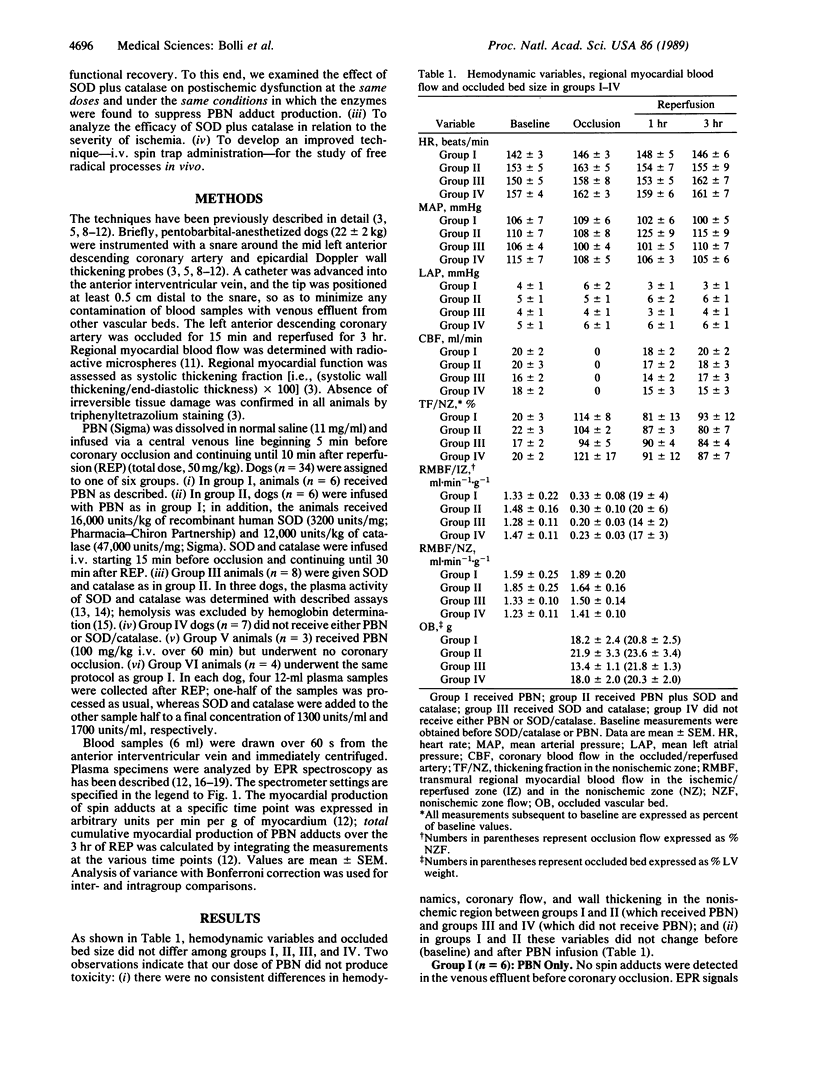
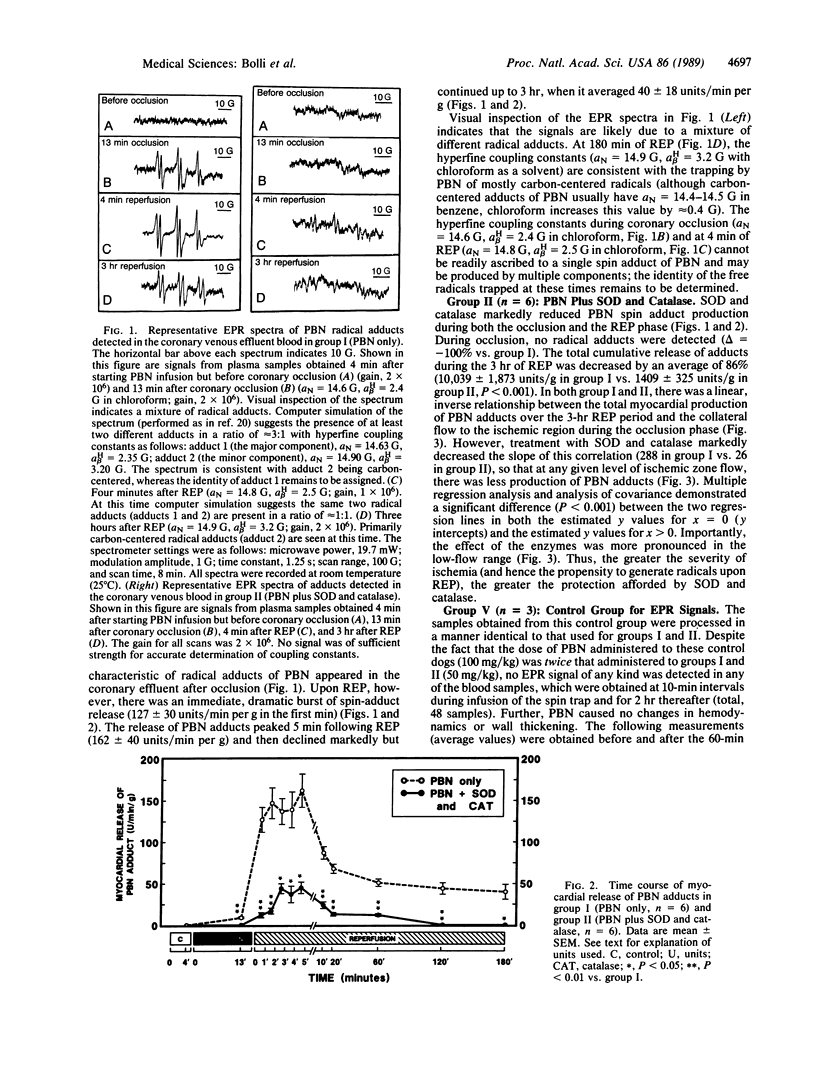
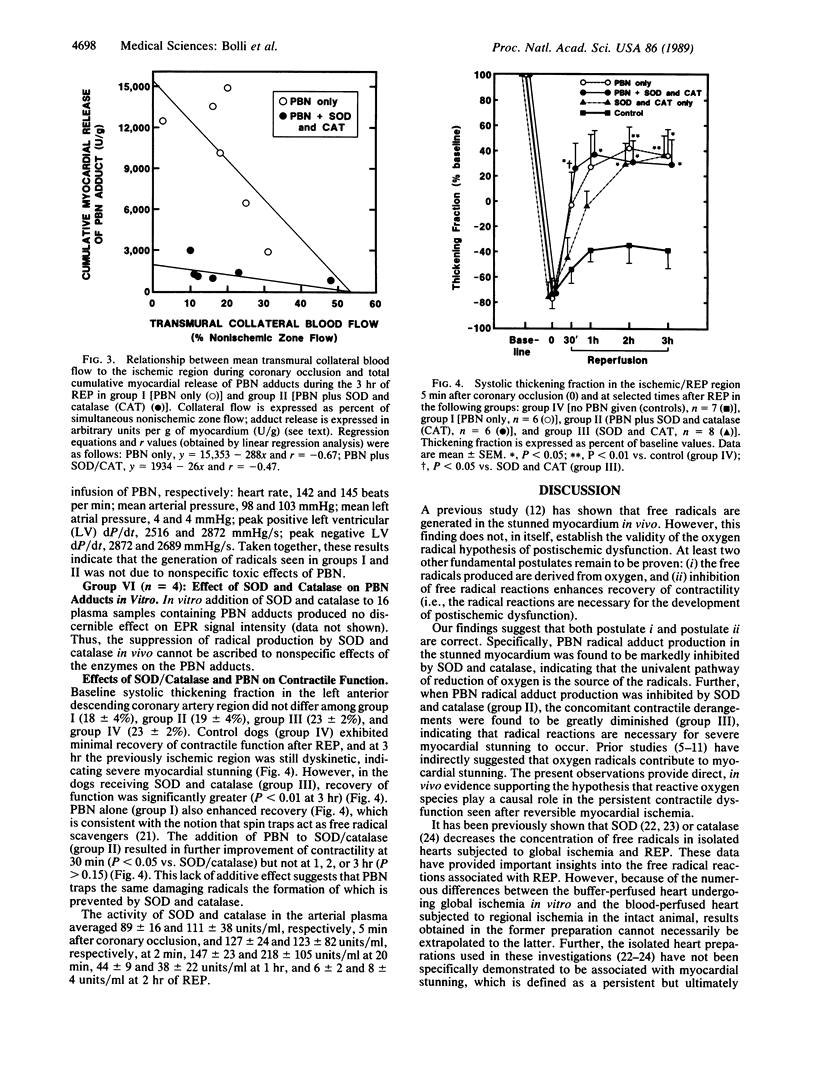
Abstract
Electron paramagnetic resonance (EPR) spectroscopy was used to investigate whether (i) the free radicals produced in the "stunned" myocardium (myocardium with postischemic contractile dysfunction) are derived from O2, (ii) inhibition of radical reactions improves function, and (iii) i.v. spin traps are effective. Open-chest dogs undergoing a 15-min coronary occlusion received an i.v. infusion of the spin trap, alpha-phenyl N-tert-butylnitrone (PBN) (50 mg/kg). In group I (n = 6), EPR signals characteristic of radical adducts of PBN appeared in the coronary venous blood during ischemia and increased dramatically after reperfusion. In group II (n = 6), which received PBN and i.v. superoxide dismutase (SOD; 16,000 units/kg) plus catalase (12,000 units/kg), myocardial production of PBN adducts was undetectable during ischemia (delta = -100%, P less than 0.01 vs. group I) and markedly inhibited after reperfusion (delta = -86%, P less than 0.001). This effect was seen at all levels of ischemic zone flow but was relatively greater in the low-flow range. In group III (n = 8), the same dosages of SOD and catalase without PBN markedly enhanced contractile recovery (measured as systolic wall thickening) after reperfusion [P less than 0.01 at 3 hr vs. controls (group IV, n = 7)]. Systemic plasma activity of SOD and catalase averaged 127 +/- 24 and 123 +/- 82 units/ml, respectively, 2 min after reperfusion. PBN produced no apparent adverse effects and actually improved postischemic contractile recovery in group I (P less than 0.05 at 3 hr vs. controls). This study shows that (i) SOD and catalase are highly effective in blocking free radical reactions in vivo, (ii) the