Abstract
Background
Septins are involved in a number of cellular processes including cytokinesis and organization of the cytoskeleton. Alterations in human septin-9 (SEPT9) levels have been linked to multiple cancers, whereas mutations in SEPT9 cause the episodic neuropathy, hereditary neuralgic amyotrophy (HNA). Despite its important function in human health, the in vivo role of SEPT9 is unknown.
Methodology/Principal Findings
Here we utilize zebrafish to study the role of SEPT9 in early development. We show that zebrafish possess two genes, sept9a and sept9b that, like humans, express multiple transcripts. Knockdown or overexpression of sept9a transcripts results in specific developmental alterations including circulation defects and aberrant epidermal development.
Conclusions/Significance
Our work demonstrates that sept9 plays an important role in zebrafish development, and establishes zebrafish as a valuable model organism for the study of SEPT9.
Introduction
Septin-9 (SEPT9, MSF) is a member of the septin gene family, a conserved family of filament forming GTPases. To date, at least 14 different septin genes have been identified in humans which, in addition to cytokinesis, also play roles in vesicle trafficking, microtubule and actin function, exocytosis, establishment of cell polarity and cell motility [1], [2]. All vertebrate septins have a highly conserved polybasic domain (PBD) followed by a GTP binding domain (GBD) homologous to those of the ras-related small GTPase family of proteins. Outside of the PBD and GBD, members of the septin family vary greatly in the length and make up of both the N- and C-termini. SEPT9 is one of three septin proteins possessing an extended N-terminus containing a proline-rich region. However, the function of this region is unknown.
The human SEPT9 gene is complex, producing at least seven mRNA transcripts encoding six distinct polypeptides through alternative splicing [3]. SEPT9 was initially identified as a fusion partner of the mixed-lineage leukemia (MLL) gene in acute myeloid leukemia patients [4]. Altered expression of SEPT9 has also been implicated in the pathogenesis of a number of cancers, with evidence for both genetic gain and allelic loss [5], [6], [7], [8]. Point mutations and intragenic duplications in SEPT9 have also been linked to hereditary neuralgic amyotrophy (HNA), an autosomal dominant episodic neuropathy primarily affecting the brachial plexus [9], [10].
In cultured cells, inhibition of SEPT9 isoforms through antibody microinjection or siRNA transfection results in cytokinesis defects, including binucleated cells, abnormal daughter cells, cells remaining attached through a short midbody bridge, and cells containing condensed DNA suggestive of apoptosis [11], [12]. Overexpression of SEPT9 isoforms in cell culture also leads to an increase in binucleated cells, an accumulation of cells in G2/M phase and an increase in the percentage of aneuploid cells leading to suppression of cell growth [13], [14]. However, overexpression of SEPT9 isoforms has also been shown to increase cell motility, and alter cellular polarity and morphology [14], [15].
No mouse knockout has been described, and so the in vivo role of SEPT9 remains unknown. Moreover, the transcriptional complexity of the SEPT9 locus will make it very difficult to study the function of specific isoforms in the mouse. In this study we use the zebrafish system to investigate the in vivo role of specific SEPT9 isoforms in early development. Zebrafish provide an excellent model for the study of genes with multiple transcripts since animals grow quickly, and can be easily genetically manipulated through the use of transgenic overexpression constructs and specific transcript inhibition using morpholino oliogonucleotides (MOs). Zebrafish possess two SEPT9 gene orthologues found on two different chromosomes, sept9a and sept9b. We found that, similar to humans, these genes express multiple mRNA transcripts that are expressed throughout development in a variety of tissues. Inhibition of all Sept9a isoforms or just the largest Sept9a isoform, sept9a_tv1, led to multiple defects in embryonic development demonstrating an essential embryonic role for this isoform. In particular, we observed an increase in apoptosis in the epidermis of all morphants and alterations in blood circulation. Overexpression of sept9a_tv1 led to similar developmental defects. Our results demonstrate the importance of sept9 during embryonic development.
Materials and Methods
Zebrafish embryos and ethics
Zebrafish were maintained, staged and injected according to standard procedures [16]. All experiments were approved by and conducted in accordance with the guidelines established by the Institutional Animal Care and Use Committee at the University of Washington, IACUC approval number: 2387-02.
