Abstract
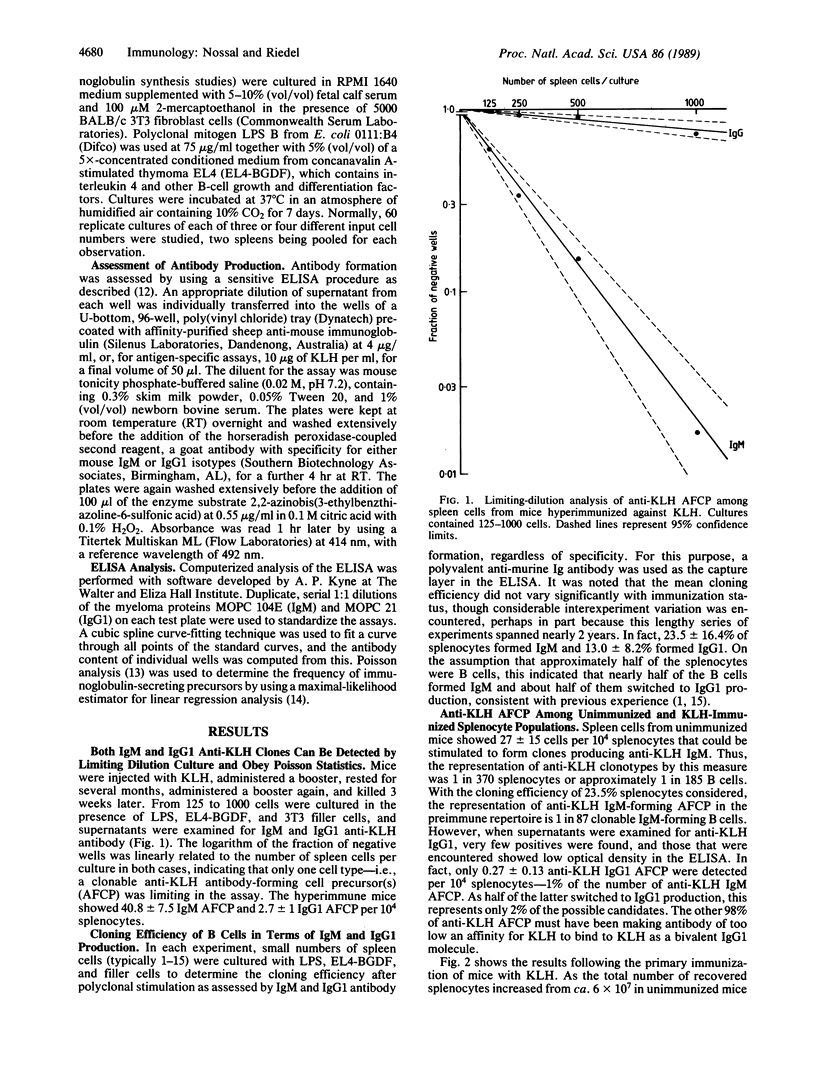
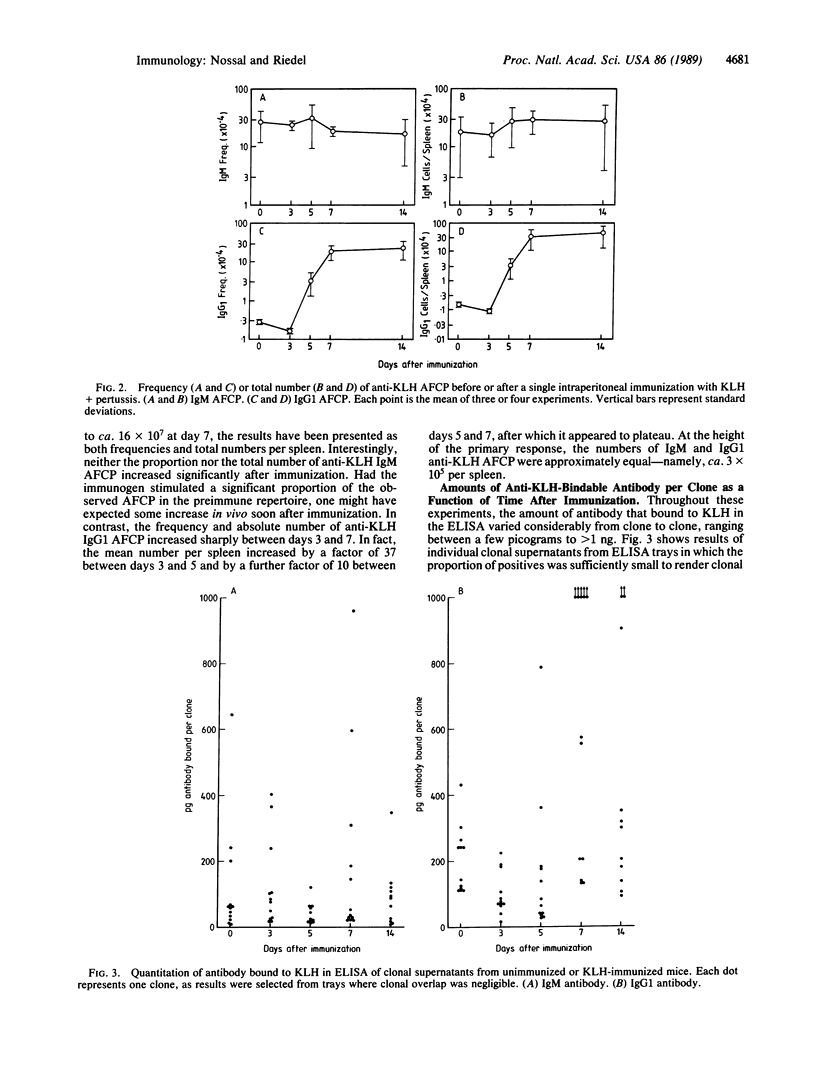
The anti-keyhole limpet hemocyanin (KLH) B-cell repertoire of unimmunized adult mice was examined by culture of splenocytes (generally 100-3000) at limiting dilution. Cells were polyclonally stimulated with Escherichia coli lipopolysaccharide (LPS) and an interleukin-4-containing lymphokine mixture in the presence of 3T3 fibroblast filler cells. After 7 days of culture, supernatants were examined for their content of anti-KLH IgM and IgG1 antibody by an enzyme-linked immunosorbent assay (ELISA). Parallel cultures of smaller numbers (generally 1-15) of splenocytes were examined to determine the cloning efficiency of B cells in terms of total IgM and IgG1 production. Whereas one spleen cell in 370 produced clones secreting anti-KLH IgM, only 1% of these produced IgG1 that could bind to KLH, despite the fact that about half of the clones switched to IgG1 production with these stimuli. In mice immunized with KLH, this situation did not change until day 5, when there was a sudden, explosive emergence of B cells that could form clones secreting anti-KLH IgG1. The absolute number of such cells in the spleen was found to rise by a factor of 350 between days 3 and 7 of immunization. Moreover, the median amount of IgG1 antibody formed per clone and binding to KLH also rose markedly. In contrast, neither the numbers nor the median KLH-binding antibody content of anti-KLH IgM clones changed significantly after immunization. The results show that the repertoire of anti-protein B cells detected through IgM formation in ELISA consists chiefly of cells producing antibody of low avidity and of doubtful in vivo significance. Assuming that the small proportion of these cells making antibody that is of sufficient avidity to bind as the IgG1 isotype are the ancestors of the many such cells found on day 7 of the primary immune response, one would have to postulate a very high recruitment and/or division rate to account for the increase in numbers and avidity that occurs. It is possible that the anti-KLH IgG1 precursors that suddenly emerge are the results of early variable region gene (V) mutations in B cells. Moreover, it is not excluded that they represent products of a subset of B cells different from those that give rise to the primary in vitro anti-KLH IgM response. The findings have implications for theories of B-cell tolerance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berek C., Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev. 1987 Apr;96:23–41. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Blier P. R., Bothwell A. A limited number of B cell lineages generates the heterogeneity of a secondary immune response. J Immunol. 1987 Dec 15;139(12):3996–4006. [PubMed] [Google Scholar]
