Abstract
Many disease states are associated with regional or systemic hypoxia. The study of healthy individuals exposed to high-altitude hypoxia offers a way to explore hypoxic adaptation without the confounding effects of disease and therapeutic interventions. Using 31P magnetic resonance spectroscopy and imaging, we investigated skeletal muscle energetics and morphology after exposure to hypobaric hypoxia in seven altitude-naïve subjects (trekkers) and seven experienced climbers. The trekkers ascended to 5300 m while the climbers ascended above 7950 m. Before the study, climbers had better mitochondrial function (evidenced by shorter phosphocreatine recovery halftime) than trekkers: 16±1 vs. 22±2 s (mean ± SE, p<0.01). Climbers had higher resting [Pi] than trekkers before the expedition and resting [Pi] was raised across both groups on their return (PRE: 2.6±0.2 vs. POST: 3.0±0.2 mM, p<0.05). There was significant muscle atrophy post-CXE (PRE: 4.7±0.2 vs. POST: 4.5±0.2 cm2, p<0.05), yet exercising metabolites were unchanged. These results suggest that, in response to high altitude hypoxia, skeletal muscle function is maintained in humans, despite significant atrophy.
Introduction
The metabolic response to impaired regional (e.g. vascular disease) or systemic (e.g. cardiopulmonary disease) oxygen delivery is hard to determine, given the confounding effects of the disease state itself and of therapeutic interventions. The study of healthy individuals exposed to altitude-related (hypobaric) hypoxia offers an approach to this problem. As yet, the pattern of response remains unclear: mitochondrial density, far from increasing [1], [2], may in fact fall [3] (albeit in the context of muscle atrophy [4]), while enzymes used in anaerobic glycolysis may be upregulated in the muscle of high altitude natives and acclimatised lowlanders [5], [6] (a suggestion supported by in vitro studies [7]). Hypoxic cachexia, although well described, remains poorly understood.
There are several hypoxia-tolerance strategies known in other mammals. For example, sea turtles reduce cellular oxygen consumption during hypoxia by prioritizing certain ATP-consuming processes at the expense of others [8]. Whether this might also be a feature of human physiology is not known. In addition, the oxygen-cost of ATP rephosphorylation might be reduced, although the notion that oxygen efficiency might be modulated in humans in response to hypoxia remains deeply controversial [9], [10].
Phosphorus magnetic resonance spectroscopy (31P-MRS) and magnetic resonance imaging (MRI) allow the non-invasive assessment of mitochondrial function, muscle cross-sectional area and phosphate metabolism in humans. We thus applied these techniques to the prospective study of two groups of healthy individuals exposed to sustained hypobaric hypoxia.
Methods
Ethics statement
The protocol was approved by the University College (University of London) Ethical Committee. Written informed consent was obtained from all participants.
Subjects and experimental outline
We studied fourteen healthy men aged 24–48 years from the Caudwell Xtreme Everest expedition (CXE) [11]. Of these, seven were altitude-naïve (‘trekkers’) and seven were experienced climbers who had previously climbed above 6800 m without incident (‘climbers’). Subjects underwent health screening to ensure that they were fit both to complete an ascent to high altitude and to take part in the planned programme of research activity. Prior to departure, all subjects attended the Oxford Centre for Clinical Magnetic Resonance Research (OCMR). On the morning after an overnight fast, their heights and weights were recorded, and body mass index (BMI) calculated.
Each subject performed a series of plantar flexion exercises in the bore of a Siemens Trio 3T clinical magnetic resonance system, with a dual-tuned 31P and 1H surface coil placed under the widest part of the right gastrocnemius. The exercise protocol was: 5 minutes rest, 5 minutes very light exercise (warm-up), 7 minutes recovery, 5 minutes at 4 W (EX1), 7 minutes recovery (REC1), 5 minutes at 5 W (EX2), 5 minutes recovery (REC2). The exercise work rates were established in pilot experiments, and were optimized to substantially deplete muscle phosphocreatine while remaining sustainable throughout the exercise periods. Muscle cross-sectional area, phosphorus metabolites, phosphocreatine (PCr) recovery halftime and intracellular pH were calculated as described below.
All the subjects ascended from Oxford to Everest Base Camp over approximately 14 days. The trekkers then descended to sea-level via Kathmandu (1300 m) over 7 days and were retested within 48 hours. All of the climbers ascended to at least the South Col (7950 m), and four successfully summitted (8848 m). The climbers then descended to sea-level over 10–17 days and were retested approximately a week after leaving Kathmandu. Twelve subjects (7 trekkers and 5 climbers) returned to OCMR following CXE.
Nuclear Magnetic Resonance Protocols
A series of gradient-echo ‘scout’ images were acquired in the transverse, sagittal and coronal planes to ensure correct positioning of the leg, and to allow for localized shimming of the region of interest (ROI). Image parameters were as follows: FOV 250×250 mm, matrix size 256×128, 3 slices per orientation, slice thickness 8 mm, TR/TE 15/5 ms, excitation flip angle 40° and bandwidth 180 Hz/Px. This was immediately followed by localized shimming of the ROI using the phasemap algorithm as implemented on the 3T system. This increased the local magnet homogeneity and reduced the linewidths in the acquired spectra.