radicals generated in the "stunned" myocardium are derived from univalent reduction of O2, and (iii) inhibition of radical reactions improves functional recovery. The results provide direct, in vivo evidence to support the hypothesis that reactive oxygen metabolites play a causal role in the myocardial "stunning" seen after brief ischemia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Blakney G. B., Dinwoodie A. J. A spectrophotometric scanning technique for the rapid determination of plasma hemoglobin. Clin Biochem. 1975 Apr;8(2):96–102. doi: 10.1016/s0009-9120(75)91005-x. [DOI] [PubMed] [Google Scholar]
- Bolli R., Patel B. S., Jeroudi M. O., Lai E. K., McCay P. B. Demonstration of free radical generation in "stunned" myocardium of intact dogs with the use of the spin trap alpha-phenyl N-tert-butyl nitrone. J Clin Invest. 1988 Aug;82(2):476–485. doi: 10.1172/JCI113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R., Patel B. S., Zhu W. X., O'Neill P. G., Hartley C. J., Charlat M. L., Roberts R. The iron chelator desferrioxamine attenuates postischemic ventricular dysfunction. Am J Physiol. 1987 Dec;253(6 Pt 2):H1372–H1380. doi: 10.1152/ajpheart.1987.253.6.H1372. [DOI] [PubMed] [Google Scholar]
- Bolli R., Zhu W. X., Hartley C. J., Michael L. H., Repine J. E., Hess M. L., Kukreja R. C., Roberts R. Attenuation of dysfunction in the postischemic 'stunned' myocardium by dimethylthiourea. Circulation. 1987 Aug;76(2):458–468. doi: 10.1161/01.cir.76.2.458. [DOI] [PubMed] [Google Scholar]
- Bolli R., Zhu W. X., Thornby J. I., O'Neill P. G., Roberts R. Time course and determinants of recovery of function after reversible ischemia in conscious dogs. Am J Physiol. 1988 Jan;254(1 Pt 2):H102–H114. doi: 10.1152/ajpheart.1988.254.1.H102. [DOI] [PubMed] [Google Scholar]
