Abstract
Matrix metalloproteases (MMPs) are a collection of enzymes capable of cleaving extracellular matrix components, growth factors, and cell-surface receptors. MMPs modulate most aspects of tumorigenesis and are highly expressed in cancer compared with normal tissues. Preclinical studies have demonstrated that head and neck squamous cell carcinomas (HNSCCs) express high levels of MMPs in vivo and that inhibition of these enzymes in vitro and in mouse models decreases invasion and metastasis. However, the clinical trials for MMP inhibitors have failed to demonstrate a significant survival advantage in most cancers. The disparity between preclinical and clinical studies has led to the reevaluation of how MMP functions in cancer and the design of clinical trials for molecularly targeted agents. Mouse model data and analysis of HNSCC tumor specimens suggests that membrane type-1 MMP (MT1-MMP) may be a critical enzyme in tumor cell invasion and survival in vivo. This accumulated data provide evidence for development of selective MT1-MMP inhibitors as therapy in HNSCC.
Keywords: matrix metalloprotease inhibitor, MMP, extracellular matrix, head and neck cancer, squamous cell carcinoma, membrane type-1 MMP, drug development, proteases
The hallmark of cancer remains regional and distant metastases. Regional metastasis in head and neck squamous cell carcinoma (HNSCC) decreases survival by almost 50%, and invasion beyond the lymph node capsule further decreases survival.1 For tumor cells to invade and metastasize they must: (1) develop motility, (2) alter cell–cell and cell–matrix adhesion, and (3) remodel the extracellular matrix.2 It was recognized in the 1980s that matrix metalloprotease (MMP) could degrade extracellular matrix (ECM) components and that this could potentiate local tumor invasion and metastasis.3
A significant amount of effort has been funneled into the development of MMP inhibitors (MPIs) to treat cancer. Although observations of MPIs in vitro and in mouse models demonstrated an impressive reduction of the invasive or metastatic phenotype, results in clinical trials have been uniformly disappointing. Multiple review articles have been written to summarize MMP data in cancer and reconcile the failure of MPI in clinical trials.4-8 Consistent with its orphan status, current summaries of MMPs in HNSCC progression are few. To this end, we will focus on what is known about MMP expression and function in HNSCC and suggest future directions for MPI therapy in head and neck cancer.
MATRIX METALLOPROTEASES
MMPs are a diverse group of zinc-dependent endopeptidases that are synthesized as latent enzymes and are activated by release of propeptide domains. More than 25 different MMPs have been identified. With the exception of the membrane-type metalloproteases that are anchored to the cell surface, MMPs are secreted and diffuse through the ECM. These enzymes are capable of cleaving most ECM components, as well as other biologically important proteins such as growth factors, other proteases, adhesion molecules, and cell surface receptors (Table 1).9-26 With a widening understanding of MMP substrates, a more complex role for these enzymes in tumor growth and metastasis has been appreciated.
Table 1.
Matrix metalloproteases commonly identified in head and neck squamous cell carcinoma.
| MMP | Common name | MMP family | Substrates | Expression | References |
|---|---|---|---|---|---|
| MMP-1 | Interstitial collagenase |
Archetypal MMPs | CN types I, II, III, V, VII, VIII, and X, and gelatin |
Fibroblasts > tumor cells, macrophages, and endothelial cells |
9-11 |
| MMP-2 | Gelatinase A or 72-kDa gelatinase |
Gelatinases | CN types IV, V, VII, and X, gelatin, laminin, elastin, and fibronectin |
Fibroblasts > tumor cells and macrophages |
12-14 |
| MMP-3 | Stromelysin-1 | Stromelysins | CN types II, IV, IX, X, and XI, gelatin, elastin, fibronectin, and proMMP-1 |
Fibroblasts > tumor cells, macrophages, and endothelial cells |
15,16 |
| MMP-7 | Matrilysin | Matrilysins | CN types IV, aggrecan, and gelatin |
Tumor cells and macrophages | 17-19 |
| MMP-8 | Collagenase-2 | Archetypal MMPs | CN types I, II, III, and V | Fibroblasts and endothelial cells | 18,20 |
| MMP-9 | Gelatinase B or 92-kDa gelatinase |
Gelatinases | CN types IV, laminin, and gelatin |
Endothelial cell, fibroblasts, tumor cells, and macrophages |
13,16,21,22 |
| MMP-10 | Stromelysin-2 | Stromelysins | CN types IV, laminin, gelatin, and fibronectin |
Fibroblasts and tumor cells | 12 |
| MMP-11 | Stromelysin-3 | Stromelysins | Laminin | Fibroblasts and tumor cells | 12 |
| MMP-13 | Collagenase-3 | Archtypal MMPs | CN types I, II, III, IV, V, IX, X, and XI, laminin, fibronectin, and gelatin |
Fibroblasts | 9,11,23 |
| MMP-14 | MT1-MMP | Membrane-anchored MMPs |
CN types I, II, and III, gelatin, fibronectin, laminin, proMMP-2, and proMMP-13 |
Fibroblasts and tumor cells | 22,24-26 |
Abbreviations: MMP, matrix metalloprotease; CN, collagen; MMP-1, membrane type-1 MMP.