Prior to the acquisition of the 31P spectra during the leg exercise protocol, three baseline scans were acquired to allow calculation of correction factors for partial saturation, caused by both the short repetition time (TR) used in the spectral acquisition, and nuclear Overhauser enhancement (NOE). The first baseline scan was acquired with a long TR to ensure complete relaxation of all resonances. The scan parameters were as follows: TR 30 s, TE 0.35 ms, bandwidth 2000 Hz, 8 averages, 512 acquired data points, excitation flip angle 90° and no NOE pulses. The second baseline scan was run with TR 500 ms, TE 0.35 ms, bandwidth 2000 Hz, 60 averages, 512 acquired data points, excitation flip angle 25° and 10 rectangular NOE pulses with pulse duration of 10 ms, inter-pulse delay of 10 ms and excitation flip angle of 180°. The third baseline scan was run with identical parameters to the second with the exception of no excitation flip angle on the NOE pulses. The ratio of the resonance areas in the first and third scans were used to calculate correction factors for partial saturation, and in the same way the second and third baseline scans were used to calculate a NOE correction factor.
In the main experiment, a series of 512 individual 31P spectra were acquired throughout the exercise protocol described above with a temporal resolution of 5 s. The acquisition parameters were: TR 500 ms, TE 0.35 ms, bandwidth 2000 Hz, 10 averages, 512 acquired data points, excitation flip angle 25° and 10 rectangular NOE pulses with pulse duration of 10 ms, inter-pulse delay of 10 ms and excitation flip angle of 180°.
Data processing
Spectra were processed using jMRUI version 2.2 [12] and quantified using a non-linear least squares algorithm (AMARES [13]). Resting ATP and total creatine concentrations were assumed to be 8.2 mM L−1 and 42 mM L−1 respectively: these commonly-used concentrations are based on extensive published values, and are reliable in healthy humans [14]. For muscle metabolite concentrations during exercise, reported values are the mean metabolite concentrations during the last minute of exercise bouts 2 and 3 (i.e. not including data from the ‘warm-up’ bout).
Calculations
The myocellular free adenosine diphosphate (ADP) concentration was calculated making the standard assumption that that the creatine kinase reaction was at equilibrium, and using an equilibrium constant allowing for changes in pH [15].
The free energy available from ATP hydrolysis (ΔG'ATP, in kJ M−1) was calculated using:
where ΔG0 is the standard free energy of ATP hydrolysis (−32.8 kJ M−1 [16]), R is the gas constant (8.31 J M−1 K−1) and T is temperature (310 K).
In the absence of large changes in pH, the halftime of PCr recovery after moderate exercise (PCrt1/2) was taken as an inverse index of mitochondrial function. PCrt1/2 was determined by fitting a monexponential equation, expressing PCr concentration as a function of time (t), of the form PCr (t) = 1 – exp (-k t), to data that had been normalized to resting values. Microsoft Solver was then used to establish the value of the rate-constant (k) that resulted in the smallest sum of the squared differences between actual and predicted PCr values, from which PCrt1/2 = ln (2)/k [17]. The values reported are the means of the recovery halftimes after both exercise bouts.
The chemical shift of the phosphate (Pi) peak relative to phosphocreatine (PCr) (σ, in parts per million) was used to determine intracellular pH, according to the equation:
Muscle cross-sectional area was measured from 1H scout images (acquired as detailed above) using a freely available stereology software package (www.easymeasure.co.uk). The measured area comprised both the soleus and gastrocnemius muscles, as these are the active muscles during plantar flexion exercise [18].
Statistical analysis
Statistical testing was carried out using SPSS 16.0 for Mac (SPSS Inc., Chicago, Illinois, USA). Shapiro-Wilk and Kolmogorov-Smirnov tests were used to establish whether data were normally distributed; no variable failed these tests of normality. Unpaired t-tests were used to test for differences between trekkers and climbers before the expedition. To quantify the significance of the effects of altitude exposure while controlling for potential differences between trekkers and climbers, a mixed analysis-of-variance was used; whether the subject was a trekker or a climber was coded as a between-subjects factor. There were, however, no significant between-subjects effects, thus the pre and post data are reported combined for brevity. Alpha was set at 0.05. All data are reported as mean ± S. E. M.
Results
Contrasts between trekkers and climbers at baseline
There was a significant difference in age between trekkers and climbers, with the climbers being 5 years older. In other respects the two groups were similar (Table 1).
Table 1. Subjects' descriptive data.
| Trekkers (n = 7) | Climbers (n = 7) | All (n = 14) | |
| Age (years) | 31±2 | 38±3 * | 35±2 |
| Weight (kg) | 74±2 | 83±6 | 78±3 |
| Height (m) | 1.80±0.02 | 1.80±0.01 | 1.80±0.01 |
| BMI (kg m−2) | 23±1 | 26±2 | 24±1 |
| Calf muscle CSA (cm2) | 4.5±0.3 | 4.8±0.2 | 4.6±0.2 |
All numbers are means ± S.E.M.
*different from trekkers at p<0.05.
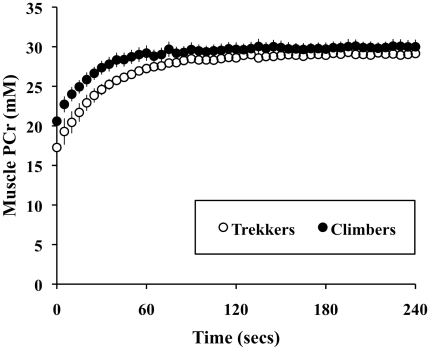
At rest, the climbers had significantly higher concentrations of muscle cytosolic inorganic phosphate than the trekkers (climbers: 2.9±0.2 s vs. trekkers: 2.3±0.2, n = 14, p<0.05) (Table 2). Other resting phosphorus metabolites and resting muscle pH were similar between the groups. During and after recovery from exercise, there were no differences in phosphorus metabolites or pH (Table 2). However, there was an unexpected difference in PCr recovery halftimes (PCrt1/2) between trekkers and climbers before the expedition, with the trekkers having longer halftimes (trekkers: 22±2 s vs. climbers: 16±1 s, n = 14, p<0.01) (Figure 1).
