittent exposures to sustained hypoxia.
There are limitations to our study. Our model of intermittent hypoxia does not entirely represent all of the events that occur during obstructive sleep apnea. Intermittent hypoxia does not cause negative intrathoracic pressure swings or obstruction of the airway. Our intermittent hypoxia model is hypocapnic, whereas sleep apnea often times produces hypercapnia. It is possible that hypercapnic hypoxia may change the myocardial and hepatic effects of intermittent hypoxia, but no data has investigated the influence of hypercapnia on Pgp expression. Second, inflammatory cytokines and inflammatory markers such as C-reactive protein have been found to be elevated in patients with OSA.39-41 Inflammation appears to influence Abcb1 mRNA and Pgp expression,42-45 but how inflammation may modulate the effects of hypoxia on Pgp is unclear. Third, we used a two-week exposure to intermittent hypoxia and cannot predict if the changes in mRNA expression we found would be maintained or augmented with a longer exposure. Fourth, our study only assessed Pgp expression and does not provide functional data about Pgp substrate distribution in hypoxic and control rats or differences in therapeutic effectiveness. Functional studies will need to be performed using a variety of models to confirm our results and determine their significance. Fifth, the clinical relevance of myocardial Pgp has not been entirely established. Knockout mice who do not have Pgp demonstrate higher myocardial concentrations of Pgp substrates compared to mice with intact Pgp.46 Furthermore, coadministration of verapamil (a Pgp inhibitor) and doxorubicin (a Pgp substrate) in mice increased the peak doxorubicin concentration in the heart by 40%.47 The increase in doxorubicin concentrations (doxorubicin is cardiotoxic) resulted in degenerative changes to the cardiac tissue and decreased survival of these mice.47 However, studies documenting the clinical importance of myocardial Pgp in humans have not been performed, and as a result, the clinical relevance of altered myocardial Pgp in our results is mostly speculative. Lastly, we used a rodent model since rats have been frequently used as a model for evaluating intermittent hypoxia. Future research should determine if similar changes are found in humans with sleep apnea.
In conclusion, we found that two weeks of intermittent hypoxia significantly increased myocardial Pgp along with hepatic and myocardial Abcb1a mRNA expression. Future research is needed to determine the clinical significance of these preliminary findings and determine if clinical sleep apnea influences Pgp function and effectiveness of Pgp substrates.
Acknowledgments
The authors wish to thank Dr. Brien Neudeck for his input and assistance with the project.
Funding: Institutional Clinical and Translational Science Award (UW-Madison) (KL2) RR025012-01 (JMD).
Footnotes
Conflict of Interest: NONE DECLARED
References
- 1.Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998;97(10):943–45. doi: 10.1161/01.cir.97.10.943. [DOI] [PubMed] [Google Scholar]
- 2.Carpagnano GE, Kharitonov SA, Resta O, et al. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124:1386–92. doi: 10.1378/chest.124.4.1386. [DOI] [PubMed] [Google Scholar]
- 3.Minoguchi K, Yokoe T, Tazaki T, et al. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:625–30. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49:1625–31. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 5.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–59. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 6.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi M, Fujimoto K, Uchikawa S, Imamura H, Kubo K. Nocturnal oxygen desaturation correlates with the severity of coronary atherosclerosis in coronary artery disease. Chest. 2003;124:936–41. doi: 10.1378/chest.124.3.936. [DOI] [PubMed] [Google Scholar]
- 8.Lavie P, Hoffstein V. Sleep apnea syndrome: a possible contributing factor to resistant hypertension. Sleep. 2001;24:721–25. doi: 10.1093/sleep/24.6.721. [DOI] [PubMed] [Google Scholar]
- 9.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of obstructive sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–77. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Whitfield LR, Stewart BH. Atorvastatin transport in the caco-2 cell model: contributions of P-glycoprotein and the proton-monocarboxylic acid co-transporter. Pharm Res. 2000;17:209–15. doi: 10.1023/a:1007525616017. [DOI] [PubMed] [Google Scholar]
- 12.Soldner A, Benet LZ, Mutschler E, Christians U. Active transport of the angiotensin-II antagonist losartan and its main metabolite EXP 3174 across MDCK-MDR1 and caco-2 cell monolayers. Br J Pharmacol. 2000;129:1235–43. doi: 10.1038/sj.bjp.0703150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. Absence of the mdr1a P-glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin and cyclosporin A. J Clin Invest. 1995;96:1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottesmann MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 15.Bellamy WT. P-glycoproteins and multidrug resistance. Annu Rev Pharmacol Toxicol. 1996;36:161–83. doi: 10.1146/annurev.pa.36.040196.001113. [DOI] [PubMed] [Google Scholar]
- 16.Fradette C, Batonga J, Teng S, Piquette-Miller M, du Souich P. Animal models of acute moderate hypoxia are associated with a down-regulation of CYP1A1, 1A2, 2B4, 2C5, and 2C16 and up-regulation of CYP3A6 and P-glycoprotein in the liver. Drug Metab Dispos. 2007;35:765–71. doi: 10.1124/dmd.106.013508. [DOI] [PubMed] [Google Scholar]