MMP activity is regulated at the transcription level. Transcription is now understood to be independently regulated with each cell type (eg, keratinocyte, melanocyte, and fibroblast) capable of displaying unique proteolytic phenotypes, such that each cancer type will likely have unique MMP profiles. MMPs can be upregulated by growth factor stimulation such as epidermal growth factors and hepatocyte growth factor.27 However, tumor cell response to growth factors have both positive and negative regulation effects of MMP transcription, depending on the stage of differentiation.5
MMP catalytic activity is also highly regulated. Cleavage of a propeptide domain allows expression of proteolytic function. Although most MMPs are secreted as inactive enzymes, membrane type 1 (MT1)-MMP is cleaved intracellularly by furin and is then expressed on the cell surface as an active protease. MT1-MMP is then capable of activating proMMP-2 to MMP-2. Tissue inhibitors of metalloproteases (TIMPs) block MMP catalytic activity at picomolar concentrations in vivo, and four homologs have been identified (TIMP-1–TIMP-4). TIMP-1 and TIMP-2 are commonly identified in HNSCC. TIMPs are secreted by fibroblasts and are also found at high levels in serum. Although the nomenclature suggests an exclusive role as inhibitors of MMPs, these molecules are known to have other biologic functions; for example, TIMP-2 is required for activation of pro-MMP-2.28 The complexity of MMP posttranscriptional regulation has implications for many studies of MMP expression in tumors. Because MMPs require processing for activation, gene or protein expression alone does not predict catalytic activity; therefore, studies that evaluate MMP expression in human tumors by immuno-histochemistry (active and inactive protein) or microarray (mRNA) provide no information of the presence of active enzymes.
MATRIX METALLOPROTEASES IN CANCER
Proteolytic enzymes are considered critical for local tumor cell invasion and distant metastasis, but not as originally hypothesized. Early understanding of MMPs in cancer cell invasion was simple; tumor cells elaborate enzymes to degrade the basement membrane and underlying collagen matrix. Using this proteolytic machinery, tumor cells migrate through dense ECM barriers and spread to local and distant sites.3 This hypothesis was supported by the wide range of ECM substrates cleaved by MMPs and the abundant MMP overexpression within tumor cells in vitro and in vivo. In fact, the initial discovery of many MMPs was a result of cloning cDNAs from tumors.
Although current evidence remains strong that certain MMPs promote invasion through ECM degradation and this function is required for cell invasion,27,29 several recent observations have fundamentally changed our understanding of how MMPs function in tumorigenesis. First, MMP substrates include proteins unrelated to ECM components such as growth factors or integrins.9,20 MMPs promote the initiation and growth of primary tumors in metastatic foci by activating growth factors or by releasing them from the matrix. MMPs process cell adhesion molecules and prevent tumor cells from responding to normal apoptotic signals.17 MMPs promote tumor angiogenesis by mobilizing or activating factors such as basic fibroblast growth factor, vascular endothelial growth factor, or transforming growth factor-beta.15,16,18
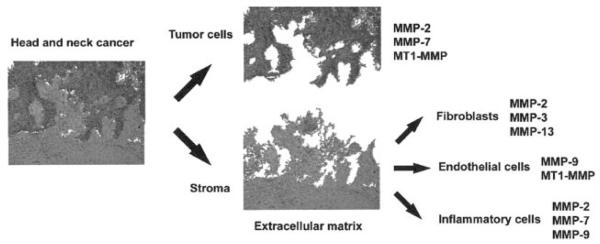
Second, the complexity of cell types expressing MMPs in the tumor mass was appreciated (Figure 1). Just as genetic dysregulation drove loss of responsiveness to normal apoptotic signals, it was theorized that MMPs were expressed primarily by malignant epithelial cells. However, most peritumoral MMPs are synthesized by host cells such as endothelial cells, fibroblasts, and inflammatory cells rather than tumor cells themselves.24,30 In fact, elegant mouse models of tumor progression in mice have demonstrated that MMP-9 expression by bone marrow–derived cells contributes to an aggressive phenotype in squa-mous cell carcinoma.21,31
FIGURE 1.
Head and neck cancer is composed of multiple cell types, each with different patterns of matrix metalloprotease (MMP) expression. The biologic complexity of MMPs in vivo was not appreciated during initial clinical trials of MMP inhibitors. Multiple cell types express MMPs.
Last, MMPs have both tumor promoting and inhibitory effects.18 Recent evidence suggests that MMPs can have a protective effect on tumor development. For example, MMPs have been found to negatively regulate neovascularization. MMP upregulation can increase conversion of plasminogen to angiostatin, which can decrease vascu-larization of transplanted xenographs.32-34 Thus, understanding the complex biology of MMPs requires understanding that MMPs are responsible for promoting cancer so that targeted MMP therapy can be developed.
Genetic models of cancer in mice have been invaluable in explaining the complexity of MMP biology in cancer. Deletions of most MMPs (including those MMPs highly expressed in HNSCC [MMP-2,35 MMP-3,36 and MMP-937]) and TIMPs have been engineered. With the exception MT1-MMP null mice, most of the MMP null mice are grossly indistinguishable from their litter mates, and fibroblasts derived from these mice display similar phenotypes on collagen.29 MT1-MMP null mice develop dwarfism and die within months of birth because of a failure to remodel type I collagen.38,39 These observations have drawn attention to the possible key role of MT1-MMP in matrix turnover.
Results from studies in genetic mouse models suggest that MMPs can impact metastasis, but the effects of growth on advanced tumors are often minimal. Injection of human tumor cells into immunosuppressed MMP-2 or MMP-9 null mice demonstrated marginal effect on tumor growth but did decrease lung metastasis by between 45% and 77%.35,40 Genetic deletions in mice have also suggested that MMPs have both growth promoting and abrogating effects in squamous cell carcinoma. For example, studies in MMP null mice have demonstrated a protective effect; deletion of MMP-8 and MMP-3 have been shown to promote skin carcinogenesis.41,42 These results have significant implications in the results of clinical trials for MMP targeted therapy.