Table 2. High-energy phosphates and pH in human skeletal muscle: trekkers vs. climbers before hypoxic exposure.
| Trekkers (n = 7) | Climbers (n = 7) | ||
| PCr (mM) | Resting | 30.7±0.8 | 31.3±0.7 |
| Exercising | 18.0±2.6 | 18.8±1.6 | |
| After recovery | 32.9±0.5 | 29.4±0.8 | |
| PCrt1/2 (s) | During recovery | 22.2±1.6 | 16.1±1.1 ** |
| Pi (mM) | Resting | 2.3±0.2 | 2.9±0.2 * |
| Exercising | 11.6±1.8 | 12.9±2.2 | |
| After recovery | 1.3±0.2 | 1.5±0.1 | |
| ADP (µM) | Resting | 28±3 | 26±2 |
| Exercising | 53±5 | 54±8 | |
| After recovery | 20±2 | 24±2 | |
| ΔG'ATP (mM J−1) | Resting | −63.3±0.5 | −62.7±0.3 |
| Exercising | −58.0±0.7 | −57.4±0.6 | |
| After recovery | −65.7±0.4 | −64.8±0.3 | |
| pH | Resting | 7.09±0.005 | 7.10±0.003 |
| Exercising | 6.94±0.05 | 7.05±0.02 | |
| After recovery | 7.08±0.02 | 7.08±0.01 |
All values are means ± S.E.M.
*different from trekkers at p<0.05;
**different from trekkers at p<0.01.
Figure 1. Muscle phosphocreatine kinetics in recovery from moderate exercise: differences between experienced climbers and altitude-naïve subjects.
Data shown are means ± S.E.M.
The effect of altitude exposure on trekkers and climbers
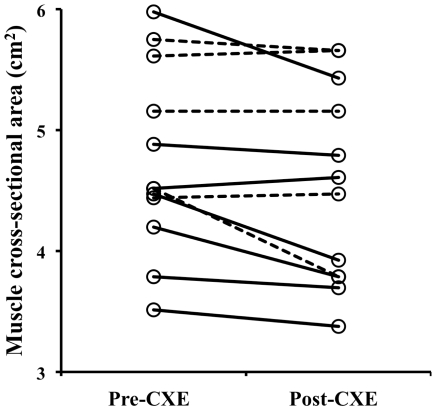
The combined subjects had a decreased calf muscle cross-sectional area following the expedition, from 4.7 to 4.5 cm2 (a loss of 4%, p<0.05) (Figure 2).
Figure 2. Individual changes in muscle cross-sectional area after a trip to high-altitude.
Dashed lines are climbers, solid lines are altitude-naïve subjects. Change in mean values is significant at p<0.05, n = 12.
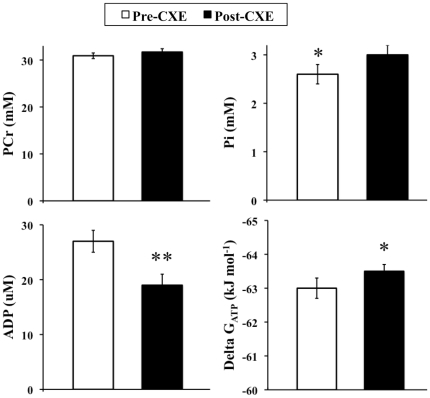
Resting muscle PCr concentration was not changed by exposure to high altitude. However, Table 3 shows that there were a number of significant effects of exposure on resting muscle phosphorus metabolites in the combined subjects. There was a 15% increase in inorganic phosphate (from 2.6 to 3.0 mM). Despite this, there was a 30% reduction in estimated resting ADP concentration, and an increase in the free energy available from ATP hydrolysis (Figure 3).
Table 3. The effect of altitude exposure on high-energy phosphates and pH in human skeletal muscle.
| PRE | POST | ||
| PCr (mM) | Resting | 30.9±0.6 | 31.7±0.7 |
| Exercising | 20.2±1.7 | 18.8±1.6 | |
| After recovery | 32.9±0.5 | 29.4±0.8 ** | |
| PCrt1/2 (s) | During recovery | 20±1 | 19±2 |
| Pi (mM) | Resting | 2.6±0.2 | 3.0±0.2 * |
| Exercising | 11.6±1.8 | 12.9±2.2 | |
| After recovery | 1.3±0.2 | 1.5±0.1 | |
| ADP (µM) | Resting | 27±2 | 19±2 ** |
| Exercising | 53±5 | 54±8 | |
| After recovery | 20±2 | 24±2 | |
| ΔG'ATP (mM J−1) | Resting | −63.0±0.3 | −63.5±0.2 * |
| Exercising | −58.0±0.7 | −57.4±0.6 | |
| After recovery | −65.7±0.4 | −64.8±0.3 | |
| pH | Resting | 7.10±0.003 | 7.06±0.006 *** |
| Exercising | 6.99±0.03 | 7.00±0.02 | |
| After recovery | 7.08±0.01 | 7.04±0.01 ** |
Values are means ± SEM (n = 12).
