Abstract
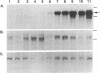
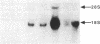
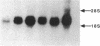
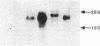
The major histocompatibility complex (MHC) class I molecules are known to serve as recognition elements for cytotoxic T cells in mediating the rejection of transplanted tumors. We demonstrate that MHC molecules may have nonimmune functions in modulating tumor cell growth in addition to their classical role in antitumor immunity. A human neuroendocrine carcinoma cell line, COLO 320, with low levels of endogenous class I expression was transfected with the murine H-2Ld gene. Eleven independent stable clones were established, four containing only pRSV-neo and seven also containing varying copy numbers of the transfected Ld gene. The ability of the different clones to grow as colonies in soft agar correlated strongly with the relative amounts of Ld antigen expression (r = 0.89; P less than 0.001). There was a weaker correlation between increased clonogenic ability and higher levels of Ld mRNA (r = 0.67; P less than 0.05). There was no correlation between clonogenic ability and relative expression of amplified c-myc gene or of integrated pRSV-neo. Furthermore, in nude mice, Ld antigen expression was associated with increased formation of metastatic lung colonies 6 weeks after intravenous injection of 10(5) cells. These observations are consistent with the concept that MHC class I antigens may have a role in modulating the growth potential of certain tumor cells independent of their involvement in immune responses.
Full text
PDF

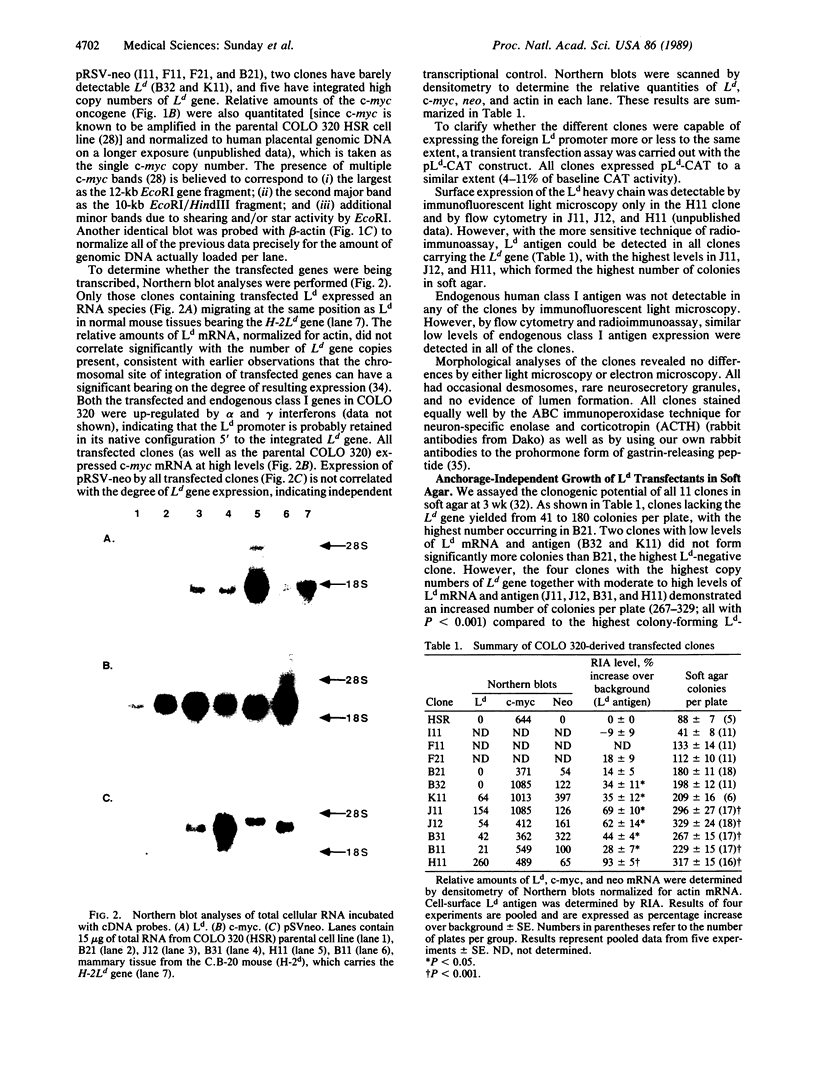
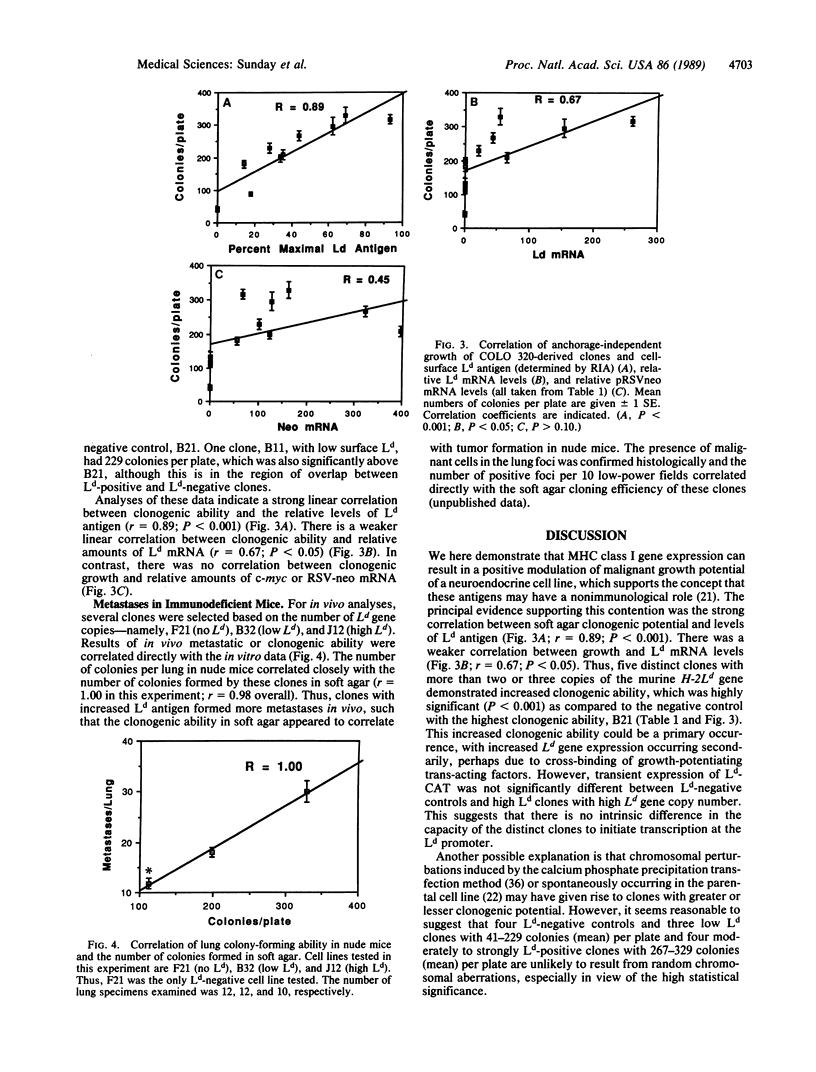

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Schwab M., Lin C. C., Varmus H. E., Bishop J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1707–1711. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artzt K. Gene mapping within the T/t complex of the mouse. III: t-Lethal genes are arranged in three clusters on chromosome 17. Cell. 1984 Dec;39(3 Pt 2):565–572. doi: 10.1016/0092-8674(84)90463-x. [DOI] [PubMed] [Google Scholar]
- Birkby C. A., Curtis A. S., McGrath M., Ripley B. D. MHC control of cell position in vitro. J Cell Sci. 1988 Feb;89(Pt 2):167–174. doi: 10.1242/jcs.89.2.167. [DOI] [PubMed] [Google Scholar]
- Bodmer W. F. The HLA system: structure and function. J Clin Pathol. 1987 Sep;40(9):948–958. doi: 10.1136/jcp.40.9.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickell P. M., Latchman D. S., Murphy D., Willison K., Rigby P. W. The class I major histocompatibility antigen gene activated in a line of SV40-transformed mouse cells is H-2Dd, not Qa/Tla. Nature. 1985 Jul 11;316(6024):162–163. doi: 10.1038/316162a0. [DOI] [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Minna J. D. Positive correlation between histological tumor involvement and generation of tumor cell colonies in agarose in specimens taken directly from patients with small-cell carcinoma of the lung. Cancer Res. 1980 Jun;40(6):1820–1823. [PubMed] [Google Scholar]