Identification and cloning of sept9 isoforms
BLAST searches using human SEPT9 were used to identify zebrafish sept9 transcripts. PCR primers were used to amplify sept9a isoforms from 24 hpf embryos. Primer sequences are available upon request.
RNA isolation and RT-PCR
RNA was isolated using the RNeasy kit (Qiagen). cDNA was prepared using Superscript polymerase (Invitrogen) using 1 ug RNA. sept9 isoforms and ef1α were amplified using transcript specific primers.
Whole-mount in situ hybridization
Embryos were processed as described [16]. The sept9a_tv1 coding region was used to generate digoxigenin-labeled probes (Roche).
Morpholino and mRNA injections
Morpholinos targeted to the splice acceptor sites of sept9a_tv1 exons 2 and 5 and sept9a_tv1 mRNA were injected into zebrafish embryos. The sequences of the morpholinos are: MO2 (5′-TGCGATGCCTGTCAGCACAGAAGAC-3′), MO5 (5′-CTCTGACCTGCACACATGAAGAACA-3′), MO2 mismatch (5′-TCCGATCCCTGTGAGCACACAACAC-3′), MO5 mismatch (5′-CTGTGAGCTGCAAACATCAACAACA-3′). Full-length sept9a_tv1 was subcloned into the pXLT vector for in vitro transcription. Messenger mRNA was synthesized using the mMessage Machine Kit (Ambion).
Acridine orange (AO) staining
For AO staining, embryos were processed as described [17].
Results
Characterization of zebrafish sept9 genes
Through a combination of genetic sequence analysis and BLAST searches using known human SEPT9 transcripts, we identified multiple mRNA transcripts produced from two different zebrafish sept9 genes, sept9a and sept9b. sept9a is located on chromosome 3, whereas sept9b is found on chromosome 6. sept9a produces transcripts homologous to the longest human SEPT9 isoforms 1, 2, and 3, the shortest human variant SEPT9_v7 (NCBI NM_001113496; named SEPT9_v5 in earlier literature [7]) and a unique transcript not identified in other vertebrates which we have denoted sept9a_tvα. Similar to humans, through the use of alternate 5′ exons, sept9a _tv1, 2, 3 and α, generate predicted protein products with unique N-termini of 32, 18, 7, and 10 amino acids respectively (Fig. 1, and Fig. 2). sept9a_tv7 encodes a truncated version of the longer transcripts. sept9b appears to express two human SEPT9_v7 homologues with alternate 5′ UTRs, sept9b_tv1 and sept9b_tv2 (Fig. 1). The predicted protein sequences of zebrafish sept9a_tv1, 2, and 3 are 73–74% similar and 61–62% identical to their human homologues, respectively (Fig. 2). sept9a_tv7 and sept9b_tv1 and _tv2 primarily encode the GTP binding domain found in all transcripts, and are highly conserved. These transcripts are 92% similar and 87% identical to each other at the amino acid level and 87–81% similar, 78–80% identical to the human sequence. We did not identify transcripts homologous to human SEPT9 transcript variants 4, 5, and 6 (NCBI NM_001113495, NM_001113492, and NM_001113494; SEPT9_v5 and v6 known as v4* and v4 in previous literature [7], [15], [18]). However, the start codon in human SEPT9_v5 and v6 is not conserved from mammals to zebrafish (Fig. 2) and to date, only a single EST of human SEPT9_v4 has been identified in a teratocarcinoma cell line, suggesting that these transcripts may not be expressed in zebrafish. It is possible that further sept9 transcripts are expressed in zebrafish yet were not identified in this analysis.
Figure 1. SEPT9 transcripts are conserved between zebrafish and mammals.
Zebrafish possess two sept9 genes, sept9a and sept9b, that encode multiple mRNA transcripts homologous to mammalian transcripts. Zebrafish also express a transcript not currently found in mammals, sept9a_tvα. Sites of morpholino splice blockers are noted.
Figure 2. SEPT9 amino acid sequence is conserved between zebrafish and mammals.
Amino-acid alignments of zebrafish Sept9a and Sept9b putative protein products show a high degree of conservation. An asterisk notes the starting methionine of human SEPT9_i5/6, which is not conserved in zebrafish. Arrowheads mark the starting methionines for human Sept9_v7, and zebrafish isoforms Sept9a_tv7 and Sept9b (both transcripts).