*different from PRE at p<0.05;
**different from PRE at p<0.01;
***different from PRE at p<0.001. There were no significant effects of trekker/climber grouping.
Figure 3. The effect of altitude exposure on resting high-energy phosphates in skeletal muscle.
* different from pre-CXE at p<0.05; ** different from pre-CXE at p<0.01. CXE = Caudwell Xtreme Everest.
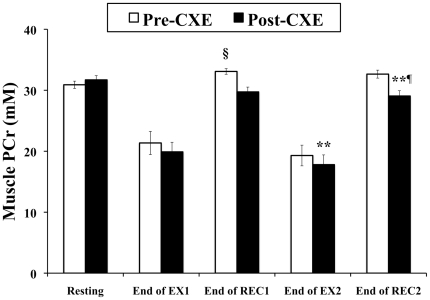
The concentrations of skeletal muscle high-energy phosphate metabolites during steady-state plantar-flexion exercise were unchanged in the combined group following exposure to altitude (Table 3); however, the mean concentration of PCr after full recovery from exercise was 11% lower following hypoxic exposure. This was due to both an absence of post-exercise phosphocreatine ‘overshoot’ and an exercise-induced loss of PCr (compared with resting values) after exposure (Figure 4).
Figure 4. The effect of altitude exposure on phosphocreatine ‘overshoot’ in skeletal muscle.
Values are means over the last minute of each period. CXE = Caudwell Xtreme Everest expedition, EXx = exercise bout x, RECx = recovery period after exercise bout x. ** different from pre-CXE at p<0.01; §End-REC1 PCr is different from resting PCr pre-CXE at p<0.05; ¶End-REC2 PCr is different from resting PCr post-CXE at p<0.05.
There was no effect of altitude exposure on PCr recovery halftimes (PRE: 20±1 s vs. POST: 19±2 s, n = 12, p>0.05).
Resting muscle pH was significantly lower post-CXE (Table 3), and although end-exercise pH was not different post-CXE, muscle pH was still lower in the acclimatised state after recovery from exercise. In addition, there was a significant difference in post-exercise/recovery muscle pH compared with resting (pre-exercise) muscle pH post-exposure, which was not present in the pre-acclimatised state (Table 3).
Discussion
The first significant finding of this study was an unexpected difference in PCr recovery halftime (and hence mitochondrial function) between climbers and trekkers at baseline, with the climbers having significantly shorter halftimes (and hence better mitochondrial function) than their altitude-naïve counterparts. The climbers also had significantly higher inorganic phosphate concentrations than trekkers (the possible significance of this difference will be discussed below). These results were particularly surprising considering the fact that none of the climbers had been to high altitude for at least 5 months. There was a significant difference in age between the groups, but this would be expected to result in the opposite effect, at least on mitochondrial function (the climbers were older, and mitochondrial function generally declines with age [19]). Nor did the climbers engage in any structured physical training prior to the expedition, being aware that differences between individuals in baseline cardiorespiratory fitness are unrelated to hypoxia tolerance (see [20] and references therein).
Several possible explanations suggest themselves. First, the climbers might have been different to the trekkers because years of climbing had selected against particular phenotypes (for example, those genetically ill-suited to high altitude hypoxia are unlikely to continually expose themselves to it). Second, altitude exposure might induce stable changes in phenotype, perhaps through epigenetic modifications. Finally, it may be that repeated hypoxic exposure causes more conventional physiological adaptations that are unusually persistent. There are no published data on the timecourse of ‘de-acclimatisation’ after hypoxic exposure. The most relevant information therefore probably comes from experiments examining the timecourse of detraining after stopping prolonged exercise training. Coyle et al. observed that subjects who had previously been well-trained but who had stopped training altogether for 84 days still had, on average, a 17% higher 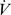 O2max than controls who had never trained at all [21]. This was due to persistent peripheral adaptations (larger mixed-arterial/venous O2 difference, and therefore better O2 extraction by skeletal muscle). So despite the fact that none of the climbers had climbed for at least 5 months prior to this study, it seems possible that the difference in mitochondrial function observed was a long-term (or even stable and transmissible) effect of exposure to high-altitude hypoxia. More work investigating these differences is clearly warranted, as are comparisons with high-altitude natives and the offspring of successful climbers.
O2max than controls who had never trained at all [21]. This was due to persistent peripheral adaptations (larger mixed-arterial/venous O2 difference, and therefore better O2 extraction by skeletal muscle). So despite the fact that none of the climbers had climbed for at least 5 months prior to this study, it seems possible that the difference in mitochondrial function observed was a long-term (or even stable and transmissible) effect of exposure to high-altitude hypoxia. More work investigating these differences is clearly warranted, as are comparisons with high-altitude natives and the offspring of successful climbers.
Exposure to hypobaric hypoxia is associated with an involuntary loss of body mass [22], whether under laboratory conditions [23], [24], [25] or in the field [26], [27]. In women, nitrogen balance is negative soon after exposure to 4300 m altitude [28] and remained negative in men throughout a 7,102 m ascent [29]. In keeping with these observations, we observed a significant reduction in muscle cross-sectional area after hypoxic exposure (Figure 2). There was no difference in the degree of atrophy between the groups (no statistically significant effect of trekker/climber grouping).
