Abstract
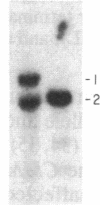
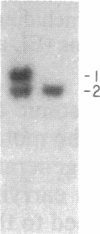
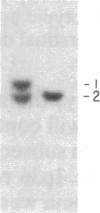
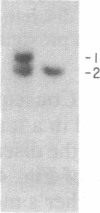
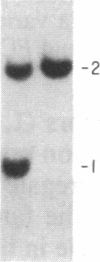
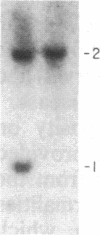
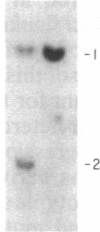
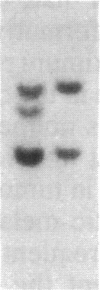
The gene for familial malignant melanoma and its precursor lesion, the dysplastic nevus, has been assigned to a region of the distal short arm of chromosome 1, which is frequently involved in karyotypic abnormalities in melanoma cells. We have examined loci on chromosome 1p for loss-of-constitutional heterozygosity in 35 melanomas and 21 melanoma cell lines to analyze the role of these abnormalities in melanocyte transformation. Loss-of-heterozygosity at loci on chromosome 1p was identified in 15/35 (43%) melanomas and 11/21 (52%) melanoma cell lines. Analysis of multiple metastases derived from the same patient and of melanoma and lymphoblastoid samples from a family with hereditary melanoma showed that the loss-of-heterozygosity at loci on distal 1p is a late event in tumor progression, rather than the second mutation that would occur if melanoma were due to a cellular recessive mechanism. Comparisons with neuroblastoma and multiple endocrine neoplasia (MEN2) suggest that the frequent 1p loss-of-heterozygosity in these malignancies is a common late event of neuroectodermal tumor progression.
Full text
PDF
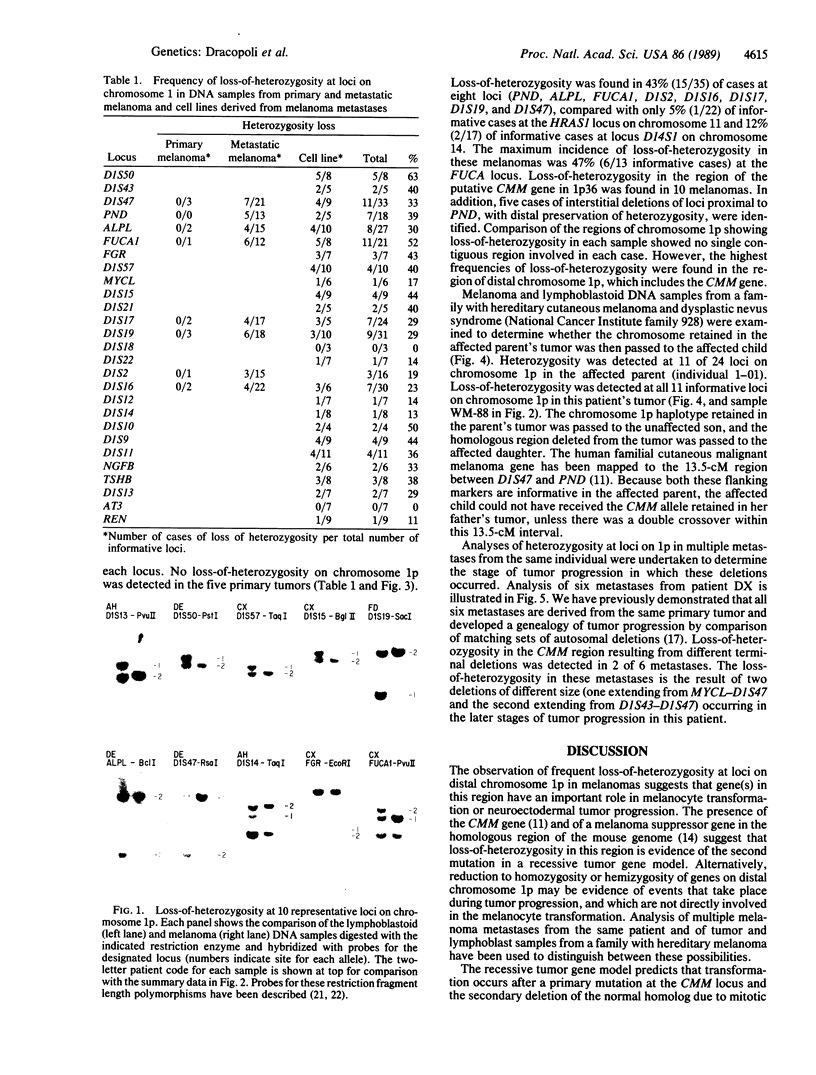
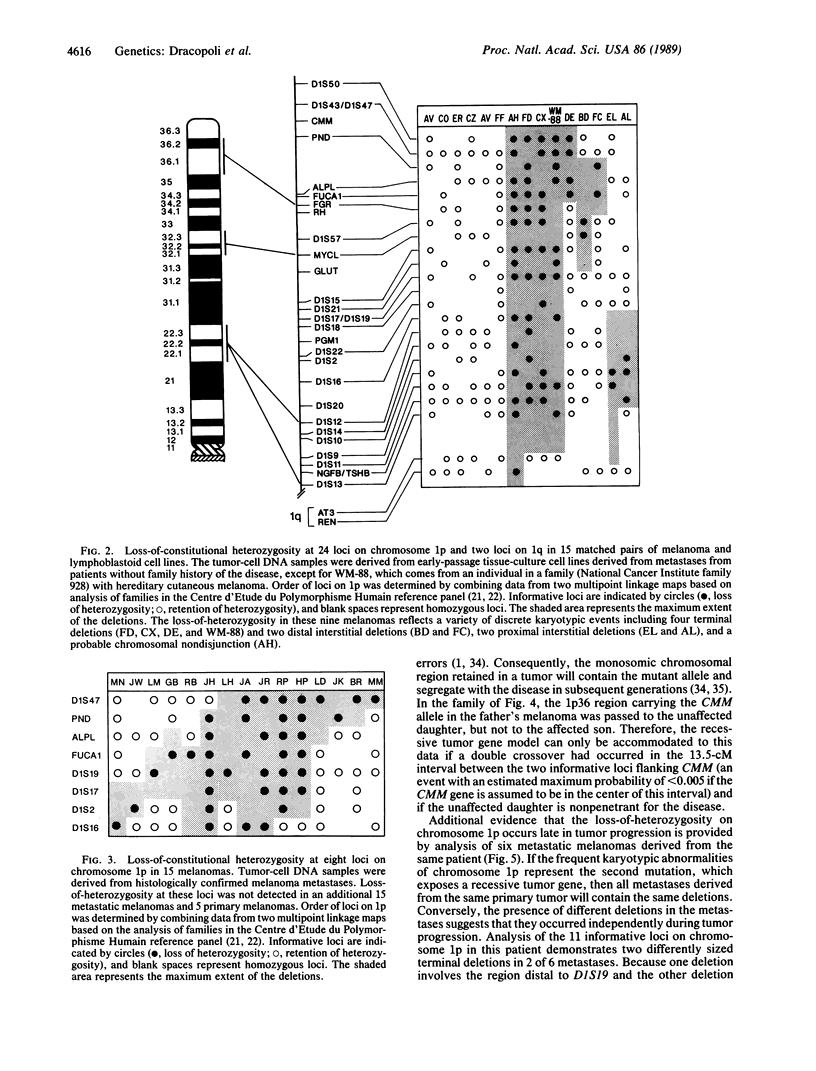
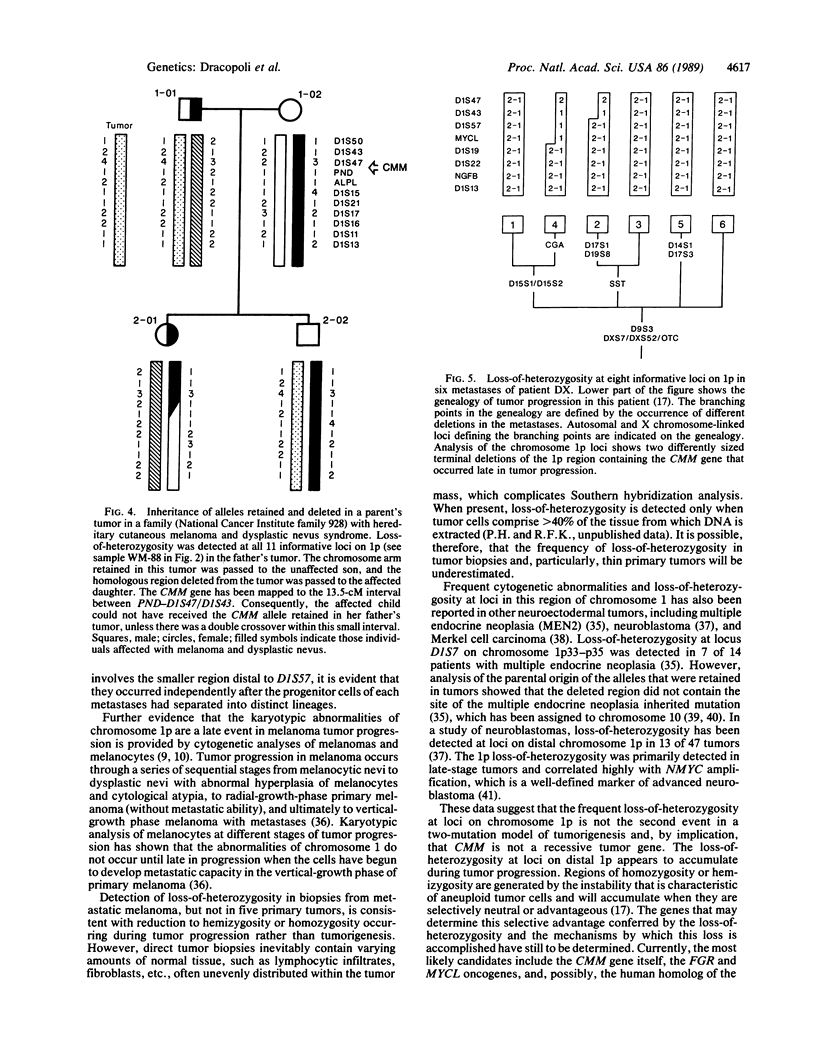

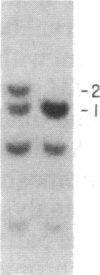
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balaban G. B., Herlyn M., Clark W. H., Jr, Nowell P. C. Karyotypic evolution in human malignant melanoma. Cancer Genet Cytogenet. 1986 Jan 1;19(1-2):113–122. doi: 10.1016/0165-4608(86)90378-x. [DOI] [PubMed] [Google Scholar]
- Bale S. J., Chakravarti A., Greene M. H. Cutaneous malignant melanoma and familial dysplastic nevi: evidence for autosomal dominance and pleiotropy. Am J Hum Genet. 1986 Feb;38(2):188–196. [PMC free article] [PubMed] [Google Scholar]