Developmental expression of zebrafish sept9 genes
To examine where sept9 message is expressed in the developing zebrafish embryo, we performed whole mount in situ hybridization using a probe to sept9a_tv1. This probe is expected to recognize all sept9a transcripts, and possibly those of sept9b. Probes designed to the individual sept9a transcripts were not synthesized because the unique regions of the longer sept9a isoforms are not large enough to probe individually. Early maternal expression of sept9 was ubiquitous (Fig. 3A) and remained so through blastula stages (Fig. 3B). During gastrulation, sept9 became more restricted to the axial mesoderm and endoderm (Fig. 3C, D). sept9 was expressed primarily in the floor plate and ventral mesoderm during segmentation (Fig. 3E–H). At 24 hours post fertilization, sept9 was expressed in the intermediate cell mass, epidermis, branchial arches, and pectoral fin bud (Fig. 3I–K).
Figure 3. Expression of sept9 genes during zebrafish development.
Detection of sept9 mRNA was carried out by whole-mount in situ hybridization using a probe targeted to all sept9 isoforms on staged embryos from 256 cells to 24 hpf. Images in A–C are lateral views, animal pole to top; D and E are dorsal views, anterior to top; F and H are dorsal posterior views; G, I and J are lateral views, K is a dorsal view, anterior to left. A–C: sept9 transcripts are ubiquitously expressed at early developmental states. D: At bud stage, sept9 is expressed in endoderm and axis. E–H: sept9 is expressed in the floor plate, ventral mesoderm and tail bud during segmentation. I–K: At 24 hpf, sept9 is expressed throughout the epidermis, branchial arches, pectoral fin, and in the intermediate cell mass. L: Transcript specific primers were used to detect sept9a and sept9b transcripts in various stages of development by RT-PCR. sept9a_tv 2, 3, and α are expressed maternally. Amplification of eIFα and total RNA without addition of reverse transcriptase were used as controls. a, axis; ep, epidermis; fp, floorplate; icm, intermediate cell mass; tb, tail bud; vm, ventral mesoderm.
While we were unable to analyze the spatial expression of specific sept9 isoforms we could, using RT-PCR, determine the temporal expression pattern of the various sept9 transcripts. Zebrafish embryos at different developmental stages were collected for cDNA preparation and subjected to RT-PCR using transcript specific primers (Fig. 3L). sept9a_tv2, 3, and α are expressed at the two cell stage consistent with the maternal sept9a expression observed in the in situs. Expression of sept9a_tv1, 7 and α, and sept9b_tv1 commence at high stage, consistent with zygotic expression, which begins at this time. All five transcripts of sept9a and both sept9b transcripts stabilize expression though the segmentation and pharyngula stages. The longest transcript, sept9a_tv1, has two phases of expression; one during the late blastula and early gastrula stages, and a second beginning during early somitogenesis.
Effect of sept9a inhibition on zebrafish development
Because a majority of sept9 transcripts, including the longest sept9a_tv1, derived from the sept9a locus, we decided to focus further studies on this set of isoforms. Therefore, we targeted all sept9a transcripts, or sept9a_tv1 only, for depletion using antisense morpholinos directed to the splice acceptor site of the fifth (MO5) or second (MO2) exons of sept9a_tv1, respectively (Fig. 1). Embryos were injected at the 1-cell stage with 1.25, 2.5 or 5 ng of morpholino. The observed phenotypes were dosage dependent and were divided into three categories which we termed class I, class II and class III based on morphology (Fig. 4J). At 48 hpf, embryos that looked similar to wild-type but had epidermal defects and minor tail perturbations were categorized as class I, those that had a curved or shortened body axis/tail in addition to the defects in class I were categorized as class II and those that had a severely shortened tail/body axis were classified as class III. Both MO5 and MO2 produced classes I and II (Fig. 4A, B, D, E). However, the severe class III phenotype (data not shown) was not observed in MO2 embryos, even at 5 ng of morpholino.
Figure 4. Charaterization of sept9a morphant and overexpression embryos.