Such weight loss does not seem to relate simply to excessive metabolic demands related to exertion. Indeed, physical activity levels (PAL, assessed as maximal exertional metabolic rate as a multiple of basal metabolic rate (BMR)) are normally 2.2–2.5 at sea level, and twice that in trained athletes. However, near Everest's summit, PAL is limited to 2.0–2.7, meaning that exertional energy loss is minimized [30]. Thus, although perceived exertion is great, actual energy expenditure is much less [31], [32]. The notion that weight loss is not purely due to excessive metabolic demands is given further credence by the observation that obese subjects exercising three times each week in 15% oxygen lose more weight than those exercising in air [33].
A variety of factors are thought to contribute to altitude-induced weight loss. First, energy expenditure may rise at altitude, due in part to an increase in BMR [34], [35], [36], [37]. This effect may be altitude-dependent: BMR has risen by 6% in men at 3,650 m [38], by 10% at 3,800 m [37], and by 27–28% in men and women by day 2–3 at 4,300 m [32], [36]. In the absence of calorie supplementation, such absolute increases in BMR do not seem sustained [36], [37], [39], [40], although BMR per unit mass may actually remain elevated [38], [41].
Second, pro-inflammatory cytokines may play a role. In eight sea-level residents, the interleukin-6 (IL-6) response to 60 min of bicycle ergometer exercise was found to be greater during exposure to acute hypoxia (4100 m altitude) than that seen in normoxia [42]. After 6 weeks of exposure to 4100 m, IL6 levels remain elevated [42], a finding in keeping with similar observations over four days of exposure to 4350 m in males [43] and with 12 days of exposure to 4300 m in women [44]. Interleukin-6 is implicated in the pathogenesis of cancer-associated weight loss, driving lipid catabolism and muscle protein catabolism [45], [46], perhaps through both lysosomal (cathepsin) and non-lysosomal (proteasome) pathways [47].
There was no decline in volume-scaled mitochondrial function (PCrt1/2) after hypoxic exposure (Table 3). Taken in the context of significant atrophy, this means that whole muscle aerobic capacity was reduced. Yet, rather surprisingly in the face of significant muscle atrophy and a loss of aerobic capacity, the expedition did not have any adverse effects on muscle function during exercise. Subjects were able to complete the same exercise tasks pre and post exposure, and exercising metabolites were unchanged (Table 3). Although at variance with previous reports that muscle mitochondrial enzyme activities (per unit of cross sectional area) are decreased by hypoxic exposure [4], our results suggest that in vivo function might somehow be maintained. Further experiments specifically targeted at illuminating changes in muscle mitochondrial function in vivo in response to hypoxic exposure are required.
When fully recovered after a period of exercise, PCr concentrations are often higher than pre-exercise values, a phenomenon known as PCr ‘overshoot’ [48]. There is very little published literature regarding the mechanisms underlying PCr overshoot in skeletal muscle. One hypothesis states that PCr overshoot is the result of a slow decay in one of the signals that directly activates oxidative phosphorylation [49]. If this were the case, then the data here suggest a tightening of off-exercise oxygen kinetics, perhaps to prevent unnecessary oxygen consumption. An alternative explanation would be that inorganic phosphate is being lost during exercise (perhaps due to calcium-phosphate precipitation [50]).
There were several changes in resting muscle high-energy phosphates after hypoxic exposure. While the changes in estimated free [ADP] will be discussed below, the increase in muscle phosphate is noteworthy because it mirrors a difference that was observed between the climbers and trekkers at baseline. There are two possible mechanisms for an increased steady-state cell [Pi]. The first is an increased Na+-dependent Pi uptake. This mechanism is poorly understood, but can be driven by an increase in insulin, possibly indirectly via effects on the Na+ gradient [51]. The second theoretical possibility is reduced permeability to Pi efflux, although convincing examples are currently lacking.
Our calculations of [ADP] rest on a number of assumptions regarding muscle metabolite (ATP and creatine) concentrations. Although these assumptions are generally sound [14], one cannot discount the possibility that the extreme conditions experienced by our subjects invalidated them. Thus our findings need to be interpreted with caution. PCr concentration was calculated from the PCr/ATP ratio (assuming an intramuscular [ATP] of 8.2 mM L−1) and was unchanged by the expedition, strongly suggesting that [ATP] was also unchanged. However, we did not directly measure creatine and the observed increase in calculated [ADP] could be accounted for by an increase in total creatine. Despite these reservations, it seems reasonable that [ADP] might have decreased in response to hypoxia. It is now widely accepted that mitochondrial oxidative rate is matched to ATP demand by feedback mediated by intracellular phosphorylation potential or some function of it. Therefore a reduction in the resting ADP concentration would indicate either a change in the control parameters linking oxidative rate to [ADP] or a reduction in resting muscle oxidative rate.
Resting (but not exercising) pH was significantly lower following exposure. This was not a result of systemic ketoacidosis, as there was no correlation between pH and β-hydroxybutyrate (correlation not shown). We therefore suggest that it was the result of either increased metabolic proton production or decreased capacity for cellular proton extrusion (for example, a reduced activity or sensitivity of Na+/H+-ATPase).
Limitations
There are a number of limitations to this study. First, the trekkers and climbers were different from each other before the study began and we have chosen to treat the subjects as a single group (as well as separately). We justify this based on an absence of any statistical evidence that the groups responded differently. Second, several of the climbers were very slow descending from altitude before revisiting Oxford. However, this has provided an unexpected benefit: because there were no differences between the responses of trekkers (who returned to Oxford immediately) and climbers, it is unlikely that the observed changes were acute responses. For example, this shows that the post-exposure reduction in muscle pH (equally present in both groups) was not due to an acute disturbance in acid-base status.