- Cole G. A., Cole G. A., Clements V. K., Garcia E. P., Ostrand-Rosenberg S. Allogeneic H-2 antigen expression is insufficient for tumor rejection. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8613–8617. doi: 10.1073/pnas.84.23.8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle A., Martin W. J., Funa K., Gazdar A., Carney D., Martin S. E., Linnoila I., Cuttitta F., Mulshine J., Bunn P. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J Exp Med. 1985 May 1;161(5):1135–1151. doi: 10.1084/jem.161.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M. Function by association? MHC antigens and membrane receptor complexes. Immunol Today. 1988 Jul-Aug;9(7-8):218–219. doi: 10.1016/0167-5699(88)91218-2. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Shykind B., Seidman J. G., Ozato K. Exon shuffling: mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982 Dec 23;300(5894):755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Allen H., Burkly L. C., Sherman D. H., Waneck G. L., Widera G. Molecular biology of the H-2 histocompatibility complex. Science. 1986 Jul 25;233(4762):437–443. doi: 10.1126/science.3726537. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Fukusato T., Gerber M. A., Thung S. N., Ferrone S., Schaffner F. Expression of HLA class I antigens on hepatocytes in liver disease. Am J Pathol. 1986 May;123(2):264–270. [PMC free article] [PubMed] [Google Scholar]
- Funa K., Gazdar A. F., Minna J. D., Linnoila R. I. Paucity of beta 2-microglobulin expression on small cell lung cancer, bronchial carcinoids and certain other neuroendocrine tumors. Lab Invest. 1986 Aug;55(2):186–193. [PubMed] [Google Scholar]
- Garcia-Espejo R., Alonso M. C., Solana R., Pera C., Peña J. Expression of HLA molecules on cells from fresh explants of human digestive tract cancer. J Immunogenet. 1986 Apr-Jun;13(2-3):211–217. doi: 10.1111/j.1744-313x.1986.tb01103.x. [DOI] [PubMed] [Google Scholar]
- Gattoni-Celli S., Willett C. G., Rhoads D. B., Simon B., Strauss R. M., Kirsch K., Isselbacher K. J. Partial suppression of anchorage-independent growth and tumorigenicity in immunodeficient mice by transfection of the H-2 class I gene H-2Ld into a human colon cancer cell line (HCT). Proc Natl Acad Sci U S A. 1988 Nov;85(22):8543–8547. doi: 10.1073/pnas.85.22.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill T. J., 3rd, Kunz H. W. Gene complex controlling growth and fertility linked to the major histocompatibility complex in the rat. Am J Pathol. 1979 Jul;96(1):185–206. [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Hämmerling G. J., Klar D., Katzav S., Segal S., Feldman M., Wallich R., Hämmerling A. Manipulation of metastasis and tumour growth by transfection with histocompatibility class I genes. J Immunogenet. 1986 Apr-Jun;13(2-3):153–157. doi: 10.1111/j.1744-313x.1986.tb01096.x. [DOI] [PubMed] [Google Scholar]