ASSESSMENT OF MATRIX METALLOPROTEASES IN HEAD AND NECK CANCER
MMPs consistently found overexpressed in head and neck cancer include MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-11, MMP-13, and MMP-14 (Table 1). However, MMP-1, MMP-2, MMP-9, and MT-1 MMP are most commonly identified in HNSCC and associated with disease progression. With the understanding that conflicting reports are abundant and negative studies unlikely to be published, it is possible to summarize the extensive MMP findings in HNSCC. Although most studies evaluate only one or two MMPs within a given head and neck site, studies have examined expression of multiple TIMPs and MMPs in oral cavity SCC.12,22 Interestingly, these studies commonly identify upregulation of TIMP-1, which is associated with a poor survival. Levels of TIMP-2 are often unchanged between tumors and adjacent normal specimens. Studies have commonly identified MMP-2 and MMP-9 associated with lymph node metastasis43 and poor outcome44 in laryngeal cancer. Microarray gene expression studies on whole HNSCC tumor samples have identified overexpression of MMP-1, MMP-2, and MMP-3.45-47
Although most studies evaluate MMP mRNA or immunohistochemical reactivity, which may not correspond to in vivo enzymatic activity, increased active MMP-2 and MMP-9 has prognostic importance in laryngeal SCC48,49 and oral cavity SCC.13,50-52 Although MT1-MMP can degrade type I collagen and other matrix components,29 MT1-MMP also activates MMP-2 and is, therefore, considered a critical enzyme controlling proteolytic activity.53 HNSCC was one of the first tumors in which MT1-MMP was identified,54 and MT1-MMP expression is reported in 75% to 100% of HNSCC tumors.22,55
Although the focus of this review is on MMPs, other proteases have been demonstrated to play a role in head and neck cancer. Plasmin is a broad-spectrum protease best known for its role in fibrin degradation in wound healing, and the urokinase–plasminogen axis has frequently been identified as agents that promote both tumor invasion and angiogenesis. Plasminogen is converted to plasmin by urokinase and tissue plasminogen activator (uPA and tPA, respectively). Urokinase has been shown stimulate HNSCC cell growth in vitro and contribute to invasion,56 although probably not through type I collagen barriers. The urokinase–plasminogen axis and MMPs have many reciprocal interactions that promote matrix degradation10,57; for example, urokinase has been shown to activate MMP-2.58 Urokinase and its receptor (uPAR) are upregulated in head and neck cancer,59,60 but expression has not been shown to correlate with outcome.59,61
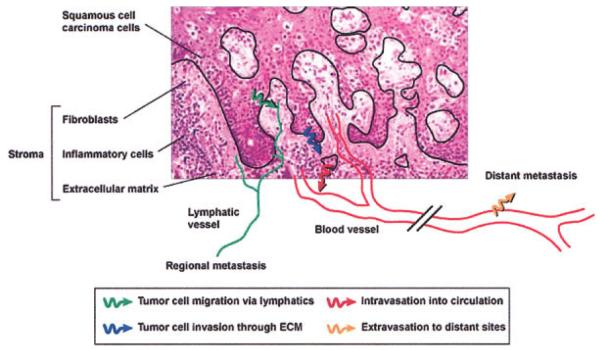
Consistent with the genetic progression model of tumorigenesis, it was originally thought that MMPs were synthesized by tumor cells to degrade the surrounding matrix; however, it is now understood that surrounding stroma cells produce most of the MMPs in tumors (Figure 2). Although HNSCC tumor cells do express some MMP-2, MMP-9, and MT1-MMP, these enzymes are primarily expressed by the surrounding fibroblasts and inflammatory cells.20,24 Also of note, tumors are continually exposed to normal serum, which contains high levels of MMP-2 and MMP-9.
FIGURE 2.
Tumor cell invasion of surrounding tissues requires multiple invasion programs. Migration of tumor cells occurs through the dense extracellular matrix (blue arrow); the tumor cells intravasate into the nearby vasculature (red arrow), followed by extravasation from the circulation into other organs. ECM, extracellular matrix.
Studies in preclinical mouse models of HNSCC have demonstrated that endogenous and synthetic inhibitors of MMPs can inhibit in vivo growth,11,62 but more dramatic effects are seen in prevention of metastasis.63-65 Multiple studies using orthotopic head and neck cancer models demonstrate an effect with MMP inhibitors; however, these models emphasize an early intervention, the need for tumor selection epidermal growth factor receptor (EGFR) status, and the lack of cellular toxicity. Using orthotopic oral SCC models that metastasize to lymph nodes, broad-spectrum synthetic MMP inhibitors have been shown to reduce the rate of cervical metastasis and improve survival, although growth rate was unaffected.64,65 Although cytotoxicity of broad-spectrum MMP inhibitors is uncommon in vitro or in vivo, some authors noted that head and neck cancer cell lines that overexpressed EGFRs were susceptible to inhibited growth by synthetic broad-spectrum inhibitors in vitro.12 These and other murine studies suggest that MPIs effectively prevent metastasis with minimal effect on growth. In fact, synthetic MMP inhibitors were found to reduce tumor growth when delivered in early stages of disease in mice, but not in late stages.66 This observation foreshadowed the outcome of MMP inhibitors in clinical trials.