Conclusions
We used magnetic resonance spectroscopy and imaging to study the effects of a trip to high altitude (Mount Everest) on a mixed cohort of altitude-naïve trekkers and experienced climbers. The climbers had unexpectedly better mitochondrial function than the trekkers at baseline. Both groups responded similarly to the hypoxic insult. Climbers had higher resting [Pi] than trekkers before the expedition and resting [Pi] was raised across both groups on their return. There was significant muscle atrophy post-CXE, yet exercising metabolites were unchanged. These results suggest that, in response to high altitude hypoxia, skeletal muscle function is maintained in humans, despite significant atrophy.
Acknowledgments
The Caudwell Xtreme Everest Research Group contributed to designing and conducting the experiments. The members of the Caudwell Xtreme Everest Research Group are as follows: Investigators—V. Ahuja, G. Aref-Adib, R. Burnham, A. Chisholm, K. Clarke, D. Coates, M. Coates, D. Cook, M. Cox, S. Dhillon, C. Dougall, P. Doyle, P. Duncan, M. Edsell, L. Edwards, L. Evans, P. Gardiner, M. Grocott, P. Gunning, N. Hart, J. Harrington, J. Harvey, C. Holloway, D. Howard, D. Hurlbut, C. Imray, C. Ince, M. Jonas, J. van der Kaaij, M. Khosravi, N. Kolfschoten, D. Levett, H. Luery, A. Luks, D. Martin, R. McMorrow, P. Meale, K. Mitchell, H. Montgomery, G. Morgan, J. Morgan, A. Murray, M. Mythen, S. Newman, M. O'Dwyer, J. Pate, T. Plant, M. Pun, P. Richards, A. Richardson, G. Rodway, J. Simpson, C. Stroud, M. Stroud, J. Stygal, B. Symons, P. Szawarski, A. Van Tulleken, C. Van Tulleken, A. Vercueil, L. Wandrag, M. Wilson, J. Windsor; Scientific Advisory Group—B. Basnyat, C. Clarke, T. Hornbein, J. Milledge, J. West.
Footnotes
Competing Interests: This project was partly funded by grants from BOC Medical (now Linde Gas Therapeutics), Lilly Critical Care, The London Clinic, Smiths Medical, Deltex Medical and The Rolex Foundation. All monies were given as unrestricted grants: the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This does not alter the authors' adherence to any of the PLoS ONE policies on sharing data and materials.
Funding: Drs. Edwards and Holloway were supported by a grant from Defence Advanced Research Projects Agency (DARPA, code: ASRJMT1). Dr Tyler was funded by the Medical Research Council, grant number G0601490. Caudwell Xtreme Everest (CXE) is a research project coordinated by the UCL Centre for Altitude, Space and Extreme Environment Medicine, University College London, United Kingdom, and funded by grants from the Association of Anaesthetists of Great Britain and Ireland, Intensive Care Society, Sir Halley Stewart Trust, John Caudwell, BOC Medical (now Linde Gas Therapeutics), Lilly Critical Care, The London Clinic, Smiths Medical, Deltex Medical and The Rolex Foundation. All monies were given as unrestricted grants: the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Some of this work was undertaken at University College London Hospital-University College London Comprehensive Biomedical Research Centre, which received a proportion of funding from the United Kingdom Department of Health's National Institute for Health Research Biomedical Research Centres funding scheme.
References
- 1.Reynafarje B. Myoglobin content and enzymatic activity of muscle and altitude adaptation. J Appl Physiol. 1962;17:301–305. doi: 10.1152/jappl.1962.17.2.301. [DOI] [PubMed] [Google Scholar]
- 2.Hochachka PW, Stanley C, Merkt J, Sumar-Kalinowski J. Metabolic meaning of elevated levels of oxidative enzymes in high altitude adapted animals: an interpretive hypothesis. Respir Physiol. 1983;52:303–313. doi: 10.1016/0034-5687(83)90087-7. [DOI] [PubMed] [Google Scholar]
- 3.Lynn EG, Lu Z, Minerbi D, Sack MN. The regulation, control, and consequences of mitochondrial oxygen utilization and disposition in the heart and skeletal muscle during hypoxia. Antioxid Redox Signal. 2007;9:1353–1361. doi: 10.1089/ars.2007.1700. [DOI] [PubMed] [Google Scholar]
- 4.Hoppeler H, Vogt M. Muscle tissue adaptations to hypoxia. J Exp Biol. 2001;204:3133–3139. doi: 10.1242/jeb.204.18.3133. [DOI] [PubMed] [Google Scholar]
- 5.Rosser BW, Hochachka PW. Metabolic capacity of muscle fibers from high-altitude natives. Eur J Appl Physiol Occup Physiol. 1993;67:513–517. doi: 10.1007/BF00241647. [DOI] [PubMed] [Google Scholar]
- 6.Green H, Roy B, Grant S, Otto C, Pipe A, et al. Human skeletal muscle exercise metabolism following an expedition to Mount Denali. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1872–1879. doi: 10.1152/ajpregu.2000.279.5.R1872. [DOI] [PubMed] [Google Scholar]