Embryos were injected at the one-cell stage with morpholinos targeted to all sept9a transcripts (MO5), sept9a_tv1 only (MO2), mismatch controls (MO5MM, MO2MM), or sept9a_tv1 mRNA with and without MO2. Morphants shown were injected with 2.5 ng morpholino. At 48 hpf, the phenotypes were assessed by morphological criteria, according to severity. A,D: Class I morphants had defects in epidermal integrity and yolk extension and minor curvature of the tail. B,E: Class II morphants had a curved body axis in addition to the defects observed in class I. Class III morphants had a severely shortened body axis (data not shown). Arrows indicate yolk extension defects. All classes exhibited defects in blood circulation. G: Coinjection of 1 pg of sept9a_tv1 mRNA with 5 ng of MO2 partially rescued the observed phenotypes. H,I: Embryos injected with as little as 4 pg of sept9a_tv1 mRNA often had phenotypes similar to those of sept9a morphants including epidermal aggregates (arrow), blood pooling, and tail edema (bracket). (OE) indicates over expression. C,F: Control mismatch morpholinos did not present a phenotype. J: Graphical representation of MO classes at various concentrations. The number of embryos tested in each experiments is indicated by (n) on top of each column. K: sept9a splice morpholinos inhibit sept9a transcript splicing. RT-PCR analysis was performed on 24 hpf wild-type embryos, embryos injected with 2.5 ng MO5 or MO2 (pooled classes I and II), or 5 bp mismatch controls. MO5 embryos show a complete loss of sept9a_tv1 and sept9a_tv7. The presence of a low level of sept9a exons 3–5 transcripts in MO5-injected embryos may be due to maternal mRNA. MO2 embryos show a decrease in sept9a_tv1 compared to wild-type while the other transcripts are not affected.
Circulating blood cells could be observed in class I embryos, however, cells were often observed pooling in the intermediate cell mass (ICM) and tail region. Classes II and III showed an absence of blood circulation, and often lacked the presence of mature hemoglobinized erythrocytes. All classes exhibited yolk extension as well as epidermal defects, most commonly seen in the tail and yolk regions. Epidermal aggregates and edema were frequently noted, often at the tip of the tail and in the ICM. Cardiac edema was regularly observed in embryos from all classes and worsened as development proceeded; class II and III embryos did not survive past 7 days.
The phenotypes resulting from inhibition of septa_tv1 or all sept9a transcripts were not regularly observed in embryos injected with mismatch controls to either morpholino (MO2MM and MO5MM; Fig. 4C, F, J). To determine if sept9a transcript levels were altered, RT-PCR was performed on 24 hpf MO5 and MO2 embryos injected with 2.5 ng morpholino (Fig. 4K). Levels of sept9a_tv1 and tv7 were undetectable in MO5 embryos, and sept9a_tv1 but not _tv7 was decreased with MO2 when compared to wild-type. MO2 also did not affect an amplicon from exons 3–5, whereas MO5 greatly reduced the level of this product. Since these exons are shared with sept9a_tv2, 3, and α (Fig. 1), the residual product may be due to perduring maternal transcripts. Morpholinos designed to sept9a_tv1 exon 3 (inhibiting transcript variants 1–3 and α) and exon 4 (inhibiting all transcripts) acceptor splice sites (Fig. 1) also produced the same classes of morphants observed in MO5 embryos (data not shown).
Co-injection of a low concentration (1 pg) of sept9a_tv1 mRNA with MO2 showed a partial rescue of the morphant phenotypes providing further evidence that at least classes I and II are a result of sept9a transcript inhibition (Fig. 4G). The phenotype of MO5 injected embryos could not be rescued by co-injection of sept9a_tv1 mRNA (data not shown). It is possible that sept9a transcripts have overlapping functions, and that over-expression of only sept9a_tv1 cannot compensate for the loss of multiple transcripts. This also complicates interpretation of the class III phenotype, as it difficult to distinguish between a phenotype caused by morpholino artifact and one caused by knocking-down additional sept9a isoforms that can not be rescued with sept9a_tv1. However, the observation that four different sept9a MOs cause similar defects whereas mismatch morpholinos result in no defect and that the morphant phenotype is rescued by co-injection of sept9a_tv1 mRNA, demonstrates that the observed morphant phenotypes are due to specific loss-of-function of sept9 and not toxicity.