- Jones E. A., Bodmer W. F. Lack of expression of HLA antigens on choriocarcinoma cell lines. Tissue Antigens. 1980 Aug;16(2):195–202. doi: 10.1111/j.1399-0039.1980.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S., Waghorne C., Man M. S., Elliott B., Breitman M. L. Alteration of the tumorigenic and metastatic properties of neoplastic cells is associated with the process of calcium phosphate-mediated DNA transfection. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1263–1267. doi: 10.1073/pnas.84.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuse W., Edidin M. Influence of the mouse major histocompatibility complex, H-2, on liver adenylate cyclase activity and on glucagon binding to liver cell membranes. Biochemistry. 1980 Jan 8;19(1):49–54. doi: 10.1021/bi00542a008. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Fisher C. A., Whelan J. P. Striking paucity of HLA-A, B, C and beta 2-microglobulin on human neuroblastoma cell lines. J Immunol. 1983 May;130(5):2471–2478. [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987 Sep 4;237(4819):1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. Experimental strategies and interpretations in the analysis of changes in MHC gene expression during tumour progression. Opposing influences of T cell and natural killer mediated resistance? J Immunogenet. 1986 Apr-Jun;13(2-3):141–151. doi: 10.1111/j.1744-313x.1986.tb01095.x. [DOI] [PubMed] [Google Scholar]
- Momburg F., Degener T., Bacchus E., Moldenhauer G., Hämmerling G. J., Möller P. Loss of HLA-A,B,C and de novo expression of HLA-D in colorectal cancer. Int J Cancer. 1986 Feb 15;37(2):179–184. doi: 10.1002/ijc.2910370203. [DOI] [PubMed] [Google Scholar]
- Momburg F., Möller P., Moldenhauer G., Hämmerling G. J. Loss of HLA-A,B,C in colorectal carcinoma is related to the degree of de-differentiation. J Immunogenet. 1986 Apr-Jun;13(2-3):195–199. doi: 10.1111/j.1744-313x.1986.tb01101.x. [DOI] [PubMed] [Google Scholar]
- Ohno S. The original function of MHC antigens as the general plasma membrane anchorage site of organogenesis-directing proteins. Immunol Rev. 1977 Jan;33:59–69. [PubMed] [Google Scholar]
- Paterson A. C., Sciot R., Kew M. C., Callea F., Dusheiko G. M., Desmet V. J. HLA expression in human hepatocellular carcinoma. Br J Cancer. 1988 Apr;57(4):369–373. doi: 10.1038/bjc.1988.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. L., Moule M. L., Delovitch T. L., Yip C. C. Class I histocompatibility antigens and insulin receptors: evidence for interactions. Proc Natl Acad Sci U S A. 1986 May;83(10):3474–3478. doi: 10.1073/pnas.83.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Guild B. C., Strominger J. L. Phosphorylation in vivo and in vitro of human histocompatibility antigens (HLA-A and HLA-B) in the carboxy-terminal intracellular domain. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6002–6006. doi: 10.1073/pnas.75.12.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Guild B. C., Strominger J. L., Veatch W. R. Purification of HLA-A2 antigen, fluorescent labeling of its intracellular region, and demonstration of an interaction between fluorescently labeled HLA-A2 antigen and lymphoblastoid cell cytoskeleton proteins in vitro. Biochemistry. 1981 Sep 15;20(19):5625–5633. doi: 10.1021/bi00522a042. [DOI] [PubMed] [Google Scholar]