- 7.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 8.Hochachka PW. Molecular/metabolic defense and rescue mechanisms for surviving oxygen lack: from genes to pathways. In: Hornbein T, Schoene R, editors. High Altitude, An Exploration of Human Adaptation. New York: Marcel Dekker; 2001. pp. 131–138. [Google Scholar]
- 9.Lundby C, Calbet JA, Sander M, van Hall G, Mazzeo RS, et al. Exercise economy does not change after acclimatization to moderate to very high altitude. Scand J Med Sci Sports. 2007;17:281–291. doi: 10.1111/j.1600-0838.2006.00530.x. [DOI] [PubMed] [Google Scholar]
- 10.Hahn AG, Gore CJ. The effect of altitude on cycling performance: a challenge to traditional concepts. Sports Med. 2001;31:533–557. doi: 10.2165/00007256-200131070-00008. [DOI] [PubMed] [Google Scholar]
- 11.Grocott M, Richardson A, Montgomery H, Mythen M. Caudwell Xtreme Everest: a field study of human adaptation to hypoxia. Crit Care. 2007;11:151. doi: 10.1186/cc5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001;31:269–286. doi: 10.1016/s0010-4825(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 13.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 14.Kemp GJ, Meyerspeer M, Moser E. Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: a quantitative review. NMR Biomed. 2007;20:555–565. doi: 10.1002/nbm.1192. [DOI] [PubMed] [Google Scholar]
- 15.Golding EM, Teague WE, Dobson GP. Adjustment of K' to varying pH and pMg for the creatine kinase, adenylate kinase and ATP hydrolysis equilibria permitting quantitative bioenergetic assessment. J Exp Biol. 1995;198:1775–1782. doi: 10.1242/jeb.198.8.1775. [DOI] [PubMed] [Google Scholar]
- 16.Jeneson JAL, Wiseman RW, Kushmerick MJ. Non-invasive quantitative 31P MRS assay of mitochondrial function in skeletal muscle in situ. Molecular and Cellular Biochemistry. 1997;174:17–22. [PubMed] [Google Scholar]
- 17.Braun M. New York: Springer; 1941. Differential Equations and their Applications. [Google Scholar]
- 18.Vandenborne K, Walter G, Leigh JS, Goelman G. pH heterogeneity during exercise in localized spectra from single human muscles. Am J Physiol Cell Physiol. 1993;265:C1332–1339. doi: 10.1152/ajpcell.1993.265.5.C1332. [DOI] [PubMed] [Google Scholar]
- 19.Short KR, Nair KS. Does aging adversely affect muscle mitochondrial function? Exerc Sport Sci Rev. 2001;29:118–123. doi: 10.1097/00003677-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Cymerman A, Reeves JT, Sutton JR, Rock PB, Groves BM, et al. Operation Everest II: maximal oxygen uptake at extreme altitude. J Appl Physiol. 1989;66:2446–2453. doi: 10.1152/jappl.1989.66.5.2446. [DOI] [PubMed] [Google Scholar]
- 21.Coyle EF, Martin WH, 3rd, Sinacore DR, Joyner MJ, Hagberg JM, et al. Time course of loss of adaptations after stopping prolonged intense endurance training. J Appl Physiol. 1984;57:1857–1864. doi: 10.1152/jappl.1984.57.6.1857. [DOI] [PubMed] [Google Scholar]
- 22.West JB, Boyer SJ, Graber DJ, Hackett PH, Maret KH, et al. Maximal exercise at extreme altitudes on Mount Everest. J Appl Physiol. 1983;55:688–698. doi: 10.1152/jappl.1983.55.3.688. [DOI] [PubMed] [Google Scholar]
- 23.Consolazio CF, Johnson HL, Krzywicki HJ, Daws TA. Metabolic aspects of acute altitude exposure (4,300 meters) in adequately nourished humans. Am J Clin Nutr. 1972;25:23–29. doi: 10.1093/ajcn/25.1.23. [DOI] [PubMed] [Google Scholar]
- 24.Consolazio CF, Matoush L, Johnson HL, Daws TA. Protein and water balances of young adults during prolonged exposure to high altitude (4,300 m). Am J Clin Nutr. 1968;21:154–161. [Google Scholar]
- 25.Rose MS, Houston CS, Fulco CS, Coates G, Sutton JR, et al. Operation Everest. II: Nutrition and body composition. J Appl Physiol. 1988;65:2545–2551. doi: 10.1152/jappl.1988.65.6.2545. [DOI] [PubMed] [Google Scholar]
- 26.Boyer SJ, Blume FD. Weight loss and changes in body composition at high altitude. J Appl Physiol. 1984;57:1580–1585. doi: 10.1152/jappl.1984.57.5.1580. [DOI] [PubMed] [Google Scholar]
- 27.Shukla V, Singh SN, Vats P, Singh VK, Singh SB, et al. Ghrelin and leptin levels of sojourners and acclimatized lowlanders at high altitude. Nutr Neurosci. 2005;8:161–165. doi: 10.1080/10284150500132823. [DOI] [PubMed] [Google Scholar]
- 28.Hannon JP, Klain GJ, Sudman DM, Sullivan FJ. Nutritional aspects of high-altitude exposure in women. Am J Clin Nutr. 1976;29:604–613. doi: 10.1093/ajcn/29.6.604. [DOI] [PubMed] [Google Scholar]
- 29.Guilland JC, Klepping J. Nutritional alterations at high altitude in man. Eur J Appl Physiol Occup Physiol. 1985;54:517–523. doi: 10.1007/BF00422963. [DOI] [PubMed] [Google Scholar]