- Conger J. D., Pike B. L., Nossal G. J. Clonal analysis of the anti-DNA repertoire of murine B lymphocytes. Proc Natl Acad Sci U S A. 1987 May;84(9):2931–2935. doi: 10.1073/pnas.84.9.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote R. J., Morrissey D. M., Houghton A. N., Beattie E. J., Jr, Oettgen H. F., Old L. J. Generation of human monoclonal antibodies reactive with cellular antigens. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2026–2030. doi: 10.1073/pnas.80.7.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J. Evolution in microcosm: the rapid somatic diversification of lymphocytes. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):761–770. doi: 10.1101/sqb.1977.041.01.087. [DOI] [PubMed] [Google Scholar]
- Dighiero G., Guilbert B., Avrameas S. Naturally occurring antibodies against nine common antigens in humans sera. II. High incidence of monoclonal Ig exhibiting antibody activity against actin and tubulin and sharing antibody specificities with natural antibodies. J Immunol. 1982 Jun;128(6):2788–2792. [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Holmberg D., Lundquist I., Coutinho A., Avrameas S. High frequency of natural autoantibodies in normal newborn mice. J Immunol. 1985 Feb;134(2):765–771. [PubMed] [Google Scholar]
- Good M. F., Boyd A. W., Nossal G. J. Analysis of true anti-hapten cytotoxic clones in limit dilution microcultures after correction for "anti-self" activity: precursor frequencies, Ly-2 and Thy-1 phenotype, specificity, and statistical methods. J Immunol. 1983 May;130(5):2046–2055. [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Adelstein S., Lavoie T. B., Smith-Gill S. J., Brink R. A., Pritchard-Briscoe H., Wotherspoon J. S., Loblay R. H., Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988 Aug 25;334(6184):676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Kaartinen M., Griffiths G. M., Hamlyn P. H., Markham A. F., Karjalainen K., Pelkonen J. L., Mäkelä O., Milstein C. Anti-oxazolone hybridomas and the structure of the oxazolone idiotype. J Immunol. 1983 Feb;130(2):937–945. [PubMed] [Google Scholar]
- MacLennan I. C., Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986 Jun;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams M. G., Nossal G. J. Clonal analysis of autoantibody-producing cell precursors in the preimmune B cell repertoire. J Immunol. 1988 Dec 15;141(12):4118–4123. [PubMed] [Google Scholar]
- McKean D., Huppi K., Bell M., Staudt L., Gerhard W., Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984 May;81(10):3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf E. S., Klinman N. R. In vitro tolerance induction of bone marrow cells: a marker for B cell maturation. J Immunol. 1977 Jun;118(6):2111–2116. [PubMed] [Google Scholar]