MATRIX METALLOPROTEASE INHIBITORS IN CLINICAL TRIALS
Development of orally bioavailable matrix metalloprotease inhibitors was started in the late 1970s for arthritis. Ironically, the drugs were found to produce musculoskeletal pain that occurred after several weeks of dosing and was reversible by discontinuing the drugs. The musculoskeletal pain was unresponsive to analgesics and was dose limiting. These side effects were noticed during early phase I clinical trials and limited duration of treatment. Musculoskeletal pain has been attributable to both inhibition of MMP-1 activity and adamalysins. There is evidence to support the latter, because a recent MMP inhibitor, BMS-275291, has reduced activity against adamalysins; musculoskeletal toxicity (grade ≥2; 36%) was not statistically different from placebo in a phase I trial.67
Phase II trials were largely omitted from the development process, and several drugs went directly to phase III trials. These large-scale randomized trials compared cytostatic MMP inhibitors with cytotoxic treatments. No significant benefit in survival or disease progression was found in patients with advanced-stage pancreatic, gastric, breast, non-small cell lung, and prostate cancer.68-70 Some clinical trials have had promising results in gastric cancer and non-small cell lung cancer.71,72 Patients with limited musculoskeletal symptoms have had improved survival in recent studies.68 A clinical trial of MMP inhibitors in head and neck cancer has not been published, but reports in other cancers imply that broad-spectrum inhibitors will have limited clinical benefit (Table 2) because of dose-limiting musculoskeletal symptoms. Addition of MPIs to conventional platinum-based cytotoxic agents is well tolerated by patients, suggesting the potential for combination therapy.73
Table 2.
Matrix metalloprotease inhibitors in clinical trials
| Inhibitor | Specificity | Outcomes | Comments |
|---|---|---|---|
| Marimastat, BB-2516 | Broad spectrum | Survival benefit in gastric cancer and glioblatoma patients No survival benefit in non-small-cell and small-cell lung cancer, or ovarian cancer patients |
Most widely studied MPI |
| Tanomastat, BAY 12-9566 | MMP-2, -3, -9 | Survival worse than placebo | Development halted after initial results |
| Prinomastat, AG3340 | Broad spectrum | No survival benefit in non-small-cell lung or prostate cancer |
|
| Neovastat AE-941 | Broad spectrum | Survival advantage in non-small-cell lung cancer | Derived from ultrafiltration of shark cartilage |
| BMS-275291 | Broad spectrum | No survival advantage in non-small-cell lung cancer in combination with carboplatin and paclitaxil |
Abbreviations: MPI, matrix metalloprotease inhibitor; MMP, matrix metalloprotease. Additional information on these trials can be found in ref. 7.
The failure of MPIs in clinical trials remains baffling in light of the overwhelming preclinical data to support in vivo antitumor activity. Although it is possible that MPIs do not have a role in human cancer, retrospective analysis of the MPI clinical trials has identified some possible reasons for their failure.
Promising results in the laboratory reflect the failure of mouse models of human cancer to predict clinical benefit when using MMP inhibitors. Although the use of immunodeficient subcutaneous xenograph models has been criticized for the failure MPI trials, most novel therapeutic agents are subject to the same handicap. However, in the case of MMP investigations, it may be especially true because of the stromal source of the MMPs and the difference between human and mouse MMP biology. For example, mice do not have a gene equivalent for MMP-1. Furthermore, deletion of MMP-2 in mice shows no discernible phenotype, but human deletion of MMP-2 results in carpal tarsal osteolysis and osteoporosis (musculoskeletal findings distinct from MMP inhibitor side effects).
Mouse data that were available were not incorporated into the design of clinical trials. As previously discussed, preclinical mouse data suggested that MMP inhibition was far more efficient in preventing metastasis than in reducing tumor size.74 MPIs have a limited cytotoxic effect and may limit metastasis in patients without necessarily altering the primary tumor.
The ability of MPIs to inhibit MMPs within tumor at the administered concentrations remains unknown. Dosing was dependent on musculoskeletal side effects rather than tissue penetration or inhibition of catalytic activity. Although recent studies show the serum levels of inhibitors are sufficient to inhibit several MMPs in vitro at the doses being administered,68 inhibition of MMPs within the tumor remains unknown.
Very little was known about specific MMP expression within tumors at the time of trial design. The MMP profile within different tumor types is highly variable. Furthermore, the tumor-promoting effects of MMPs were not appreciated.
Currently, the only MPI approved by the U. S. Food and Drug Administration is doxycycline hyclate (Periostat, CollaGenex Pharmaceuticals, Inc, Newton, PA). This agent is an orally available, potent MMP-1 inhibitor. It is used as an adjunct to prevent periodontitis; although it has no antibacteria activity, as a collagenase inhibitor, it can prevent host-derived MMPs from degrading tooth-supporting structures. Currently multiple matrix metalloprotease inhibitors are being assessed in clinical trials for activities against psoriasis, acne, arthritis, cancer, and congestive heart failure.75
MT1-MMP AS A TARGET FOR SELECTIVE INHIBITION IN HEAD AND NECK CANCER
Significant data support selective MT1-MMP inhibition in HNSCC. These data come from expression studies in human tumors, genetic mouse models, and an improved understanding of MMP biology. Although multiple MMPs have been shown to be important in tumorigenesis, MT1-MMP is consistently reported to be overexpressed in head and neck cancer. Indeed, one of the first reports of MT-1 expression in human cancers was in head and neck cancer.54 Elevated MT1-MMP expression in HNSCC has been shown to positively correlate with an aggressive pattern of invasion, poor survival, and lymph node metastasis.12,76-78 Increased activity of the MT1-MMP activator, furin, is associated with more aggressive in vitro and in vivo tumor activity in HNSCC.79,80 In vitro and in vivo data similarly suggest that overexpression of MT1-MMP promotes HNSCC tumor cell invasion.25,81
MT1-MMP plays a critical role in tumor cell invasion and angiogenesis through several known mechanisms: (1) activation of MMP-2,77,82 (2) cleavage of the cell adhesion molecule CD44 from the cell surface,83,84 (3) degradation of type I collagen and fibrin substrates,29,85,86 and (4) enabling cell survival in three-dimensional matrices.87 As a membrane-anchored collagenase, MT1-MMP remains localized to the cell surface, unlike secreted MMPs. This enables cells a higher degree of control over the immediate microenvironment; cells can target adjacent tissues or other proteins for cleavage.