Effect of sept9a_tv1 overexpression on zebrafish development
Recent studies in cultured cells have shown that human SEPT9 appears to be highly regulated [12], [13], [14]. While attempting rescue of sept9a morphant embryos, we found that increased levels of sept9a_tv1 mRNA led to a number of embryonic developmental defects including alterations in convergence and extension, dorsalization, and cyclopia. However, the phenotypes did not clearly group into classes like the sept9a morphant embryos. Interestingly, many of the phenotypes were similar to those observed in sept9a morphants including cardiac and tail fin edema, a curved tail and/or body axis, a loss of circulating blood cells with concentrated pools of cells in the tail region and ICM, and epidermal defects including regions of aggregated cells (Fig. 4H, I). Thus, some phenotypes were observed with both gain and loss of sept9a_tv1 function, whereas other phenotypes were only found in gain-of-function experiments.
Knock-down and overexpression of sept9a cause an increase in cell death
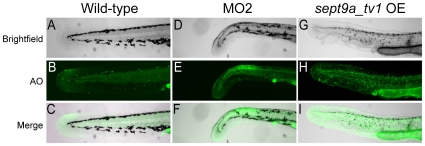
Alterations in human SEPT9 have been shown to cause defects in cytokinesis, leading to changes in cell morphology and decreases in cellular growth [12], [14]. To determine if the defects observed in the tails of sept9a_tv1 morphant and overexpression (OE) embryos included an increase in apoptotic cells, we used acridine orange (AO) to mark cell death. AO-positive cells were rarely observed in wild-type embryos, yet both sept9a MO2 and sept9a_tv1 OE embryos showed an increase in apoptotic cells in the tail indicating cell death (Fig. 5). These data suggest that both loss- and gain-of-function of sept9a in zebrafish lead to an increase in cell death possibly through defects in cell division.
Figure 5. Knockdown and overexpression (OE) of sept9a_tv1 results in an increase in apoptotic cells in the tail.
Embryos at the one-cell stage were injected with 2.5 ng of MO2 or 4 pg of sept9a_tv1 mRNA and analyzed for acridine orange (AO) staining at 24 hpf. A–C: The tail fin of wild-type embryos is negative for AO indicating few apoptotic cells. D–I: Both class II MO2 and sept9a_v1 OE embryos show an increase in AO staining indicating increased cell death.
Discussion
In this study we have shown that, like humans, zebrafish express multiple sept9 transcripts. These transcripts are expressed throughout development in different tissues types including the ventral mesoderm and axis at early developmental stages, and the epidermis at later stages. We have demonstrated that inhibition and overexpression of sept9a transcripts in zebrafish embryos lead to a myriad of phenotypes including edema, loss of blood circulation, tail fin malformations, loss of epidermal integrity and increased cell death. Additionally, we have provided evidence that multiple sept9a MOs targeted to different splice sites yield similar phenotypes, and that overexpression of sept9a_tv1 causes developmental defects similar to those observed with the MOs. Thus, too much or too little sept9a function is deleterious for many embryonic cells, indicating that cells need to carefully regulate sept9a levels. The correct levels of sept9a, therefore, are needed to maintain tissue integrity and to allow normal cell division.
The fact that zebrafish posses two sept9 orthologues (sept9a and sept9b) is not unusual, given the proposed genomic duplication event that occurred in teleost fish [19]. The two orthologues appear to have evolved such that only sept9a expresses longer isoforms possessing a proline-rich region, while both genes express shorter isoforms primarily consisting of a GTP-binding domain. The predicted polypeptides are highly similar to mammalian SEPT9 proteins, suggesting possible overlapping functions. While zebrafish do not appear to express homologues to human SEPT9 transcripts 5 and 6, they do express two additional variant 7 transcripts from sept9b. It is possible that these transcripts are regulated in a manner similar to SEPT9_v5 and v6 [18]. Further studies are required to determine if sept9a and sept9b have overlapping functions in zebrafish.