- NOSSAL G. J., ADA G. L., AUSTIN C. M. ANTIGENS IN IMMUNITY. IV. CELLULAR LOCALIZATION OF 125-I- AND 131-I-LABELLED FLAGELLA IN LYMPH NODES. Aust J Exp Biol Med Sci. 1964 Jun;42:311–330. [PubMed] [Google Scholar]
- Nossal G. J. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Single cell studies on the antibody-forming potential of fractionated, hapten-specific B lymphocytes. Immunology. 1976 Feb;30(2):189–202. [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Alderson M. R., Nossal G. J. T-independent activation of single B cells: an orderly analysis of overlapping stages in the activation pathway. Immunol Rev. 1987 Oct;99:119–152. doi: 10.1111/j.1600-065x.1987.tb01175.x. [DOI] [PubMed] [Google Scholar]
- Pike B. L., Nossal G. J. A high-efficiency cloning system for single hapten-specific B lymphocytes that is suitable for assay of putative growth and differentiation factors. Proc Natl Acad Sci U S A. 1985 May;82(10):3395–3399. doi: 10.1073/pnas.82.10.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar B. S., Saegusa J., Onodera T., Notkins A. L. Lymphocytes capable of making monoclonal autoantibodies that react with multiple organs are a common feature of the normal B cell repertoire. J Immunol. 1984 Dec;133(6):2815–2817. [PubMed] [Google Scholar]
- Ragimbeau J., Avrameas S. Single lipopolysaccharide-reactive B cells in the non-immune mouse spleen cell population secrete natural multispecific autoantibodies. Scand J Immunol. 1987 Jul;26(1):29–35. doi: 10.1111/j.1365-3083.1987.tb02231.x. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Förster I., Cumano A. Evolutionary and somatic selection of the antibody repertoire in the mouse. Science. 1987 Nov 20;238(4830):1088–1094. doi: 10.1126/science.3317826. [DOI] [PubMed] [Google Scholar]
- Sanz I., Capra J. D. The genetic origin of human autoantibodies. J Immunol. 1988 May 15;140(10):3283–3285. [PubMed] [Google Scholar]
- Underwood J. R., Pedersen J. S., Chalmers P. J., Toh B. H. Hybrids from normal, germ free, nude and neonatal mice produce monoclonal autoantibodies to eight different intracellular structures. Clin Exp Immunol. 1985 May;60(2):417–426. [PMC free article] [PubMed] [Google Scholar]
- Yoshida N., Radbruch A., Rajewsky K. Ig gene rearrangement and expression in the progeny of B-cell progenitors in the course of clonal expansion in bone marrow cultures. EMBO J. 1987 Sep;6(9):2735–2741. doi: 10.1002/j.1460-2075.1987.tb02567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Shortman K. The separation of different cell classes from lymphoid organs. IX. A simple and rapid method for removal of damaged cells from lymphoid cell suspensions. J Immunol Methods. 1973 Apr;2(3):293–301. doi: 10.1016/0022-1759(73)90055-0. [DOI] [PubMed] [Google Scholar]