- Bale S. J., Dracopoli N. C., Tucker M. A., Clark W. H., Jr, Fraser M. C., Stanger B. Z., Green P., Donis-Keller H., Housman D. E., Greene M. H. Mapping the gene for hereditary cutaneous malignant melanoma-dysplastic nevus to chromosome 1p. N Engl J Med. 1989 May 25;320(21):1367–1372. doi: 10.1056/NEJM198905253202102. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock S. C., Harris J. F., Schwartz C. E., Ward J. H., Hershgold E. J., Skolnick M. H. Hereditary thrombosis in a Utah kindred is caused by a dysfunctional antithrombin III gene. Am J Hum Genet. 1985 Jan;37(1):32–41. [PMC free article] [PubMed] [Google Scholar]
- Bodmer W. F., Bailey C. J., Bodmer J., Bussey H. J., Ellis A., Gorman P., Lucibello F. C., Murday V. A., Rider S. H., Scambler P. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987 Aug 13;328(6131):614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- Breakefield X. O., Orloff G., Castiglione C., Coussens L., Axelrod F. B., Ullrich A. Structural gene for beta-nerve growth factor not defective in familial dysautonomia. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4213–4216. doi: 10.1073/pnas.81.13.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Hansen M. F., Nordenskjold M., Kock E., Maumenee I., Squire J. A., Phillips R. A., Gallie B. L. Genetic origin of mutations predisposing to retinoblastoma. Science. 1985 Apr 26;228(4698):501–503. doi: 10.1126/science.3983638. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Schaefer I. M., Rotwein P. S., Piccini N., Gross K. W., Naylor S. L. Human renin gene is on chromosome 1. Somat Cell Mol Genet. 1984 Jul;10(4):415–421. doi: 10.1007/BF01535637. [DOI] [PubMed] [Google Scholar]
- Cowan J. M., Halaban R., Francke U. Cytogenetic analysis of melanocytes from premalignant nevi and melanomas. J Natl Cancer Inst. 1988 Sep 21;80(14):1159–1164. doi: 10.1093/jnci/80.14.1159. [DOI] [PubMed] [Google Scholar]