RT-PCR analysis of human tissues has shown that a majority of SEPT9 transcripts are expressed in almost every tissue type tested [6], [7] and cultured cell lines express different combinations of SEPT9 proteins depending on the line [11], [12], [14], [20]. However, whether different SEPT9 polypeptides interact with one another and the individual function of each transcript remains to be determined. We found that zebrafish express multiple sept9 transcripts from two different genes, and confirmed the role of sept9 during zebrafish development. Moreover, we observed the same spectrum of phenotypes when we eliminated the largest sept9a isoform, sept9a_tv1, as when we eliminated all sept9a isoforms, providing the first evidence that the smaller isoforms cannot compensate for a lack of the largest isoform.
Recently, a number of sept9a transcripts were identified in an analysis of hematopoietic genes isolated from zebrafish kidney marrow [21] and expression of sept9b was shown to be increased in embryos overexpressing etsrp, a transcription factor required for vasculogenesis and primitive myelopoiesis in zebrafish [22]. This studies support the hypothesis that sept9 genes play a role in hematopoiesis. However, the pericardial edema, loss of blood circulation and tail malformations observed in both sept9a morphant and OE embryos are also consistent with defects in osmoregulation observed when epidermal barrier function is lost [23] or if fish are exposed to toxins that impair homeostasis of the skin or kidney [24], [25]. Zebrafish will be a good model system for future studies examining the roles of various sept9 isoforms in developmental processes such as hematopoiesis and epidermal development.
Acknowledgments
M. L. would like to thank Dr. Phillip Chance for his support and guidance, and Dr. Dan Doherty for intellectual contributions and thoughtful discussions.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by NIH grant (GM079203) to D.K. and NIH grant (NS38181) to Phillip Chance and an NRSA fellowship (F32HD053189) and University of the Pacific Start-up funds to D.C.W. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hall PA, Russell SE. The pathobiology of the septin gene family. J Pathol. 2004;204:489–505. doi: 10.1002/path.1654. [DOI] [PubMed] [Google Scholar]
- 2.Kinoshita M. The septins. Genome Biol. 2003;4:236. doi: 10.1186/gb-2003-4-11-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McIlhatton MA, Burrows JF, Donaghy PG, Chanduloy S, Johnston PG, et al. Genomic organization, complex splicing pattern and expression of a human septin gene on chromosome 17q25.3. Oncogene. 2001;20:5930–5939. doi: 10.1038/sj.onc.1204752. [DOI] [PubMed] [Google Scholar]
- 4.Osaka M, Rowley JD, Zeleznik-Le NJ. MSF (MLL septin-like fusion), a fusion partner gene of MLL, in a therapy-related acute myeloid leukemia with a t(11;17)(q23;q25). Proc Natl Acad Sci U S A. 1999;96:6428–6433. doi: 10.1073/pnas.96.11.6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell SE, McIlhatton MA, Burrows JF, Donaghy PG, Chanduloy S, et al. Isolation and mapping of a human septin gene to a region on chromosome 17q, commonly deleted in sporadic epithelial ovarian tumors. Cancer Res. 2000;60:4729–4734. [PubMed] [Google Scholar]
- 6.Burrows JF, Chanduloy S, McIlhatton MA, Nagar H, Yeates K, et al. Altered expression of the septin gene, SEPT9, in ovarian neoplasia. J Pathol. 2003;201:581–588. doi: 10.1002/path.1484. [DOI] [PubMed] [Google Scholar]
- 7.Scott M, Hyland PL, McGregor G, Hillan KJ, Russell SE, et al. Multimodality expression profiling shows SEPT9 to be overexpressed in a wide range of human tumours. Oncogene. 2005;24:4688–4700. doi: 10.1038/sj.onc.1208574. [DOI] [PubMed] [Google Scholar]
- 8.Amir S, Wang R, Matzkin H, Simons JW, Mabjeesh NJ. MSF-A interacts with hypoxia-inducible factor-1alpha and augments hypoxia-inducible factor transcriptional activation to affect tumorigenicity and angiogenesis. Cancer Res. 2006;66:856–866. doi: 10.1158/0008-5472.CAN-05-2738. [DOI] [PubMed] [Google Scholar]