As previously discussed, deletion of MT1-MMP results in a lethal mutation in the mouse model; this is not true in other MMP mouse deletion. Studies comparing fibroblasts derived from MMP-2, MMP-3, MMP-8, MMP-9, and MMP-13 gene-deleted mice demonstrates that only MT1-MMP null fibroblasts display an invasive phenotype in type I collagen.29 Data from our laboratory are consistent with these findings. FaDu HNSCCs cells were cocultured with wild-type, MMP-2 null, MMP-9 null, or MT1-MMP null fibroblasts in vitro and in vivo. Wild-type, MMP-2 null, and MMP-9 null fibroblasts, but not MT1-MMP null fibroblasts, spontaneously invaded into type I collagen gels. Wild-type fibroblasts stimulated HNSCC tumor cell invasion in coculture, which was unaffected by combination with MMP-9 null fibroblasts, reduced with MMP-2 null fibroblasts, but completely abrogated in MT1-MMP null fibroblasts. Co-injection of HNSCC tumor cells with fibroblasts in an orthotopic oral cavity SCID model demonstrated some reduction of tumor volume using MMP-9 and MMP-2 null fibroblasts (48% and 49%, respectively) compared with wild-type fibroblasts. Consistent with in vitro studies, MT1-MMP null fibroblasts when coinjected with HNSCC cells resulted in a 90% reduction in tumor volume compared with HNSCC cells injected with wild-type fibroblasts. These data suggest that fibroblast-derived MT1-MMP plays a significant role in HNSCC progression and may be an important molecular target in head and neck cancer (Rosenthal, 2005).
The preclinical evidence suggests an important role for MT1-MMP in HNSCC, but selective inhibition requires a better understanding of MT1-MMP biology. A total of six MT-MMPs exists, and little is known about the biologic function of these enzymes. Furthermore, an in vivo assay of MT1-MMP catalytic activity should be developed to determine therapeutic levels in patients.88 Difficulties with the development of selective inhibitors may be a function of conformational variability at the catalytic site.89 The MMP responsible for musculoskeletal pain has not been identified and could be MT1-MMP (based on the phenotype of MT1-MMP null mice). Furthermore, MT1-MMP null mice have defects in early postnatal development that result in dwarfism, but it is unknown whether treatment with MT1-MMP–specific inhibitors will negatively impact mature bone remodeling. Ultimately, the evidence for MT1-MMP selective inhibition will require a clinical trial. An advance in the area of MPIs in another disease process may pave the way for additional clinical trials with MPIs in cancer.
CONCLUSION
MMP inhibitors were one of the first molecularly targeted agents to be brought to clinical trials. The failure of these agents in phase III clinical trials is primarily considered a result of applying a clinical trial endpoints and patient criteria established for conventional cytotoxic agents. A broader understanding of MMPs in tumors and their clinical implications will likely result in MMP-specific inhibitors that do not have dose-limiting musculoskeletal side effects. Evidence suggests that MT1-MMP is a logical target for such therapy in head and neck cancer. With additional understanding of MT1-MMP function in human tumors, selective inhibition is likely to act as an anti-invasion and antimetastatic therapeutic agent.
Acknowledgment
Alice Rhodes provided invaluable administrative assistance with this manuscript.
Contract grant sponsor: This work was supported by National Institutes of Health grant R03 DE16049 (ELR).
REFERENCES
- 1.Myers JN, Greenberg JS, Mo V, Roberts D. Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer. 2001;92(12):3030–3036. doi: 10.1002/1097-0142(20011215)92:12<3030::aid-cncr10148>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 2.Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80(Suppl 8):1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Liotta LA. Tumor invasion and metastases–role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986;46(1):1–7. [PubMed] [Google Scholar]
- 4.Freije JM, Balbin M, Pendas AM, Sanchez LM, Puente XS, Lopez-Otin C. Matrix metalloproteinases and tumor progression. Adv Exp Med Biol. 2003;532:91–107. doi: 10.1007/978-1-4615-0081-0_9. [DOI] [PubMed] [Google Scholar]
- 5.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2(9):657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 6.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411(6835):375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 7.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 8.Matrisian LM, Sledge GW, Jr, Mohla S. Extracellular proteolysis and cancer: meeting summary and future directions. Cancer Res. 2003;63(19):6105–6109. [PubMed] [Google Scholar]
- 9.Mueller MM, Fusenig NE. Tumor-stroma interactions directing phenotype and progression of epithelial skin tumor cells. Differentiation. 2002;70(9–10):486–497. doi: 10.1046/j.1432-0436.2002.700903.x. [DOI] [PubMed] [Google Scholar]
- 10.Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol. 1995;7(5):728–735. doi: 10.1016/0955-0674(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 11.Ala-aho R, et al. Targeted inhibition of human collagenase-3 (MMP-13) expression inhibits squamous cell carcinoma growth in vivo. Oncogene. 2004;23(30):5111–5123. doi: 10.1038/sj.onc.1207678. [DOI] [PubMed] [Google Scholar]
- 12.P OC, Rhys-Evans PH, Eccles SA. Expression of matrix metalloproteinases and their inhibitors correlates with invasion and metastasis in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2001;127(7):813–820. [PubMed] [Google Scholar]