- Ponder B. A., Wilkinson M. M., Wood M., Westwood J. H. Immunohistochemical demonstration of H2 antigens in mouse tissue sections. J Histochem Cytochem. 1983 Jul;31(7):911–919. doi: 10.1177/31.7.6343482. [DOI] [PubMed] [Google Scholar]
- Quinn L. A., Moore G. E., Morgan R. T., Woods L. K. Cell lines from human colon carcinoma with unusual cell products, double minutes, and homogeneously staining regions. Cancer Res. 1979 Dec;39(12):4914–4924. [PubMed] [Google Scholar]
- Shoyab M., McDonald V. L., Bradley J. G., Todaro G. J. Amphiregulin: a bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate-treated human breast adenocarcinoma cell line MCF-7. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6528–6532. doi: 10.1073/pnas.85.17.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Peptide growth factors are multifunctional. Nature. 1988 Mar 17;332(6161):217–219. doi: 10.1038/332217a0. [DOI] [PubMed] [Google Scholar]
- Sunday M. E., Kaplan L. M., Motoyama E., Chin W. W., Spindel E. R. Gastrin-releasing peptide (mammalian bombesin) gene expression in health and disease. Lab Invest. 1988 Jul;59(1):5–24. [PubMed] [Google Scholar]
- Tanaka K., Isselbacher K. J., Khoury G., Jay G. Reversal of oncogenesis by the expression of a major histocompatibility complex class I gene. Science. 1985 Apr 5;228(4695):26–30. doi: 10.1126/science.3975631. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Yoshioka T., Bieberich C., Jay G. Role of the major histocompatibility complex class I antigens in tumor growth and metastasis. Annu Rev Immunol. 1988;6:359–380. doi: 10.1146/annurev.iy.06.040188.002043. [DOI] [PubMed] [Google Scholar]
- Townes T. M., Lingrel J. B., Chen H. Y., Brinster R. L., Palmiter R. D. Erythroid-specific expression of human beta-globin genes in transgenic mice. EMBO J. 1985 Jul;4(7):1715–1723. doi: 10.1002/j.1460-2075.1985.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallich R., Bulbuc N., Hämmerling G. J., Katzav S., Segal S., Feldman M. Abrogation of metastatic properties of tumour cells by de novo expression of H-2K antigens following H-2 gene transfection. Nature. 1985 May 23;315(6017):301–305. doi: 10.1038/315301a0. [DOI] [PubMed] [Google Scholar]
- Weis J. H., Reiss C. S., Mulligan R. C., Burakoff S. J., Seidman J. G. H-2Ld antigen encoded by a recombinant retrovirus genome is expressed on the surface of infected cells. Mol Cell Biol. 1985 Jun;5(6):1379–1384. doi: 10.1128/mcb.5.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Yamazaki K., Beauchamp G. K., Bard J., Thomas L., Boyse E. A. Distinctive urinary odors governed by the major histocompatibility locus of the mouse. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5817–5820. doi: 10.1073/pnas.78.9.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman R. J., Gaillard E. T., Goldin A. Metastatic potential of four human melanoma xenografts in young athymic mice following tail vein inoculation. Cancer Res. 1987 May 1;47(9):2305–2310. [PubMed] [Google Scholar]