- Braunwald E., Kloner R. A. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation. 1982 Dec;66(6):1146–1149. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- Charlat M. I., O'Neill P. G., Egan J. M., Abernethy D. R., Michael L. H., Myers M. L., Roberts R., Bolli R. Evidence for a pathogenetic role of xanthine oxidase in the "stunned" myocardium. Am J Physiol. 1987 Mar;252(3 Pt 2):H566–H577. doi: 10.1152/ajpheart.1987.252.3.H566. [DOI] [PubMed] [Google Scholar]
- Gross G. J., Farber N. E., Hardman H. F., Warltier D. C. Beneficial actions of superoxide dismutase and catalase in stunned myocardium of dogs. Am J Physiol. 1986 Mar;250(3 Pt 2):H372–H377. doi: 10.1152/ajpheart.1986.250.3.H372. [DOI] [PubMed] [Google Scholar]
- Heyndrickx G. R., Millard R. W., McRitchie R. J., Maroko P. R., Vatner S. F. Regional myocardial functional and electrophysiological alterations after brief coronary artery occlusion in conscious dogs. J Clin Invest. 1975 Oct;56(4):978–985. doi: 10.1172/JCI108178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. H., Arroyo C. M., Dickens B. F., Weglicki W. B. Spin-trapping evidence that graded myocardial ischemia alters post-ischemic superoxide production. Free Radic Biol Med. 1987;3(2):153–159. doi: 10.1016/s0891-5849(87)80011-4. [DOI] [PubMed] [Google Scholar]
- Lai E. K., Crossley C., Sridhar R., Misra H. P., Janzen E. G., McCay P. B. In vivo spin trapping of free radicals generated in brain, spleen, and liver during gamma radiation of mice. Arch Biochem Biophys. 1986 Jan;244(1):156–160. doi: 10.1016/0003-9861(86)90104-9. [DOI] [PubMed] [Google Scholar]