- 13.Patel BP, et al. Activation of MMP-2 and MMP-9 in patients with oral squamous cell carcinoma. J Surg Oncol. 2005;90(2):81–88. doi: 10.1002/jso.20240. [DOI] [PubMed] [Google Scholar]
- 14.Arenas-Huertero FJ, et al. Matrix metalloproteinases expressed in squamous cell carcinoma of the oral cavity: correlation with clinicopathologic features and neo-adjuvant chemotherapy response. J Exp Clin Cancer Res. 1999;18(3):279–284. [PubMed] [Google Scholar]
- 15.Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10(6):415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- 16.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 17.Fingleton B, Vargo-Gogola T, Crawford HC, Matrisian LM. Matrilysin [MMP-7] expression selects for cells with reduced sensitivity to apoptosis. Neoplasia. 2001;3(6):459–468. doi: 10.1038/sj.neo.7900190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeClerck YA, Mercurio AM, Stack MS, et al. Proteases, extracellular matrix, and cancer: a workshop of the path B study section. Am J Pathol. 2004;164(4):1131–1139. doi: 10.1016/S0002-9440(10)63200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunha GR, Matrisian LM. It’s not my fault, blame it on my microenvironment. Differentiation. 2002;70(9–10):469–472. doi: 10.1046/j.1432-0436.2002.700901.x. [DOI] [PubMed] [Google Scholar]
- 20.Lynch CC, Matrisian LM. Matrix metalloproteinases in tumor-host cell communication. Differentiation. 2002;70(9–10):561–573. doi: 10.1046/j.1432-0436.2002.700909.x. [DOI] [PubMed] [Google Scholar]
- 21.Coussens LM, et al. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103(3):481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurahara S, et al. Expression of MMPS, MT-MMP, and TIMPs in squamous cell carcinoma of the oral cavity: correlations with tumor invasion and metastasis. Head Neck. 1999;21(7):627–638. doi: 10.1002/(sici)1097-0347(199910)21:7<627::aid-hed7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Mandic R, et al. Expression of MMP-3, MMP-13, TIMP-2 and TIMP-3 in the VX2 carcinoma of the New Zealand white rabbit. Anticancer Res. 2002;22(6A):3281–3284. [PubMed] [Google Scholar]
- 24.Rosenthal EL, et al. Expression of proteolytic enzymes in head and neck cancer-associated fibroblasts. Arch Otolaryngol Head Neck Surg. 2004;130(8):943–947. doi: 10.1001/archotol.130.8.943. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal EL, et al. Role of membrane type 1-matrix metalloproteinase and gelatinase A in head and neck squamous cell carcinoma invasion in vitro. Otolaryngol Head Neck Surg. 1999;121(4):337–343. doi: 10.1016/S0194-5998(99)70217-2. [DOI] [PubMed] [Google Scholar]
- 26.Tokumaru Y, et al. Activation of matrix metalloproteinase-2 in head and neck squamous cell carcinoma: studies of clinical samples and in vitro cell lines co-cultured with fibroblasts. Cancer Lett. 2000;150(1):15–21. doi: 10.1016/s0304-3835(99)00371-7. [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal EL, Johnson TM, Allen ED, Apel IJ, Punturieri A, Weiss SJ. Role of the plasminogen activator and matrix metalloproteinase systems in epidermal growth factor- and scatter factor-stimulated invasion of carcinoma cells. Cancer Res. 1998;58(22):5221–5230. [PubMed] [Google Scholar]
- 28.Itoh Y, Takamura A, Ito N, et al. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 2001;20(17):4782–4793. doi: 10.1093/emboj/20.17.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabeh F, Ota I, Holmbeck K, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167(4):769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shekhar MP, et al. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res. 2001;61(4):1320–1326. [PubMed] [Google Scholar]
- 31.Yang L, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6(4):409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 32.Pozzi A, LeVine WF, Gardner HA. Low plasma levels of matrix metalloproteinase 9 permit increased tumor angiogenesis. Oncogene. 2002;21(2):272–281. doi: 10.1038/sj.onc.1205045. [DOI] [PubMed] [Google Scholar]
- 33.Pozzi A, et al. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci U S A. 2000;97(5):2202–2207. doi: 10.1073/pnas.040378497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patterson BC, Sang QA. Angiostatin-converting enzyme activities of human matrilysin (MMP-7) and gelatinase B/ type IV collagenase (MMP-9) J Biol Chem. 1997;272(46):28823–28825. doi: 10.1074/jbc.272.46.28823. [DOI] [PubMed] [Google Scholar]
- 35.Itoh T, et al. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58(5):1048–1051. [PubMed] [Google Scholar]
- 36.Wilson CL, et al. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci U S A. 1997;94(4):1402–1407. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z, et al. Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J Exp Med. 1998;188(3):475–482. doi: 10.1084/jem.188.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmbeck K, et al. MT1-MMP: a tethered collagenase. J Cell Physiol. 2004;200(1):11–19. doi: 10.1002/jcp.20065. [DOI] [PubMed] [Google Scholar]
- 39.Holmbeck K, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99(1):81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 40.Itoh T, et al. Experimental metastasis is suppressed in MMP-9-deficient mice. Clin Exp Metastasis. 1999;17(2):177–181. doi: 10.1023/a:1006603723759. [DOI] [PubMed] [Google Scholar]