- Darby J. K., Johnsen J., Nakashima P., Willems P. J., O'Brien J. S., Fowler M. L., Shows T. B., Shooter E. M., Cavalli-Sforza L. L. Pvu II RFLP at the human chromosome 1 alpha-L-fucosidase gene locus (FUCA1). Nucleic Acids Res. 1986 Dec 9;14(23):9543–9543. doi: 10.1093/nar/14.23.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Green P., Helms C., Cartinhour S., Weiffenbach B., Stephens K., Keith T. P., Bowden D. W., Smith D. R., Lander E. S. A genetic linkage map of the human genome. Cell. 1987 Oct 23;51(2):319–337. doi: 10.1016/0092-8674(87)90158-9. [DOI] [PubMed] [Google Scholar]
- Dracopoli N. C., Alhadeff B., Houghton A. N., Old L. J. Loss of heterozygosity at autosomal and X-linked loci during tumor progression in a patient with melanoma. Cancer Res. 1987 Aug 1;47(15):3995–4000. [PubMed] [Google Scholar]
- Dracopoli N. C., Houghton A. N., Old L. J. Loss of polymorphic restriction fragments in malignant melanoma: implications for tumor heterogeneity. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1470–1474. doi: 10.1073/pnas.82.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracopoli N. C., Rose E., Whitfield G. K., Guidon P. T., Jr, Bale S. J., Chance P. A., Kourides I. A., Housman D. E. Two thyroid hormone regulated genes, the beta-subunits of nerve growth factor (NGFB) and thyroid stimulating hormone (TSHB), are located less than 310 kb apart in both human and mouse genomes. Genomics. 1988 Aug;3(2):161–167. doi: 10.1016/0888-7543(88)90148-6. [DOI] [PubMed] [Google Scholar]
- Dracopoli N. C., Stanger B. Z., Ito C. Y., Call K. M., Lincoln S. E., Lander E. S., Housman D. E. A genetic linkage map of 27 loci from PND to FY on the short arm of human chromosome I. Am J Hum Genet. 1988 Oct;43(4):462–470. [PMC free article] [PubMed] [Google Scholar]
- Dracopoli N. C., Stanger B. Z., Lager M., Housman D. E. Localization of the FGR protooncogene on the genetic linkage map of human chromosome 1p. Genomics. 1988 Aug;3(2):124–128. doi: 10.1016/0888-7543(88)90142-5. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B., Feinberg A. P. Somatic deletion and duplication of genes on chromosome 11 in Wilms' tumours. Nature. 1984 May 10;309(5964):176–178. doi: 10.1038/309176a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fong C. T., Dracopoli N. C., White P. S., Merrill P. T., Griffith R. C., Housman D. E., Brodeur G. M. Loss of heterozygosity for the short arm of chromosome 1 in human neuroblastomas: correlation with N-myc amplification. Proc Natl Acad Sci U S A. 1989 May;86(10):3753–3757. doi: 10.1073/pnas.86.10.