Abstract
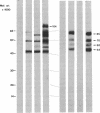
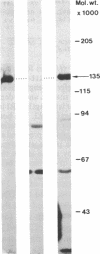
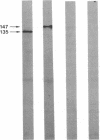
Theileria annulata is an economically important protozoan parasite that threatens an estimated 250 million cattle with the disease tropical theileriosis. Development of a defined subunit vaccine is one means of trying to develop control measures against the disease. To this end we have characterized a surface antigen complex of the infective stage (sporozoite), by using a monoclonal antibody that neutralizes sporozoite infectivity in vitro. We have cloned the gene coding for this complex and have demonstrated that a fusion protein expressed from a fragment of this gene elicits strong neutralizing antibodies. Furthermore we provide data on the structure and expression of this gene. In particular we show that the region of the gene, expressed in one clone, codes for a protein segment relatively rich in proline residues. Also we demonstrate that expression of this gene appears to be stage specific, transcripts being present only in the sporoblast and sporozoite stages. The relevance of these findings to the production of a defined subunit vaccine is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dobbelaere D. A., Spooner P. R., Barry W. C., Irvin A. D. Monoclonal antibody neutralizes the sporozoite stage of different Theileria parva stocks. Parasite Immunol. 1984 Jul;6(4):361–370. doi: 10.1111/j.1365-3024.1984.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Nussenzweig R. Progress on Theileria vaccine. Nature. 1985 Aug 8;316(6028):484–485. doi: 10.1038/316484a0. [DOI] [PubMed] [Google Scholar]
- FULTON J. D., GRANT P. T. The sulphur requirements of the erythrocytic from of Plasmodium knowlesi. Biochem J. 1956 Jun;63(2):274–282. doi: 10.1042/bj0630274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring M. R., Shiels B. R., Northemann W., de Bruijn M. H., Kan C. C., Chain A. C., Noonan D. J., Fey G. H. Sequence of rat liver alpha 2-macroglobulin and acute phase control of its messenger RNA. J Biol Chem. 1987 Jan 5;262(1):446–454. [PubMed] [Google Scholar]
- Gill B. S., Bhattacharyulu Y., Kaur D. Immunisation against bovine tropical theileriasis (Theileria annulata infection). Res Vet Sci. 1976 Sep;21(2):146–149. [PubMed] [Google Scholar]
- Hall R., Hyde J. E., Goman M., Simmons D. L., Hope I. A., Mackay M., Scaife J., Merkli B., Richle R., Stocker J. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. 1984 Sep 27-Oct 3Nature. 311(5984):379–382. doi: 10.1038/311379a0. [DOI] [PubMed] [Google Scholar]
- Irvin A. D., Dobbelaere D. A., Mwamachi D. M., Minami T., Spooner P. R., Ocama J. G. Immunisation against East Coast fever: correlation between monoclonal antibody profiles of Theileria parva stocks and cross immunity in vivo. Res Vet Sci. 1983 Nov;35(3):341–346. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Lecocq J. P. New versatile cloning and sequencing vectors based on bacteriophage M13. Gene. 1983 Dec;26(1):91–99. doi: 10.1016/0378-1119(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Melrose T. R., Brown C. G., Sharma R. D. Glucose phosphate isomerase isoenzyme patterns in bovine lymphoblastoid cell lines infected with Theileria annulata and T parva, with an improved enzyme visualisation method using meldola blue. Res Vet Sci. 1980 Nov;29(3):298–304. [PubMed] [Google Scholar]
- Musoke A. J., Nantulya V. M., Rurangirwa F. R., Buscher G. Evidence for a common protective antigenic determinant on sporozoites of several Theileria parva strains. Immunology. 1984 Jun;52(2):231–238. [PMC free article] [PubMed] [Google Scholar]
- Pearson T. W., Lundin L. B., Dolan T. T., Stagg D. A. Cell-mediated immunity to Theileria-transformed cell lines. Nature. 1979 Oct 25;281(5733):678–680. doi: 10.1038/281678a0. [DOI] [PubMed] [Google Scholar]
- Pearson T. W., Pinder M., Roelants G. E., Kar S. K., Lundin L. B., Mayor-Withey K. S., Hwett R. S. Methods for derivation and detection of anti-parasite monoclonal antibodies. J Immunol Methods. 1980;34(2):141–154. doi: 10.1016/0022-1759(80)90168-4. [DOI] [PubMed] [Google Scholar]
- Preston P. M., Brown C. G. Inhibition of lymphocyte invasion by sporozoites and the transformation of trophozoite infected lymphocytes in vitro by serum from Theileria annulata immune cattle. Parasite Immunol. 1985 May;7(3):301–314. doi: 10.1111/j.1365-3024.1985.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Preston P. M., Brown C. G., Spooner R. L. Cell-mediated cytotoxicity in Theileria annulata infection of cattle with evidence for BoLA restriction. Clin Exp Immunol. 1983 Jul;53(1):88–100. [PMC free article] [PubMed] [Google Scholar]
- Preston P. M., McDougall C., Wilkie G., Shiels B. R., Tait A., Brown C. G. Specific lysis of Theileria annulata-infected lymphoblastoid cells by a monoclonal antibody recognizing an infection-associated antigen. Parasite Immunol. 1986 Jul;8(4):369–380. doi: 10.1111/j.1365-3024.1986.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Robinson P. M. Theileriosis annulata and its transmission-a review. Trop Anim Health Prod. 1982 Feb;14(1):3–12. doi: 10.1007/BF02281092. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein E., Büscher G., Friedhoff K. T. Lichtmikroskopische Untersuchungen über die Entwicklung von Theileria annulata (Dschunkowsky and Luhs, 1904) in Hyalomma anatolicum excavatum (Koch, 1844). I. Die Entwicklung im Darm vollgesogener Nymphen. Z Parasitenkd. 1975 Dec 23;48(2):123–136. doi: 10.1007/BF00389643. [DOI] [PubMed] [Google Scholar]
- Shiels B. R., McDougall C., Tait A., Brown C. G. Identification of infection-associated antigens in Theileria annulata transformed cells. Parasite Immunol. 1986 Jan;8(1):69–77. doi: 10.1111/j.1365-3024.1986.tb00834.x. [DOI] [PubMed] [Google Scholar]
- Shiels B., McDougall C., Tait A., Brown C. G. Antigenic diversity of Theileria annulata macroschizonts. Vet Parasitol. 1986 May;21(1):1–10. doi: 10.1016/0304-4017(86)90137-8. [DOI] [PubMed] [Google Scholar]
- Shortman K., Williams N., Adams P. The separation of different cell classes from lymphoid organs. V. Simple procedures for the removal of cell debris. Damaged cells and erythroid cells from lymphoid cell suspensions. J Immunol Methods. 1972 May;1(3):273–287. doi: 10.1016/0022-1759(72)90005-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Walker A. R., McKellar S. B. Observations on the separation of Theileria sporozoites from ticks. Int J Parasitol. 1983 Jun;13(3):313–318. doi: 10.1016/0020-7519(83)90044-9. [DOI] [PubMed] [Google Scholar]
- Walker A. R., McKellar S. B. The maturation of Theileria annulata in Hyalomma anatolicum anatolicum stimulated by incubation or feeding to produce sporozoites. Vet Parasitol. 1983 Aug;13(1):13–21. doi: 10.1016/0304-4017(83)90016-x. [DOI] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]