- McCay P. B., Lai E. K., Poyer J. L., DuBose C. M., Janzen E. G. Oxygen- and carbon-centered free radical formation during carbon tetrachloride metabolism. Observation of lipid radicals in vivo and in vitro. J Biol Chem. 1984 Feb 25;259(4):2135–2143. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Myers M. L., Bolli R., Lekich R. F., Hartley C. J., Roberts R. Enhancement of recovery of myocardial function by oxygen free-radical scavengers after reversible regional ischemia. Circulation. 1985 Oct;72(4):915–921. doi: 10.1161/01.cir.72.4.915. [DOI] [PubMed] [Google Scholar]
- Myers M. L., Bolli R., Lekich R. F., Hartley C. J., Roberts R. N-2-mercaptopropionylglycine improves recovery of myocardial function after reversible regional ischemia. J Am Coll Cardiol. 1986 Nov;8(5):1161–1168. doi: 10.1016/s0735-1097(86)80396-5. [DOI] [PubMed] [Google Scholar]
- Poyer J. L., McCay P. B., Weddle C. C., Downs P. E. In vivo spin-trapping of radicals formed during halothane metabolism. Biochem Pharmacol. 1981 Jun 15;30(12):1517–1519. doi: 10.1016/0006-2952(81)90375-0. [DOI] [PubMed] [Google Scholar]
- Przyklenk K., Kloner R. A. Superoxide dismutase plus catalase improve contractile function in the canine model of the "stunned myocardium". Circ Res. 1986 Jan;58(1):148–156. doi: 10.1161/01.res.58.1.148. [DOI] [PubMed] [Google Scholar]
- Reinke L. A., Lai E. K., DuBose C. M., McCay P. B. Reactive free radical generation in vivo in heart and liver of ethanol-fed rats: correlation with radical formation in vitro. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9223–9227. doi: 10.1073/pnas.84.24.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. A., Hess M. L. The oxygen free radical system: a fundamental mechanism in the production of myocardial necrosis. Prog Cardiovasc Dis. 1986 May-Jun;28(6):449–462. doi: 10.1016/0033-0620(86)90027-7. [DOI] [PubMed] [Google Scholar]
- Zweier J. L. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988 Jan 25;263(3):1353–1357. [PubMed] [Google Scholar]