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frossard P. M., Coleman R. T. Human atrial natriuretic peptides (ANP) gene locus: BglI RFLP. Nucleic Acids Res. 1986 Nov 25;14(22):9223–9223. doi: 10.1093/nar/14.22.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M. H., Goldin L. R., Clark W. H., Jr, Lovrien E., Kraemer K. H., Tucker M. A., Elder D. E., Fraser M. C., Rowe S. Familial cutaneous malignant melanoma: autosomal dominant trait possibly linked to the Rh locus. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6071–6075. doi: 10.1073/pnas.80.19.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlyn M., Thurin J., Balaban G., Bennicelli J. L., Herlyn D., Elder D. E., Bondi E., Guerry D., Nowell P., Clark W. H. Characteristics of cultured human melanocytes isolated from different stages of tumor progression. Cancer Res. 1985 Nov;45(11 Pt 2):5670–5676. [PubMed] [Google Scholar]
- Jonasson J., Povey S., Harris H. The analysis of malignancy by cell fusion. VII. Cytogenetic analysis of hybrids between malignant and diploid cells and of tumours derived from them. J Cell Sci. 1977 Apr;24:217–254. doi: 10.1242/jcs.24.1.217. [DOI] [PubMed] [Google Scholar]
- Kidd K. K., Kidd J. R., Castiglione C. M., Sparkes R. S., Egeland J. A., Bakker E. The anonymous RFLP locus D1S2 is close to PGM1 on chromosome 1. Hum Hered. 1988;38(1):22–26. doi: 10.1159/000153749. [DOI] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Mathew C. G., Smith B. A., Thorpe K., Wong Z., Royle N. J., Jeffreys A. J., Ponder B. A. Deletion of genes on chromosome 1 in endocrine neoplasia. Nature. 1987 Aug 6;328(6130):524–526. doi: 10.1038/328524a0. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Leppert M., O'Connell P., Wolff R., Holm T., Culver M., Martin C., Fujimoto E., Hoff M., Kumlin E. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987 Mar 27;235(4796):1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- Nau M. M., Brooks B. J., Battey J., Sausville E., Gazdar A. F., Kirsch I. R., McBride O. W., Bertness V., Hollis G. F., Minna J. D. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature. 1985 Nov 7;318(6041):69–73. doi: 10.1038/318069a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Potter M., Sanford K. K., Parshad R., Tarone R. E., Price F. M., Mock B., Huppi K. Genes on chromosomes 1 and 4 in the mouse are associated with repair of radiation-induced chromatin damage. Genomics. 1988 Apr;2(3):257–262. doi: 10.1016/0888-7543(88)90010-9. [DOI] [PubMed] [Google Scholar]
- Reeve A. E., Housiaux P. J., Gardner R. J., Chewings W. E., Grindley R. M., Millow L. J. Loss of a Harvey ras allele in sporadic Wilms' tumour. Nature. 1984 May 10;309(5964):174–176. doi: 10.1038/309174a0. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Martuza R. L., Gusella J. F. Loss of genes on chromosome 22 in tumorigenesis of human acoustic neuroma. Nature. 1986 Aug 14;322(6080):644–647. doi: 10.1038/322644a0. [DOI] [PubMed] [Google Scholar]
- Simpson N. E., Kidd K. K., Goodfellow P. J., McDermid H., Myers S., Kidd J. R., Jackson C. E., Duncan A. M., Farrer L. A., Brasch K. Assignment of multiple endocrine neoplasia type 2A to chromosome 10 by linkage. Nature. 1987 Aug 6;328(6130):528–530. doi: 10.1038/328528a0. [DOI] [PubMed] [Google Scholar]
- Solomon E., Voss R., Hall V., Bodmer W. F., Jass J. R., Jeffreys A. J., Lucibello F. C., Patel I., Rider S. H. Chromosome 5 allele loss in human colorectal carcinomas. Nature. 1987 Aug 13;328(6131):616–619. doi: 10.1038/328616a0. [DOI] [PubMed] [Google Scholar]
- Sozzi G., Bertoglio M. G., Pilotti S., Rilke F., Pierotti M. A., Della Porta G. Cytogenetic studies in primary and metastatic neuroendocrine Merkel cell carcinoma. Cancer Genet Cytogenet. 1988 Jan;30(1):151–158. doi: 10.1016/0165-4608(88)90104-5. [DOI] [PubMed] [Google Scholar]
- Stoler A., Bouck N. Identification of a single chromosome in the normal human genome essential for suppression of hamster cell transformation. Proc Natl Acad Sci U S A. 1985 Jan;82(2):570–574. doi: 10.1073/pnas.82.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]