- 17.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–94. [PubMed] [Google Scholar]
- 18.Wartenberg M, Ling FC, Muschen M, et al. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. Faseb J. 2003;17:503–05. doi: 10.1096/fj.02-0358fje. [DOI] [PubMed] [Google Scholar]
- 19.Liu M, Li D, Aneja R, et al. PO2-dependent differential regulation of multidrug resistance 1 gene expression by the c-Jun NH2-terminal kinase pathway. J Biol Chem. 2007;282:17581–86. doi: 10.1074/jbc.M702206200. [DOI] [PubMed] [Google Scholar]
- 20.Bachmakov I, Werner U, Endress B, Auge D, Fromm MF. Characterization of beta-adrenoreceptor antagonists as substrates and inhibitors of the drug transporter p-glycoprotein. Fund Clinical Pharmacology. 2006;20:273–82. doi: 10.1111/j.1472-8206.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 21.Sakaeda T, Takara K, Kakumoto M, et al. Simvastatin and lovastatin, but not pravastatin interact with MDR1. J Pharm Pharmacol. 2002;54:419–23. doi: 10.1211/0022357021778493. [DOI] [PubMed] [Google Scholar]
- 22.Cornwell MM, Pastan I, Gottesman MM. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem. 1987;262:2166–70. [PubMed] [Google Scholar]
- 23.Kakumoto M, Takara K, Sakaeda T, Tanigawara Y, Kita T, Okumura K. MDR1-mediated interaction of digoxin with antiarrhythmic or antianginal drugs. Biol Pharm Bull. 2002;25:1604–07. doi: 10.1248/bpb.25.1604. [DOI] [PubMed] [Google Scholar]
- 24.Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol. 2004;286:388–93. doi: 10.1152/ajpheart.00683.2003. [DOI] [PubMed] [Google Scholar]
- 25.Sawilowsky S. Misconceptions leading to choosing the t test over the Wilcoxon Mann-Whitney test for shift in location parameter. JMASM. 2005;4:598–600. [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Anderson NL, Seilhamer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis. 1997;18:533–37. doi: 10.1002/elps.1150180333. [DOI] [PubMed] [Google Scholar]
- 28.Anderson NL, Anderson NG. Proteome and proteomics: new technologies, new concepts, and new words. Electrophoresis. 1998;19:1853–61. doi: 10.1002/elps.1150191103. [DOI] [PubMed] [Google Scholar]
- 29.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–30. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Hoog CL, Mann M. Proteomics. Annu Rev Genomics Hum Genet. 2004;5:267–293. doi: 10.1146/annurev.genom.4.070802.110305. [DOI] [PubMed] [Google Scholar]
- 31.Hedge PS, White IR, Debouck C. Interplay of transcriptomics and proteomics. Curr Opin Biotechnol. 2003;14:647–51. doi: 10.1016/j.copbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene. 2001;265:11–23. doi: 10.1016/s0378-1119(01)00350-x. [DOI] [PubMed] [Google Scholar]
- 33.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–78. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 34.Panisello P, Torrella JR, Pages T, Viscor G. Capillary supply and fiber morphometry in rat myocardium after intermittent exposure to hypobaric hypoxia. High Alt Med Biol. 2007;8:322–30. doi: 10.1089/ham.2007.1030. [DOI] [PubMed] [Google Scholar]
- 35.Meissner K, Sperker B, Karsten C, et al. Expression and localization of P-glycoprotein in human heart: effects of cardiomyopathy. J Histochem Cytochem. 2002;50:1351–56. doi: 10.1177/002215540205001008. [DOI] [PubMed] [Google Scholar]
- 36.Beaulieu E, Demeule M, Ghitescu L, Beliveau R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J. 1997;326:539–44. doi: 10.1042/bj3260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cayre A, Moins N, Finat-Duclos F, Maublant J, Albuisson E, Verrelle P. In vitro detection of the MDR phenotype in rat myocardium: use of PCR, [3H]daunomycin and MDR reversing agents. Anticancer Drugs. 1996;7:833–37. doi: 10.1097/00001813-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Lazarowski AJ, Garcia Rivello HJ, Vera Janavel GL, et al. Cardiomyocytes of chronically ischemic pig hearts express the MDR-1 gene-encoded p-glycoprotein. J Histochem Cytochem. 2005;53:845–50. doi: 10.1369/jhc.4A6542.2005. [DOI] [PubMed] [Google Scholar]
- 39.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–64. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 40.Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Yokoe T, Minoguchi K, Matsuo H, et al. elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 42.Piquette-Miller M, Pak A, Kim H, Anarai R, Shahzamiani A. Decreased expression and activity of PGP in rat liver during acute inflammation. Pharm Res. 1998;15:706–11. doi: 10.1023/a:1011962818051. [DOI] [PubMed] [Google Scholar]
- 43.Hartmann G, Kim H, Piquette-Miller M. Regulation of the hepatic multidrug resistance gene expression by endotoxin and inflammatory cytokines in mice. Int Immunopharmacol. 2001;1:189–99. doi: 10.1016/s0162-3109(00)00271-x. [DOI] [PubMed] [Google Scholar]
- 44.Sukhai M, Yong A, Pak A, Piquette-Miller M. Decreased expression of P-glycoprotein in interleukin-1beta and interleukin-6 treated rat hepatocytes. Inflamm Res. 2001;50:362–70. doi: 10.1007/PL00000257. [DOI] [PubMed] [Google Scholar]
- 45.Hirsch-Ernst KI, Ziemann C, Foth H, Kozian D, Schmitz-Salue C, Kahl GF. Induction of mdr1b mRNA and PGP expression by TNF-alpha in primary rat hepatocyte cultures. J Cell Physiol. 1998;176:506–15. doi: 10.1002/(SICI)1097-4652(199809)176:3<506::AID-JCP7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 46.Schinkel AH, Smit JJ, van Tellingen O, et al. Disruption of the mouse mdr1a p-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 47.Sridhar R, Dwivedi C, Anderson J, et al. Effects of verapamil on the acute toxicity of doxorubicin in vivo. J Natl Cancer Inst. 1992;84:1653–60. doi: 10.1093/jnci/84.21.1653. [DOI] [PubMed] [Google Scholar]