- 41.Balbin M, et al. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35(3):252–257. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]
- 42.McCawley LJ, et al. A protective role for matrix metalloproteinase-3 in squamous cell carcinoma. Cancer Res. 2004;64(19):6965–6972. doi: 10.1158/0008-5472.CAN-04-0910. [DOI] [PubMed] [Google Scholar]
- 43.Xie M, Sun Y, Li Y. Expression of matrix metalloproteinases in supraglottic carcinoma and its clinical implication for estimating lymph node metastases. Laryngoscope. 2004;114(12):2243–2248. doi: 10.1097/01.mlg.0000149467.18822.59. [DOI] [PubMed] [Google Scholar]
- 44.Katayama A, et al. Expressions of matrix metalloproteinases in early-stage oral squamous cell carcinoma as predictive indicators for tumor metastases and prognosis. Clin Cancer Res. 2004;10(2):634–640. doi: 10.1158/1078-0432.ccr-0864-02. [DOI] [PubMed] [Google Scholar]
- 45.Chung CH, et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5(5):489–500. doi: 10.1016/s1535-6108(04)00112-6. [DOI] [PubMed] [Google Scholar]
- 46.Villaret DB, et al. Identification of genes overexpressed in head and neck squamous cell carcinoma using a combination of complementary DNA subtraction and microarray analysis. Laryngoscope. 2000;110(3 Pt 1):374–381. doi: 10.1097/00005537-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Nagata M, et al. Identification of potential biomarkers of lymph node metastasis in oral squamous cell carcinoma by cDNA microarray analysis. Int J Cancer. 2003;106(5):683–689. doi: 10.1002/ijc.11283. [DOI] [PubMed] [Google Scholar]
- 48.Christopoulos TA, et al. Diagnostic and classification value of metalloproteinases in squamous human laryngeal carcinoma. Int J Oncol. 2004;25(2):481–485. [PubMed] [Google Scholar]
- 49.Bogusiewicz M, et al. Activity of matrix metalloproteinases-2 and -9 in advanced laryngeal cancer. Otolaryngol Head Neck Surg. 2003;128(1):132–136. doi: 10.1067/mhn.2003.8. [DOI] [PubMed] [Google Scholar]
- 50.Yoshizaki T, et al. Expression of tissue inhibitor of matrix metalloproteinase-2 correlates with activation of matrix metalloproteinase-2 and predicts poor prognosis in tongue squamous cell carcinoma. Int J Cancer. 2001;95(1):44–50. doi: 10.1002/1097-0215(20010120)95:1<44::aid-ijc1008>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 51.Yorioka CW, et al. Matrix metalloproteinase-2 and -9 activities correlate with the disease-free survival of oral squamous cell carcinoma patients. Int J Oncol. 2002;20(1):189–194. [PubMed] [Google Scholar]
- 52.Shimada T, et al. Enhanced production and activation of progelatinase A mediated by membrane-type 1 matrix metalloproteinase in human oral squamous cell carcinomas: implications for lymph node metastasis. Clin Exp Metastasis. 2000;18(2):179–188. doi: 10.1023/a:1006749501682. [DOI] [PubMed] [Google Scholar]
- 53.Sato H, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370(6484):61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 54.Okada A, et al. Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci U S A. 1995;92(7):2730–2734. doi: 10.1073/pnas.92.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Birkedal-Hansen B, et al. MMP and TIMP gene expression in head and neck squamous cell carcinomas and adjacent tissues. Oral Dis. 2000;6(6):376–382. doi: 10.1111/j.1601-0825.2000.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt M, Grunsfelder P. Urokinase-type plasminogen activator expression and proliferation stimulation in head and neck squamous cell carcinoma in vitro and in situ. Arch Otolaryngol Head Neck Surg. 2001;127(6):679–682. doi: 10.1001/archotol.127.6.679. [DOI] [PubMed] [Google Scholar]
- 57.Eeckhout Y, Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keski-Oja J, et al. Proteolytic processing of the 72,000-Da type IV collagenase by urokinase plasminogen activator. Exp Cell Res. 1992;202(2):471–476. doi: 10.1016/0014-4827(92)90101-d. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt M, et al. Urokinase receptor up-regulation in head and neck squamous cell carcinoma. Head Neck. 2000;22(5):498–504. doi: 10.1002/1097-0347(200008)22:5<498::aid-hed9>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 60.Pacheco MM, et al. Differential expression of c-jun and c-fos mRNAs in squamous cell carcinoma of the head and neck: associations with uPA, gelatinase B, and matrilysin mRNAs. Head Neck. 2002;24(1):24–32. doi: 10.1002/hed.10009. [DOI] [PubMed] [Google Scholar]
- 61.Pasini FS, et al. Transforming growth factor beta1, urokinase-type plasminogen activator and plasminogen activator inhibitor-1 mRNA expression in head and neck squamous carcinoma and normal adjacent mucosa. Head Neck. 2001;23(9):725–732. doi: 10.1002/hed.1103. [DOI] [PubMed] [Google Scholar]
- 62.Ahonen M, et al. Antitumor activity and bystander effect of adenovirally delivered tissue inhibitor of metalloproteinases-3. Mol Ther. 2002;5(6):705–715. doi: 10.1006/mthe.2002.0606. [DOI] [PubMed] [Google Scholar]
- 63.Maekawa K, et al. Inhibition of cervical lymph node metastasis by marimastat (BB-2516) in an orthotopic oral squamous cell carcinoma implantation model. Clin Exp Metastasis. 2002;19(6):513–518. doi: 10.1023/a:1020329411957. [DOI] [PubMed] [Google Scholar]
- 64.P OC, Rhys-Evans P, Eccles S. A synthetic matrix metalloproteinase inhibitor prevents squamous carcinoma cell proliferation by interfering with epidermal growth factor receptor autocrine loops. Int J Cancer. 2002;100(5):527–533. doi: 10.1002/ijc.10531. [DOI] [PubMed] [Google Scholar]