- 30.Westerterp KR. Limits to sustainable human metabolic rate. J Exp Biol. 2001;204:3183–3187. doi: 10.1242/jeb.204.18.3183. [DOI] [PubMed] [Google Scholar]
- 31.Westerterp KR, Kayser B. Body mass regulation at altitude. Eur J Gastroenterol Hepatol. 2006;18:1–3. doi: 10.1097/00042737-200601000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Butterfield GE, Gates J, Fleming S, Brooks GA, Sutton JR, et al. Increased energy intake minimizes weight loss in men at high altitude. J Appl Physiol. 1992;72:1741–1748. doi: 10.1152/jappl.1992.72.5.1741. [DOI] [PubMed] [Google Scholar]
- 33.Netzer NC, Chytra R, Kupper T. Low intense physical exercise in normobaric hypoxia leads to more weight loss in obese people than low intense physical exercise in normobaric sham hypoxia. Sleep Breath. 2008;12:129–134. doi: 10.1007/s11325-007-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill MB, Pugh LG. Basal Metabolism and Respiration in Men Living at 5,800 M (19,000 Ft). J Appl Physiol. 1964;19:949–954. doi: 10.1152/jappl.1964.19.5.949. [DOI] [PubMed] [Google Scholar]
- 35.Grover RF. Basal Oxygen Uptake of Man at High Altitude. J Appl Physiol. 1963;18:909–912. doi: 10.1152/jappl.1963.18.5.909. [DOI] [PubMed] [Google Scholar]
- 36.Hannon JP, Sudman DM. Basal metabolic and cardiovascular function of women during altitude acclimatization. J Appl Physiol. 1973;34:471–477. doi: 10.1152/jappl.1973.34.4.471. [DOI] [PubMed] [Google Scholar]
- 37.Kellogg RH, Pace N, Archibald ER, Vaughan BE. Respiratory response to inspired CO2 during acclimatization to an altitude of 12, 470 feet. J Appl Physiol. 1957;11:65–71. doi: 10.1152/jappl.1957.11.1.65. [DOI] [PubMed] [Google Scholar]
- 38.Stock MJ, Norgan NG, Ferro-Luzzi A, Evans E. Effect of altitude on dietary-induced thermogenesis at rest and during light exercise in man. J Appl Physiol. 1978;45:345–349. doi: 10.1152/jappl.1978.45.3.345. [DOI] [PubMed] [Google Scholar]
- 39.Mawson JT, Braun B, Rock PB, Moore LG, Mazzeo R, et al. Women at altitude: energy requirement at 4,300 m. J Appl Physiol. 2000;88:272–281. doi: 10.1152/jappl.2000.88.1.272. [DOI] [PubMed] [Google Scholar]
- 40.Mathew L, Purkayastha SS, Gupta JS, Malhotra MS. Body temperature and basal metabolic changes during acclimatization to altitude (3,500 m) in man. Indian J Physiol Pharmacol. 1976;20:197–202. [PubMed] [Google Scholar]
- 41.Armellini F, Zamboni M, Robbi R, Todesco T, Bissoli L, et al. The effects of high altitude trekking on body composition and resting metabolic rate. Horm Metab Res. 1997;29:458–461. doi: 10.1055/s-2007-979077. [DOI] [PubMed] [Google Scholar]
- 42.Lundby C, Steensberg A. Interleukin-6 response to exercise during acute and chronic hypoxia. Eur J Appl Physiol. 2004;91:88–93. doi: 10.1007/s00421-003-0935-y. [DOI] [PubMed] [Google Scholar]
- 43.Klausen B, Toubro S, Astrup A. Age and sex effects on energy expenditure. Am J Clin Nutr. 1997;65:895–907. doi: 10.1093/ajcn/65.4.895. [DOI] [PubMed] [Google Scholar]
- 44.Mazzeo RS, Donovan D, Fleshner M, Butterfield GE, Zamudio S, et al. Interleukin-6 response to exercise and high-altitude exposure: influence of alpha-adrenergic blockade. J Appl Physiol. 2001;91:2143–2149. doi: 10.1152/jappl.2001.91.5.2143. [DOI] [PubMed] [Google Scholar]
- 45.Barton BE. IL-6-like cytokines and cancer cachexia: consequences of chronic inflammation. Immunol Res. 2001;23:41–58. doi: 10.1385/IR:23:1:41. [DOI] [PubMed] [Google Scholar]
- 46.Barton BE, Murphy TF. Cancer cachexia is mediated in part by the induction of IL-6-like cytokines from the spleen. Cytokine. 2001;16:251–257. doi: 10.1006/cyto.2001.0968. [DOI] [PubMed] [Google Scholar]
- 47.Tisdale MJ. Loss of skeletal muscle in cancer: biochemical mechanisms. Front Biosci. 2001;6:D164–174. doi: 10.2741/tisdale. [DOI] [PubMed] [Google Scholar]
- 48.Korzeniewski B, Zoladz JA. Some factors determining the PCr recovery overshoot in skeletal muscle. Biophysical Chemistry. 2005;116:129–136. doi: 10.1016/j.bpc.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Korzeniewski B. Regulation of oxidative phosphorylation in different muscles and various experimental conditions. Biochem J. 2003;375:799–804. doi: 10.1042/BJ20030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen DG, Westerblad H. Role of phosphate and calcium stores in muscle fatigue. J Physiol. 2001;536:657–665. doi: 10.1111/j.1469-7793.2001.t01-1-00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polgreen KE, Kemp GJ, Leighton B, Radda GK. Modulation of Pi transport in skeletal muscle by insulin and IGF-1. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1994;1223:279–284. doi: 10.1016/0167-4889(94)90238-0. [DOI] [PubMed] [Google Scholar]