- 9.Kuhlenbaumer G, Hannibal MC, Nelis E, Schirmacher A, Verpoorten N, et al. Mutations in SEPT9 cause hereditary neuralgic amyotrophy. Nat Genet. 2005;37:1044–1046. doi: 10.1038/ng1649. [DOI] [PubMed] [Google Scholar]
- 10.Landsverk ML, Ruzzo EK, Mefford HC, Buysse K, Buchan JG, et al. Duplication within the SEPT9 gene associated with a founder effect in North American families with hereditary neuralgic amyotrophy. Hum Mol Genet. 2009;18:1200–1208. doi: 10.1093/hmg/ddp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata K, Kawajiri A, Matsui S, Takagishi M, Shiromizu T, et al. Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J Biol Chem. 2003;278:18538–18543. doi: 10.1074/jbc.M205246200. [DOI] [PubMed] [Google Scholar]
- 12.Surka MC, Tsang CW, Trimble WS. The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol Biol Cell. 2002;13:3532–3545. doi: 10.1091/mbc.E02-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson C, Church SW, Nagar HA, Price J, Hall PA, et al. Properties of SEPT9 isoforms and the requirement for GTP binding. J Pathol. 2004;203:519–527. doi: 10.1002/path.1551. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez ME, Peterson EA, Privette LM, Loffreda-Wren JL, Kalikin LM, et al. High SEPT9_v1 expression in human breast cancer cells is associated with oncogenic phenotypes. Cancer Res. 2007;67:8554–8564. doi: 10.1158/0008-5472.CAN-07-1474. [DOI] [PubMed] [Google Scholar]
- 15.Chacko AD, Hyland PL, McDade SS, Hamilton PW, Russell SH, et al. SEPT9_v4 expression induces morphological change, increased motility and disturbed polarity. J Pathol. 2005;206:458–465. doi: 10.1002/path.1794. [DOI] [PubMed] [Google Scholar]
- 16.Westerfield M. Eugene: University of Oregon Press; 1993. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio). [Google Scholar]
- 17.Webb AE, Driever W, Kimelman D. psoriasis regulates epidermal development in zebrafish. Dev Dyn. 2008;237:1153–1164. doi: 10.1002/dvdy.21509. [DOI] [PubMed] [Google Scholar]
- 18.McDade SS, Hall PA, Russell SE. Translational control of SEPT9 isoforms is perturbed in disease. Hum Mol Genet. 2007;16:742–752. doi: 10.1093/hmg/ddm003. [DOI] [PubMed] [Google Scholar]
- 19.Meyer A, Schartl M. Gene and genome duplications in vertebrates: the one-to-four (-to-eight in fish) rule and the evolution of novel gene functions. Curr Opin Cell Biol. 1999;11:699–704. doi: 10.1016/s0955-0674(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 20.Sudo K, Ito H, Iwamoto I, Morishita R, Asano T, et al. SEPT9 sequence alternations causing hereditary neuralgic amyotrophy are associated with altered interactions with SEPT4/SEPT11 and resistance to Rho/Rhotekin-signaling. Hum Mutat. 2007;28:1005–1013. doi: 10.1002/humu.20554. [DOI] [PubMed] [Google Scholar]
- 21.Song HD, Sun XJ, Deng M, Zhang GW, Zhou Y, et al. Hematopoietic gene expression profile in zebrafish kidney marrow. Proc Natl Acad Sci U S A. 2004;101:16240–16245. doi: 10.1073/pnas.0407241101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez GA, Veldman MB, Zhao Y, Burgess S, Lin S. Discovery and characterization of novel vascular and hematopoietic genes downstream of etsrp in zebrafish. PLoS One. 2009;4:e4994. doi: 10.1371/journal.pone.0004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiener TK, Selptsova-Friedrich I, Hunziker W. Tjp3/zo-3 is critical for epidermal barrier function in zebrafish embryos. Dev Biol. 2008;316:36–49. doi: 10.1016/j.ydbio.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 24.Hentschel DM, Park KM, Cilenti L, Zervos AS, Drummond I, et al. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol. 2005;288:F923–929. doi: 10.1152/ajprenal.00386.2004. [DOI] [PubMed] [Google Scholar]
- 25.Hill AJ, Bello SM, Prasch AL, Peterson RE, Heideman W. Water permeability and TCDD-induced edema in zebrafish early-life stages. Toxicol Sci. 2004;78:78–87. doi: 10.1093/toxsci/kfh056. [DOI] [PubMed] [Google Scholar]