- 65.Yamashita T, et al. The inhibitory effect of matrix metalloproteinase inhibitor ONO-4817 on lymph node metastasis in tongue carcinoma. Anticancer Res. 2003;23(3B):2297–2302. [PubMed] [Google Scholar]
- 66.Bergers G, et al. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284(5415):808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 67.Miller KD, et al. A randomized phase II feasibility trial of BMS-275291 in patients with early stage breast cancer. Clin Cancer Res. 2004;10(6):1971–1975. doi: 10.1158/1078-0432.ccr-03-0968. [DOI] [PubMed] [Google Scholar]
- 68.Sparano JA, et al. Randomized phase III trial of marimastat versus placebo in patients with metastatic breast cancer who have responding or stable disease after first-line chemotherapy: Eastern Cooperative Oncology Group trial E2196. J Clin Oncol. 2004;22(23):4683–4690. doi: 10.1200/JCO.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 69.Evans JD, et al. A phase II trial of marimastat in advanced pancreatic cancer. Br J Cancer. 2001;85(12):1865–1870. doi: 10.1054/bjoc.2001.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bramhall SR, et al. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87(2):161–167. doi: 10.1038/sj.bjc.6600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Latreille J, et al. Phase I/II trial of the safety and efficacy of AE-941 (Neovastat) in the treatment of non-small-cell lung cancer. Clin Lung Cancer. 2003;4(4):231–236. doi: 10.3816/clc.2003.n.003. [DOI] [PubMed] [Google Scholar]
- 72.Bramhall SR, et al. Marimastat as maintenance therapy for patients with advanced gastric cancer: a randomised trial. Br J Cancer. 2002;86(12):1864–1870. doi: 10.1038/sj.bjc.6600310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Douillard JY, et al. Randomized phase II feasibility study of combining the matrix metalloproteinase inhibitor BMS-275291 with paclitaxel plus carboplatin in advanced non-small cell lung cancer. Lung Cancer. 2004;46(3):361–368. doi: 10.1016/j.lungcan.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 74.Sledge GW, Jr, et al. Effect of matrix metalloproteinase inhibitor batimastat on breast cancer regrowth and metastasis in athymic mice. J Natl Cancer Inst. 1995;87(20):1546–1550. doi: 10.1093/jnci/87.20.1546. [DOI] [PubMed] [Google Scholar]
- 75.Peterson JT. Matrix metalloproteinase inhibitor development and the remodeling of drug discovery. Heart Fail Rev. 2004;9(1):63–79. doi: 10.1023/B:HREV.0000011395.11179.af. [DOI] [PubMed] [Google Scholar]
- 76.Janot F, et al. Prognostic value of clinicopathological parameters in head and neck squamous cell carcinoma: a prospective analysis. Br J Cancer. 1996;73(4):531–538. doi: 10.1038/bjc.1996.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imanishi Y, et al. Clinical significance of expression of membrane type 1 matrix metalloproteinase and matrix metalloproteinase-2 in human head and neck squamous cell carcinoma. Hum Pathol. 2000;31(8):895–904. doi: 10.1053/hupa.2000.9756. [DOI] [PubMed] [Google Scholar]
- 78.Yoshizaki T, et al. Increased expression of membrane type 1-matrix metalloproteinase in head and neck carcinoma. Cancer. 1997;79(1):139–144. doi: 10.1002/(sici)1097-0142(19970101)79:1<139::aid-cncr20>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 79.Bassi DE, et al. Increased furin activity enhances the malignant phenotype of human head and neck cancer cells. Am J Pathol. 2003;162(2):439–447. doi: 10.1016/s0002-9440(10)63838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bassi DE, et al. Elevated furin expression in aggressive human head and neck tumors and tumor cell lines. Mol Carcinog. 2001;31(4):224–232. doi: 10.1002/mc.1057. [DOI] [PubMed] [Google Scholar]
- 81.Pauli BU, Knudson W. Tumor invasion: a consequence of destructive and compositional matrix alterations. Hum Pathol. 1988;19(6):628–639. doi: 10.1016/s0046-8177(88)80168-0. [DOI] [PubMed] [Google Scholar]
- 82.Jiang A, Pei D. Distinct roles of catalytic and pexin-like domains in MT-MMP mediated proMMP-2 activation and collagenolysis. J Biol Chem. 2003;278(40):38765–38771. doi: 10.1074/jbc.M306618200. [DOI] [PubMed] [Google Scholar]
- 83.Hotary KB, et al. Matrix metalloproteinases (MMPs) regulate fibrin-invasive activity via MT1-MMP-dependent and -independent processes. J Exp Med. 2002;195(3):295–308. doi: 10.1084/jem.20010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang A, et al. Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc Natl Acad Sci U S A. 2001;98(24):13693–13698. doi: 10.1073/pnas.241293698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kajita M, et al. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153(5):893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mori H, et al. CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J. 2002;21(15):3949–3959. doi: 10.1093/emboj/cdf411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hotary KB, et al. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114(1):33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 88.McIntyre JO, et al. Development of a novel fluorogenic proteolytic beacon for in vivo detection and imaging of tumour-associated matrix metalloproteinase-7 activity. Biochem J. 2004;377(Pt 3):617–628. doi: 10.1042/BJ20030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bertini I, et al. Conformational variability of matrix metalloproteinases: Beyond a single 3D structure. Proc Natl Acad Sci U S A. 2005;102(15):5334–5339. doi: 10.1073/pnas.0407106102. [DOI] [PMC free article] [PubMed] [Google Scholar]